 Found 186 hits with Last Name = 'subramanian' and Initial = 'n'
Found 186 hits with Last Name = 'subramanian' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 21.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 904-14 (2001)
Article DOI: 10.1016/S0893-133X(01)00285-8
BindingDB Entry DOI: 10.7270/Q21V5CHQ |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50068974
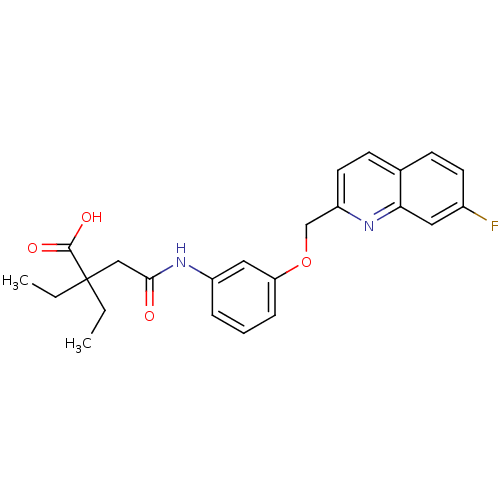
(2,2-Diethyl-N-[3-(7-fluoro-quinolin-2-ylmethoxy)-p...)Show SMILES CCC(CC)(CC(=O)Nc1cccc(OCc2ccc3ccc(F)cc3n2)c1)C(O)=O Show InChI InChI=1S/C24H25FN2O4/c1-3-24(4-2,23(29)30)14-22(28)27-18-6-5-7-20(13-18)31-15-19-11-9-16-8-10-17(25)12-21(16)26-19/h5-13H,3-4,14-15H2,1-2H3,(H,27,28)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro for antagonistic activity against LTD4 receptor in guinea pig ileum |
Bioorg Med Chem Lett 8: 965-70 (1999)
BindingDB Entry DOI: 10.7270/Q2V123XT |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50215369
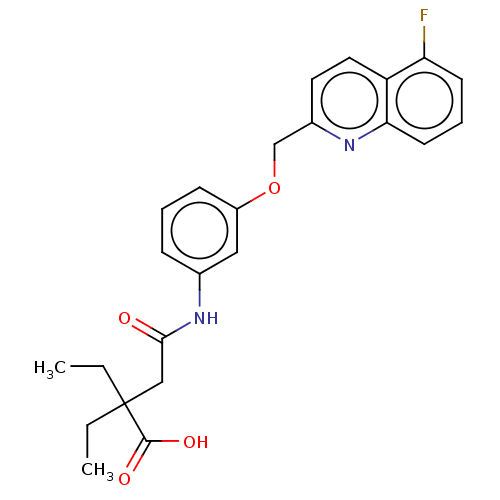
(CHEMBL368290)Show SMILES CCC(CC)(CC(=O)Nc1cccc(OCc2ccc3c(F)cccc3n2)c1)C(O)=O Show InChI InChI=1S/C24H25FN2O4/c1-3-24(4-2,23(29)30)14-22(28)27-16-7-5-8-18(13-16)31-15-17-11-12-19-20(25)9-6-10-21(19)26-17/h5-13H,3-4,14-15H2,1-2H3,(H,27,28)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro for antagonistic activity against LTD4 receptor in guinea pig ileum |
Bioorg Med Chem Lett 8: 965-70 (1999)
BindingDB Entry DOI: 10.7270/Q2V123XT |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50215411
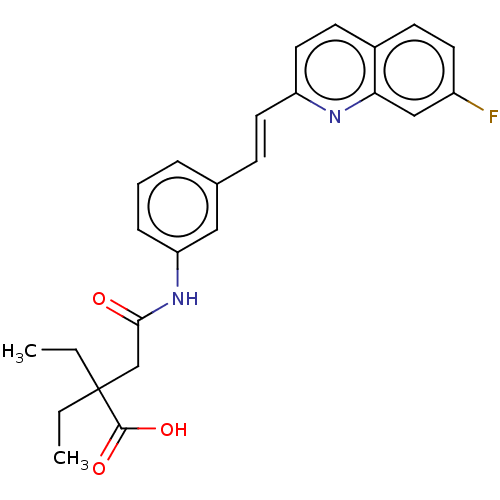
(CHEMBL425675)Show SMILES CCC(CC)(CC(=O)Nc1cccc(\C=C\c2ccc3ccc(F)cc3n2)c1)C(O)=O Show InChI InChI=1S/C25H25FN2O3/c1-3-25(4-2,24(30)31)16-23(29)28-21-7-5-6-17(14-21)8-12-20-13-10-18-9-11-19(26)15-22(18)27-20/h5-15H,3-4,16H2,1-2H3,(H,28,29)(H,30,31)/b12-8+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro for antagonistic activity against LTD4 receptor in guinea pig ileum |
Bioorg Med Chem Lett 8: 965-70 (1999)
BindingDB Entry DOI: 10.7270/Q2V123XT |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50215410
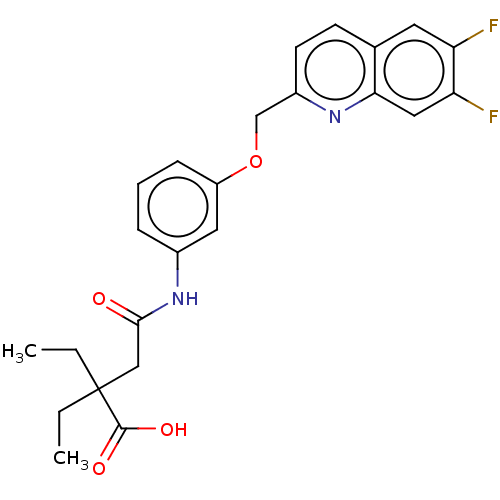
(CHEMBL369421)Show SMILES CCC(CC)(CC(=O)Nc1cccc(OCc2ccc3cc(F)c(F)cc3n2)c1)C(O)=O Show InChI InChI=1S/C24H24F2N2O4/c1-3-24(4-2,23(30)31)13-22(29)28-16-6-5-7-18(11-16)32-14-17-9-8-15-10-19(25)20(26)12-21(15)27-17/h5-12H,3-4,13-14H2,1-2H3,(H,28,29)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro for antagonistic activity against LTD4 receptor in guinea pig ileum |
Bioorg Med Chem Lett 8: 965-70 (1999)
BindingDB Entry DOI: 10.7270/Q2V123XT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50106901
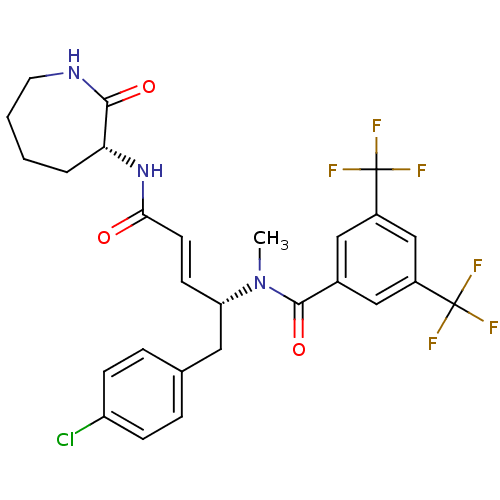
(CHEMBL104954 | N-[(E)-(R)-1-(4-Chloro-benzyl)-3-((...)Show SMILES CN([C@H](Cc1ccc(Cl)cc1)\C=C\C(=O)N[C@@H]1CCCCNC1=O)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H26ClF6N3O3/c1-37(25(40)17-13-18(26(29,30)31)15-19(14-17)27(32,33)34)21(12-16-5-7-20(28)8-6-16)9-10-23(38)36-22-4-2-3-11-35-24(22)39/h5-10,13-15,21-22H,2-4,11-12H2,1H3,(H,35,39)(H,36,38)/b10-9+/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-Sar9-substance P binding to Tachykinin receptor 1 in bovine retinal membranes |
Bioorg Med Chem Lett 10: 1467-70 (2000)
BindingDB Entry DOI: 10.7270/Q2M907X9 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50106901

(CHEMBL104954 | N-[(E)-(R)-1-(4-Chloro-benzyl)-3-((...)Show SMILES CN([C@H](Cc1ccc(Cl)cc1)\C=C\C(=O)N[C@@H]1CCCCNC1=O)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H26ClF6N3O3/c1-37(25(40)17-13-18(26(29,30)31)15-19(14-17)27(32,33)34)21(12-16-5-7-20(28)8-6-16)9-10-23(38)36-22-4-2-3-11-35-24(22)39/h5-10,13-15,21-22H,2-4,11-12H2,1H3,(H,35,39)(H,36,38)/b10-9+/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro binding affinity for Tachykinin receptor 1 of bovine retinal membranes using [3H]-Sar9-substance P as radioligand |
Bioorg Med Chem Lett 11: 3081-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V40THD |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50106898
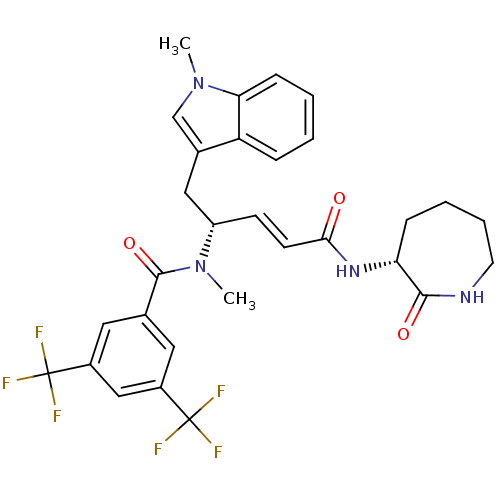
(CHEMBL317632 | N-Methyl-N-[(E)-(R)-1-(1-methyl-1H-...)Show SMILES CN([C@H](Cc1cn(C)c2ccccc12)\C=C\C(=O)N[C@@H]1CCCCNC1=O)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C30H30F6N4O3/c1-39-17-19(23-7-3-4-9-25(23)39)15-22(10-11-26(41)38-24-8-5-6-12-37-27(24)42)40(2)28(43)18-13-20(29(31,32)33)16-21(14-18)30(34,35)36/h3-4,7,9-11,13-14,16-17,22,24H,5-6,8,12,15H2,1-2H3,(H,37,42)(H,38,41)/b11-10+/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro binding affinity for Tachykinin receptor 1 of bovine retinal membranes using [3H]-Sar9-substance P as radioligand |
Bioorg Med Chem Lett 11: 3081-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V40THD |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50115967

(2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...)Show SMILES CN(O)C(=O)c1ccc(-c2ccc(Cl)cc2)c(c1)N(C)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H17ClF6N2O3/c1-32(21(34)15-9-16(23(26,27)28)12-17(10-15)24(29,30)31)20-11-14(22(35)33(2)36)5-8-19(20)13-3-6-18(25)7-4-13/h3-12,36H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50115966
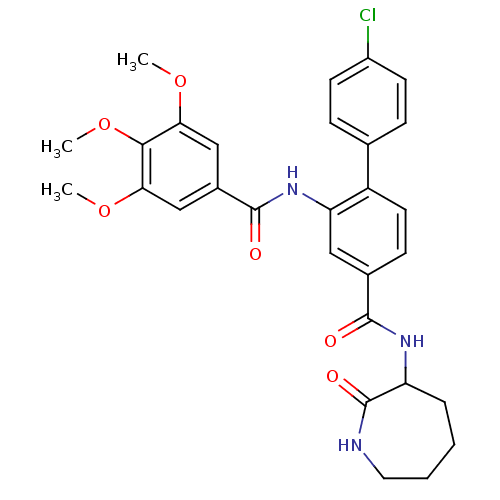
(4'-Chloro-2-(3,4,5-trimethoxy-benzoylamino)-biphen...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)Nc1cc(ccc1-c1ccc(Cl)cc1)C(=O)NC1CCCCNC1=O Show InChI InChI=1S/C29H30ClN3O6/c1-37-24-15-19(16-25(38-2)26(24)39-3)28(35)33-23-14-18(9-12-21(23)17-7-10-20(30)11-8-17)27(34)32-22-6-4-5-13-31-29(22)36/h7-12,14-16,22H,4-6,13H2,1-3H3,(H,31,36)(H,32,34)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50089567
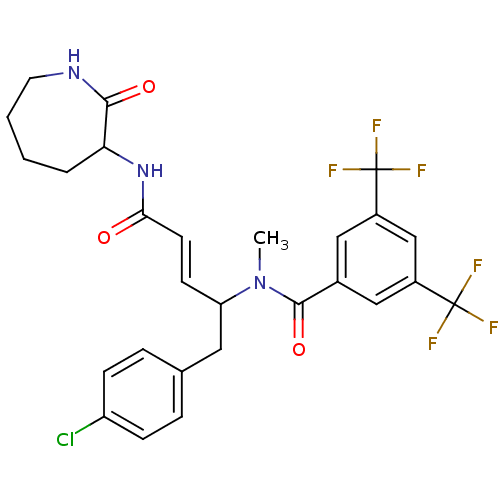
(CHEMBL35857 | N-[(E)-1-(4-Chloro-benzyl)-3-(2-oxo-...)Show SMILES CN(C(Cc1ccc(Cl)cc1)\C=C\C(=O)NC1CCCCNC1=O)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H26ClF6N3O3/c1-37(25(40)17-13-18(26(29,30)31)15-19(14-17)27(32,33)34)21(12-16-5-7-20(28)8-6-16)9-10-23(38)36-22-4-2-3-11-35-24(22)39/h5-10,13-15,21-22H,2-4,11-12H2,1H3,(H,35,39)(H,36,38)/b10-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-Sar9-substance P binding to Tachykinin receptor 1 in bovine retinal membranes |
Bioorg Med Chem Lett 10: 1467-70 (2000)
BindingDB Entry DOI: 10.7270/Q2M907X9 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50403920
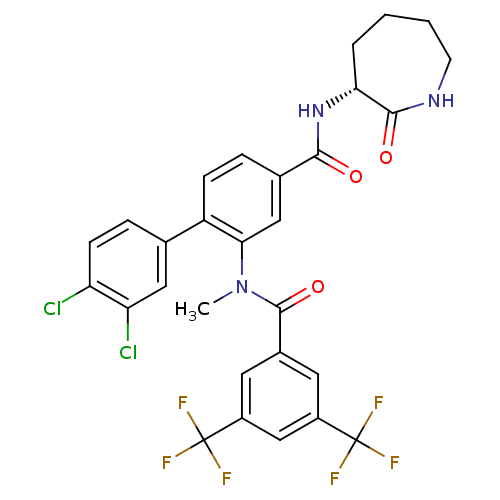
(CHEMBL2112212)Show SMILES CN(C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cc(ccc1-c1ccc(Cl)c(Cl)c1)C(=O)N[C@@H]1CCCCNC1=O |r| Show InChI InChI=1S/C29H23Cl2F6N3O3/c1-40(27(43)17-10-18(28(32,33)34)14-19(11-17)29(35,36)37)24-13-16(25(41)39-23-4-2-3-9-38-26(23)42)5-7-20(24)15-6-8-21(30)22(31)12-15/h5-8,10-14,23H,2-4,9H2,1H3,(H,38,42)(H,39,41)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50068987
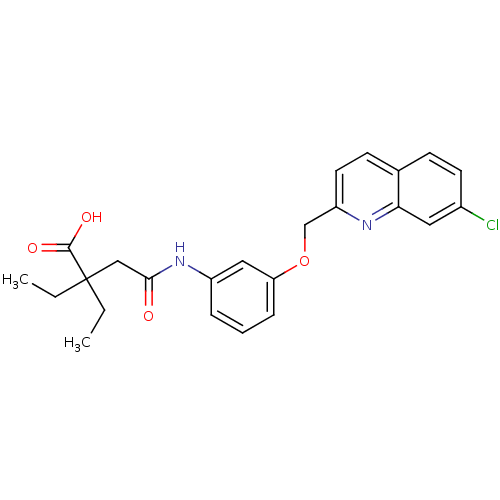
(CHEMBL175119 | N-[3-(7-Chloro-quinolin-2-ylmethoxy...)Show SMILES CCC(CC)(CC(=O)Nc1cccc(OCc2ccc3ccc(Cl)cc3n2)c1)C(O)=O Show InChI InChI=1S/C24H25ClN2O4/c1-3-24(4-2,23(29)30)14-22(28)27-18-6-5-7-20(13-18)31-15-19-11-9-16-8-10-17(25)12-21(16)26-19/h5-13H,3-4,14-15H2,1-2H3,(H,27,28)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro for antagonistic activity against LTD4 receptor in guinea pig ileum |
Bioorg Med Chem Lett 8: 965-70 (1999)
BindingDB Entry DOI: 10.7270/Q2V123XT |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50068980
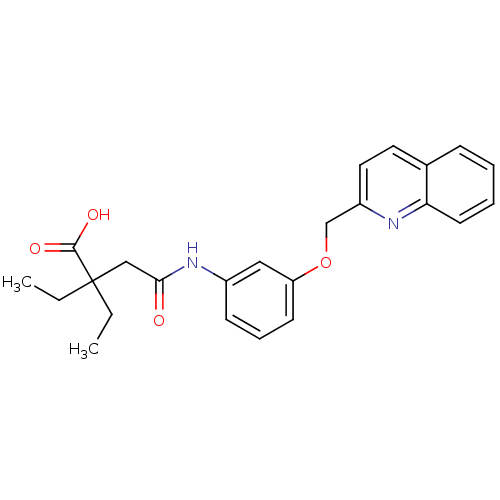
(2,2-Diethyl-N-[3-(quinolin-2-ylmethoxy)-phenyl]-su...)Show SMILES CCC(CC)(CC(=O)Nc1cccc(OCc2ccc3ccccc3n2)c1)C(O)=O Show InChI InChI=1S/C24H26N2O4/c1-3-24(4-2,23(28)29)15-22(27)26-18-9-7-10-20(14-18)30-16-19-13-12-17-8-5-6-11-21(17)25-19/h5-14H,3-4,15-16H2,1-2H3,(H,26,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro for antagonistic activity against LTD4 receptor in guinea pig ileum |
Bioorg Med Chem Lett 8: 965-70 (1999)
BindingDB Entry DOI: 10.7270/Q2V123XT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50115965

(2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...)Show SMILES CN(C)CCCNC(=O)c1ccc(-c2ccc(Cl)cc2)c(c1)N(C)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C28H26ClF6N3O2/c1-37(2)12-4-11-36-25(39)18-7-10-23(17-5-8-22(29)9-6-17)24(15-18)38(3)26(40)19-13-20(27(30,31)32)16-21(14-19)28(33,34)35/h5-10,13-16H,4,11-12H2,1-3H3,(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50215370
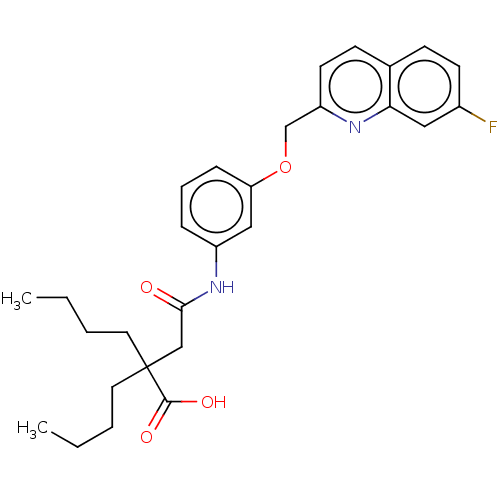
(CHEMBL174265)Show SMILES CCCCC(CCCC)(CC(=O)Nc1cccc(OCc2ccc3ccc(F)cc3n2)c1)C(O)=O Show InChI InChI=1S/C28H33FN2O4/c1-3-5-14-28(27(33)34,15-6-4-2)18-26(32)31-22-8-7-9-24(17-22)35-19-23-13-11-20-10-12-21(29)16-25(20)30-23/h7-13,16-17H,3-6,14-15,18-19H2,1-2H3,(H,31,32)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro for antagonistic activity against LTD4 receptor in guinea pig ileum |
Bioorg Med Chem Lett 8: 965-70 (1999)
BindingDB Entry DOI: 10.7270/Q2V123XT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50403918
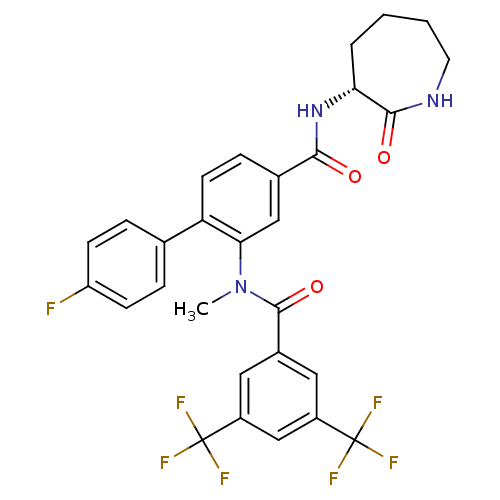
(CHEMBL2113739)Show SMILES CN(C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cc(ccc1-c1ccc(F)cc1)C(=O)N[C@@H]1CCCCNC1=O |r| Show InChI InChI=1S/C29H24F7N3O3/c1-39(27(42)18-12-19(28(31,32)33)15-20(13-18)29(34,35)36)24-14-17(7-10-22(24)16-5-8-21(30)9-6-16)25(40)38-23-4-2-3-11-37-26(23)41/h5-10,12-15,23H,2-4,11H2,1H3,(H,37,41)(H,38,40)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50106899
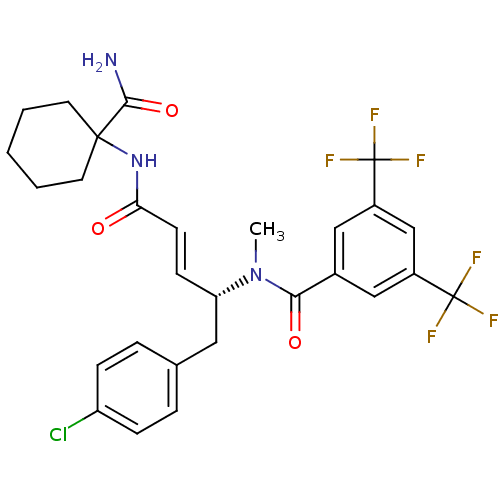
(CHEMBL105600 | N-[(E)-(R)-3-(1-Carbamoyl-cyclohexy...)Show SMILES CN([C@H](Cc1ccc(Cl)cc1)\C=C\C(=O)NC1(CCCCC1)C(N)=O)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C28H28ClF6N3O3/c1-38(24(40)18-14-19(27(30,31)32)16-20(15-18)28(33,34)35)22(13-17-5-7-21(29)8-6-17)9-10-23(39)37-26(25(36)41)11-3-2-4-12-26/h5-10,14-16,22H,2-4,11-13H2,1H3,(H2,36,41)(H,37,39)/b10-9+/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro binding affinity for Tachykinin receptor 1 of bovine retinal membranes using [3H]-Sar9-substance P as radioligand |
Bioorg Med Chem Lett 11: 3081-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V40THD |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50106900

(CHEMBL103979 | N-Methyl-N-[(E)-(R)-1-naphthalen-2-...)Show SMILES CN([C@H](Cc1ccc2ccccc2c1)\C=C\C(=O)N[C@@H]1CCCCNC1=O)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C31H29F6N3O3/c1-40(29(43)22-16-23(30(32,33)34)18-24(17-22)31(35,36)37)25(15-19-9-10-20-6-2-3-7-21(20)14-19)11-12-27(41)39-26-8-4-5-13-38-28(26)42/h2-3,6-7,9-12,14,16-18,25-26H,4-5,8,13,15H2,1H3,(H,38,42)(H,39,41)/b12-11+/t25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro binding affinity for Tachykinin receptor 1 of bovine retinal membranes using [3H]-Sar9-substance P as radioligand |
Bioorg Med Chem Lett 11: 3081-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V40THD |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50106891
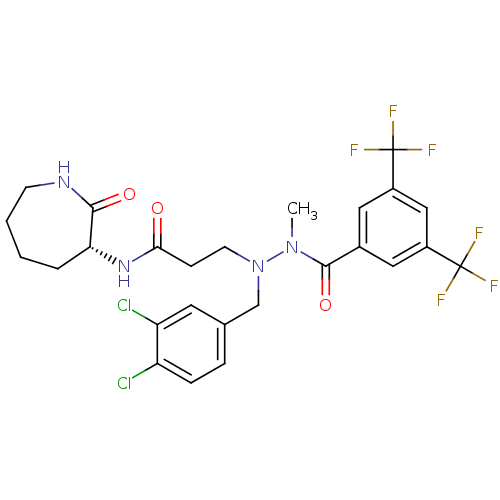
(3-[N'-(3,5-Bis-trifluoromethyl-benzoyl)-N-(3,4-dic...)Show SMILES CN(N(CCC(=O)N[C@@H]1CCCCNC1=O)Cc1ccc(Cl)c(Cl)c1)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C26H26Cl2F6N4O3/c1-37(24(41)16-11-17(25(29,30)31)13-18(12-16)26(32,33)34)38(14-15-5-6-19(27)20(28)10-15)9-7-22(39)36-21-4-2-3-8-35-23(21)40/h5-6,10-13,21H,2-4,7-9,14H2,1H3,(H,35,40)(H,36,39)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro binding affinity for Tachykinin receptor 1 of bovine retinal membranes using [3H]-Sar9-substance P as radioligand |
Bioorg Med Chem Lett 11: 3081-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V40THD |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50215372
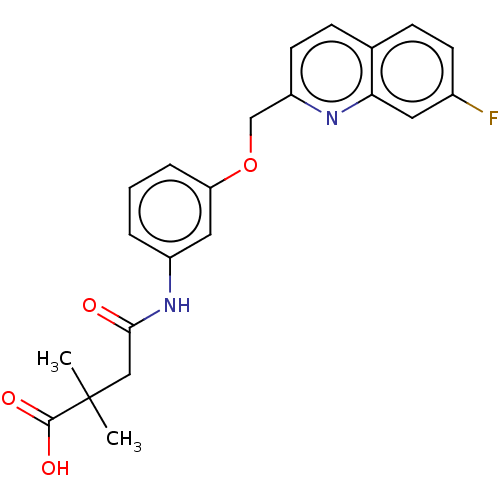
(CHEMBL175419)Show SMILES CC(C)(CC(=O)Nc1cccc(OCc2ccc3ccc(F)cc3n2)c1)C(O)=O Show InChI InChI=1S/C22H21FN2O4/c1-22(2,21(27)28)12-20(26)25-16-4-3-5-18(11-16)29-13-17-9-7-14-6-8-15(23)10-19(14)24-17/h3-11H,12-13H2,1-2H3,(H,25,26)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro for antagonistic activity against LTD4 receptor in guinea pig ileum |
Bioorg Med Chem Lett 8: 965-70 (1999)
BindingDB Entry DOI: 10.7270/Q2V123XT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
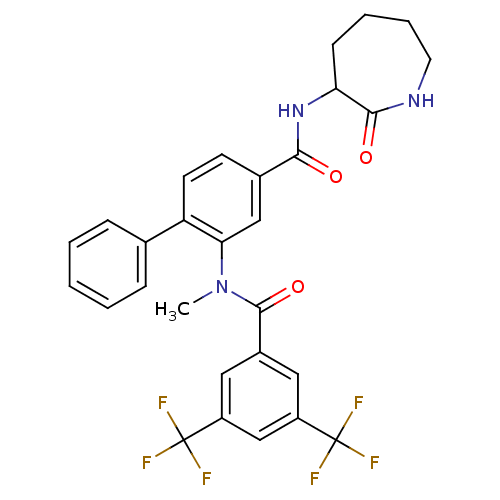
(Homo sapiens (Human)) | BDBM50115959
(2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...)Show SMILES CN(C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cc(ccc1-c1ccccc1)C(=O)NC1CCCCNC1=O Show InChI InChI=1S/C29H25F6N3O3/c1-38(27(41)19-13-20(28(30,31)32)16-21(14-19)29(33,34)35)24-15-18(10-11-22(24)17-7-3-2-4-8-17)25(39)37-23-9-5-6-12-36-26(23)40/h2-4,7-8,10-11,13-16,23H,5-6,9,12H2,1H3,(H,36,40)(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data