

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50256379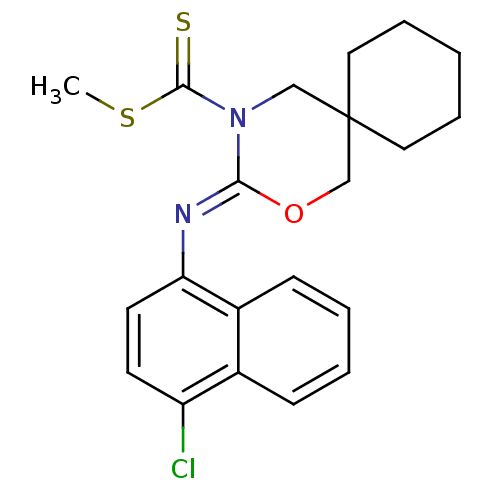 (CHEMBL482356 | methyl 3-(4-chloronaphthalen-1-ylim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to mouse CB1 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172430 (US9090618, ZA53 | US9598411, Ref. No. ZA53) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50256378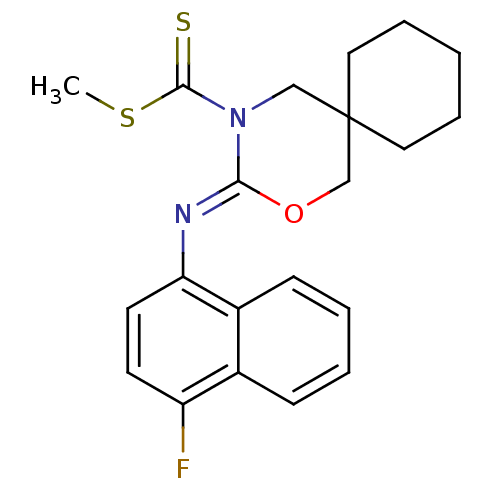 (CHEMBL482355 | methyl 3-(4-fluoronaphthalen-1-ylim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50256315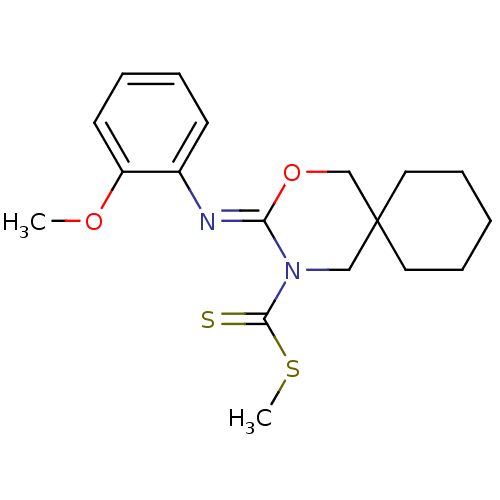 (CHEMBL469895 | methyl 3-(2-methoxyphenylimino)-2-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50256318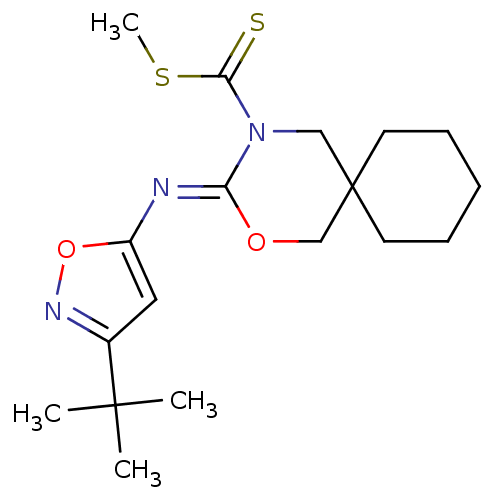 (CHEMBL481508 | methyl 3-(3-tert-butylisoxazol-5-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50256218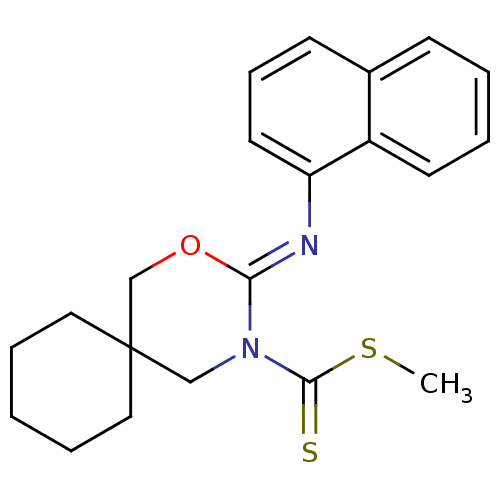 (CHEMBL516406 | methyl 3-(naphthalen-1-ylimino)-2-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50256379 (CHEMBL482356 | methyl 3-(4-chloronaphthalen-1-ylim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172419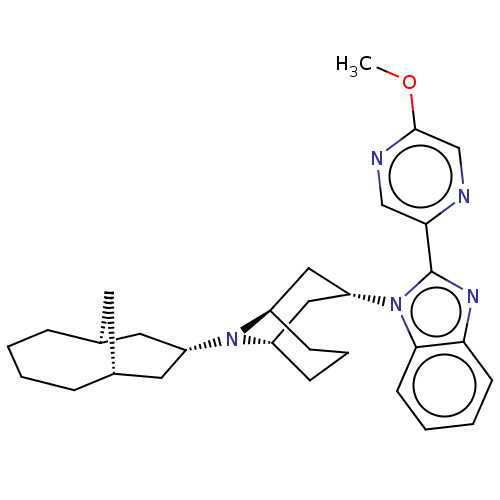 (US9090618, ZA42 | US9598411, Ref. No. ZA42) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50256318 (CHEMBL481508 | methyl 3-(3-tert-butylisoxazol-5-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50256319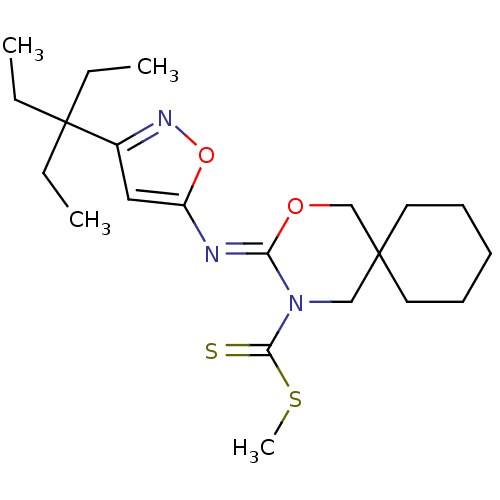 (CHEMBL519288 | methyl 3-(3-(3-ethylpentan-3-yl)iso...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50256317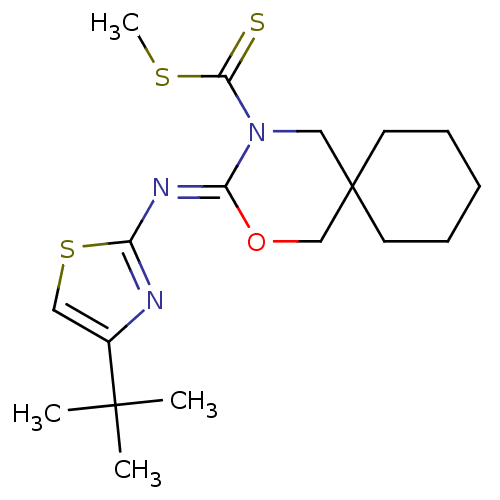 (CHEMBL511790 | methyl 3-(4-tert-butylthiazol-2-yli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50256431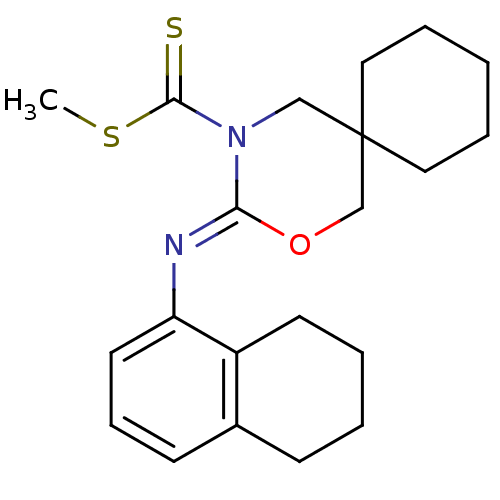 (CHEMBL480578 | methyl 3-(5,6,7,8-tetrahydronaphtha...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50256319 (CHEMBL519288 | methyl 3-(3-(3-ethylpentan-3-yl)iso...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50256317 (CHEMBL511790 | methyl 3-(4-tert-butylthiazol-2-yli...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172418 (US9090618, ZA41 | US9598411, Ref. No. ZA41) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50284800 (((5R,6S)-9-Bromo-6-ethyl-2,3-dioxo-2,3,6,7-tetrahy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity measured by using [3H]5,7-dichlorokynurenic acid (DCKA) for the glycine binding site of NMDA receptor | Bioorg Med Chem Lett 5: 1527-1532 (1995) Article DOI: 10.1016/0960-894X(95)00243-M BindingDB Entry DOI: 10.7270/Q2K35TKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50256219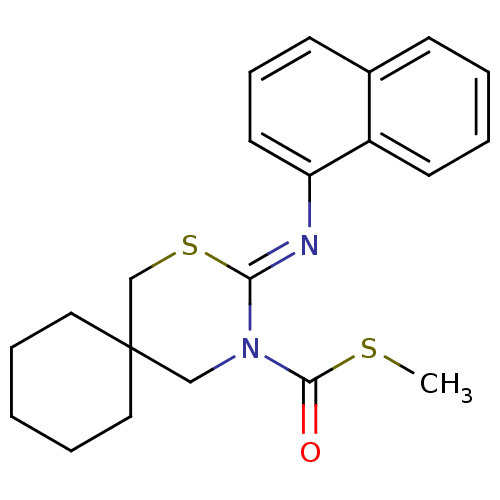 (CHEMBL475772 | S-methyl 3-(naphthalen-1-ylimino)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50256380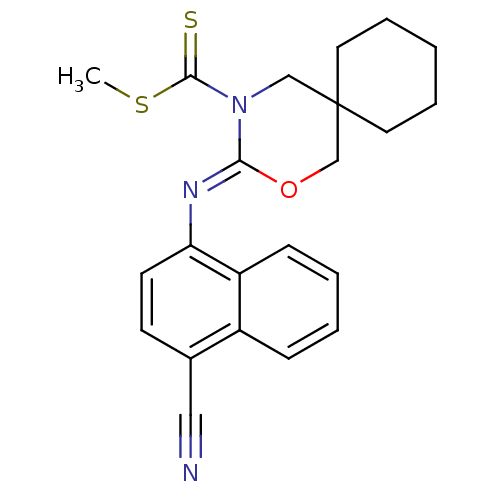 (CHEMBL482357 | methyl 3-(4-cyanonaphthalen-1-ylimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50256433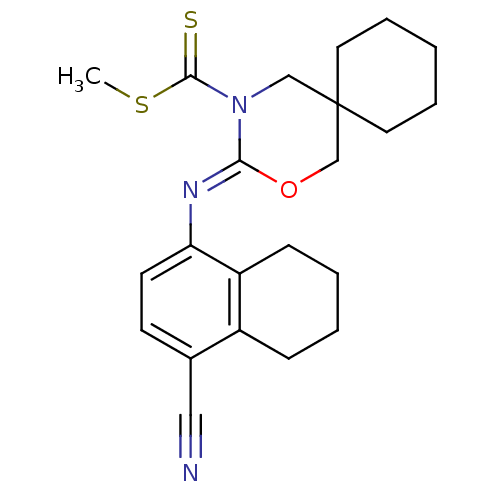 (CHEMBL481888 | methyl 3-(4-cyano-5,6,7,8-tetrahydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172382 (US9090618, H27b(i) | US9598411, Ref. No. H27b(i)) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172433 (US9090618, ZA56 | US9598411, Ref. No. ZA56) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172417 (US9090618, ZA40 | US9598411, Ref. No. ZA40) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50256381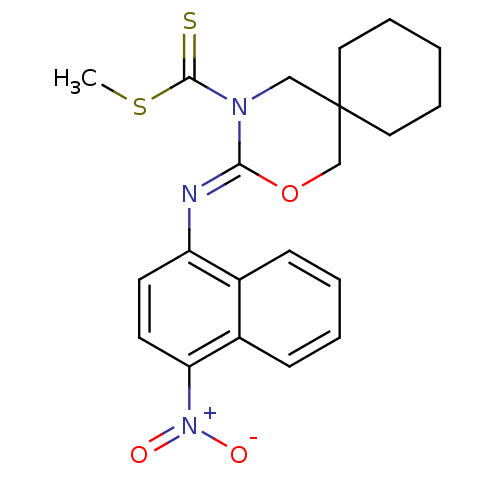 (CHEMBL519635 | methyl 3-(4-nitronaphthalen-1-ylimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50038047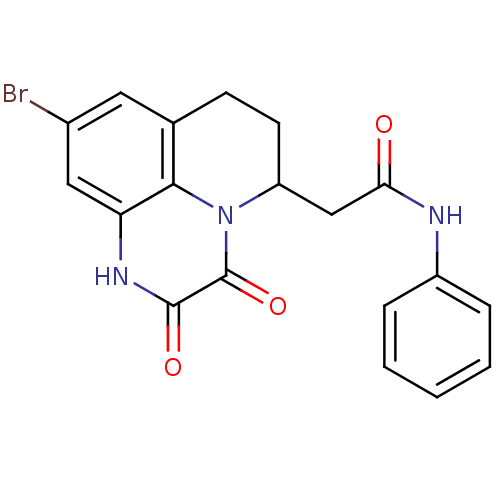 (2-(9-Bromo-2,3-dioxo-2,3,6,7-tetrahydro-1H,5H-pyri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity for the glycine binding site of the NMDA receptor using [3H]- 5,7- dichloro -kynurenic acid as radio-ligand | Bioorg Med Chem Lett 5: 1533-1536 (1995) Article DOI: 10.1016/0960-894X(95)00244-N BindingDB Entry DOI: 10.7270/Q2FB52WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50038046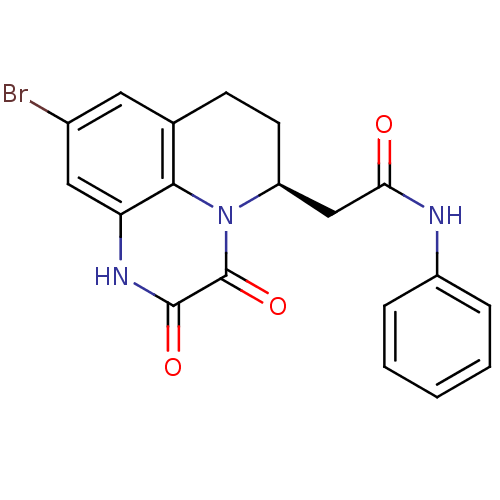 (2-((S)-9-Bromo-2,3-dioxo-2,3,6,7-tetrahydro-1H,5H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity measured by using [3H]5,7-dichlorokynurenic acid (DCKA) for the glycine binding site of NMDA receptor | Bioorg Med Chem Lett 5: 1527-1532 (1995) Article DOI: 10.1016/0960-894X(95)00243-M BindingDB Entry DOI: 10.7270/Q2K35TKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172413 (US9090618, ZA35 | US9598411, Ref. No. ZA35) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50284808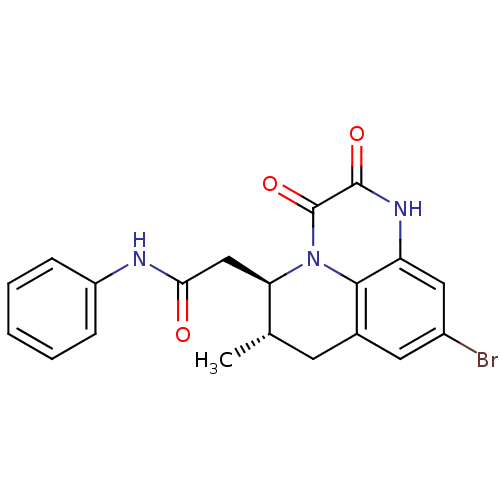 (2-((5R,6S)-9-Bromo-6-methyl-2,3-dioxo-2,3,6,7-tetr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity for the glycine binding site of the NMDA receptor using [3H]- 5,7- dichloro -kynurenic acid as radio-ligand | Bioorg Med Chem Lett 5: 1533-1536 (1995) Article DOI: 10.1016/0960-894X(95)00244-N BindingDB Entry DOI: 10.7270/Q2FB52WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50284808 (2-((5R,6S)-9-Bromo-6-methyl-2,3-dioxo-2,3,6,7-tetr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity measured by using [3H]5,7-dichlorokynurenic acid (DCKA) for the glycine binding site of NMDA receptor | Bioorg Med Chem Lett 5: 1527-1532 (1995) Article DOI: 10.1016/0960-894X(95)00243-M BindingDB Entry DOI: 10.7270/Q2K35TKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50284815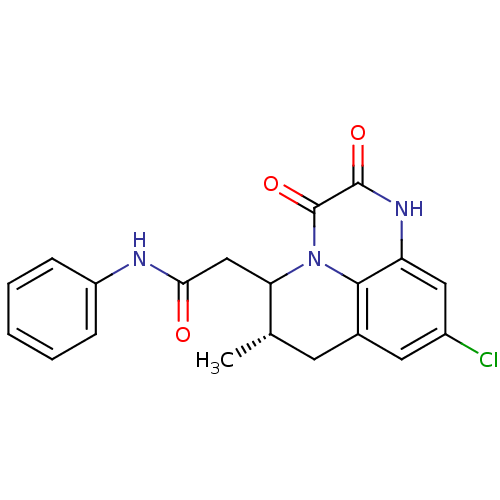 (2-((S)-9-Chloro-6-methyl-2,3-dioxo-2,3,6,7-tetrahy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity for the glycine binding site of the NMDA receptor using [3H]- 5,7- dichloro -kynurenic acid as radio-ligand | Bioorg Med Chem Lett 5: 1533-1536 (1995) Article DOI: 10.1016/0960-894X(95)00244-N BindingDB Entry DOI: 10.7270/Q2FB52WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50284801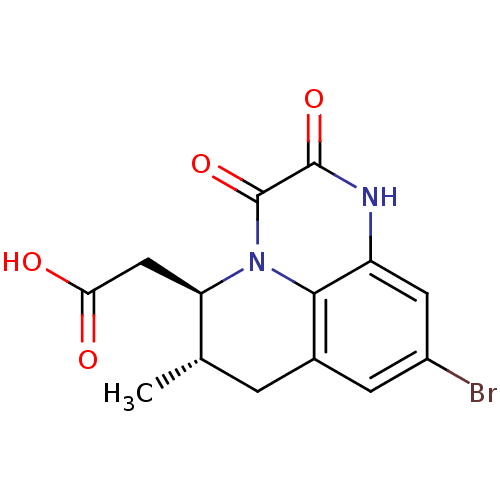 (((5R,6S)-9-Bromo-6-methyl-2,3-dioxo-2,3,6,7-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity measured by using [3H]5,7-dichlorokynurenic acid (DCKA) for the glycine binding site of NMDA receptor | Bioorg Med Chem Lett 5: 1527-1532 (1995) Article DOI: 10.1016/0960-894X(95)00243-M BindingDB Entry DOI: 10.7270/Q2K35TKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50284801 (((5R,6S)-9-Bromo-6-methyl-2,3-dioxo-2,3,6,7-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity for the glycine binding site of the NMDA receptor using [3H]- 5,7- dichloro -kynurenic acid as radio-ligand | Bioorg Med Chem Lett 5: 1533-1536 (1995) Article DOI: 10.1016/0960-894X(95)00244-N BindingDB Entry DOI: 10.7270/Q2FB52WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50256379 (CHEMBL482356 | methyl 3-(4-chloronaphthalen-1-ylim...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50284805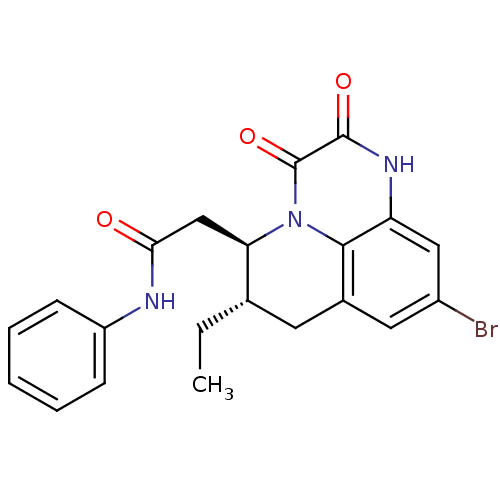 (2-((5R,6S)-9-Bromo-6-ethyl-2,3-dioxo-2,3,6,7-tetra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity measured by using [3H]5,7-dichlorokynurenic acid (DCKA) for the glycine binding site of NMDA receptor | Bioorg Med Chem Lett 5: 1527-1532 (1995) Article DOI: 10.1016/0960-894X(95)00243-M BindingDB Entry DOI: 10.7270/Q2K35TKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172438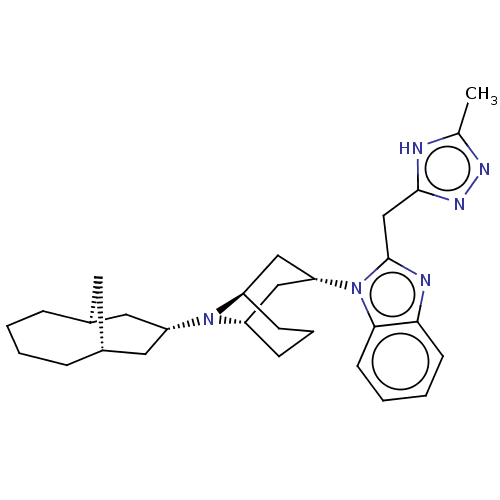 (US9090618, ZA61 | US9598411, Ref. No. ZA61) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172440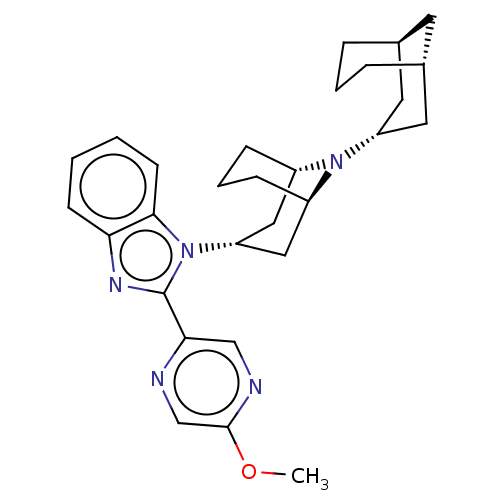 (US9090618, ZA63 | US9598411, Ref. No. ZA63) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172444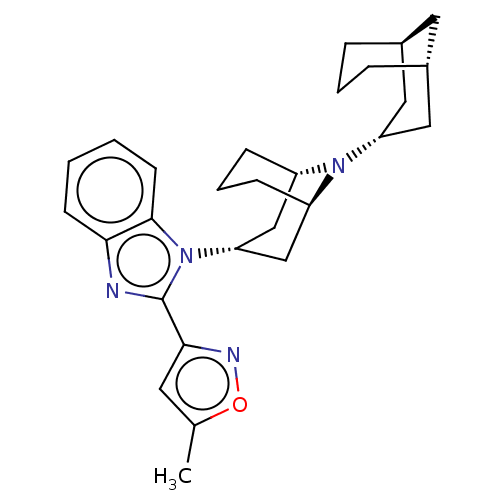 (US9090618, ZA67 | US9598411, Ref. No. ZA67) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50284813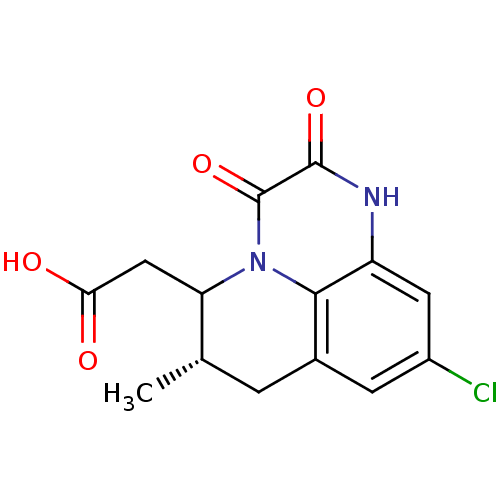 (((S)-9-Chloro-6-methyl-2,3-dioxo-2,3,6,7-tetrahydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity for the glycine binding site of the NMDA receptor using [3H]- 5,7- dichloro -kynurenic acid as radio-ligand | Bioorg Med Chem Lett 5: 1533-1536 (1995) Article DOI: 10.1016/0960-894X(95)00244-N BindingDB Entry DOI: 10.7270/Q2FB52WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50257064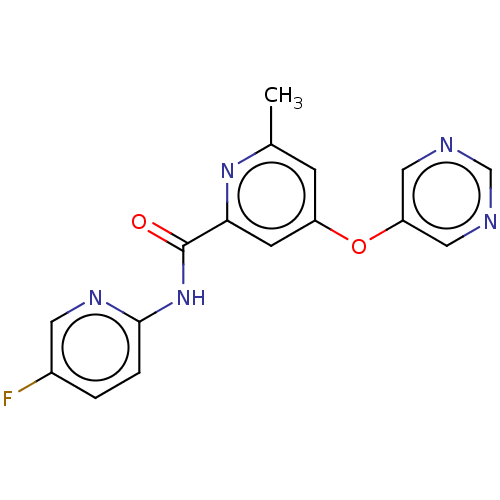 (CHEMBL2386850) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Radiology and Radiological Sciences, Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center , Nashville, Tennessee 37232, United States. Curated by ChEMBL | Assay Description Displacement of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from rat mGlu5 receptor expressed in HEK293A cell membranes after 1 hr by scintillatio... | J Med Chem 60: 5072-5085 (2017) Article DOI: 10.1021/acs.jmedchem.7b00410 BindingDB Entry DOI: 10.7270/Q2JH3PM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172437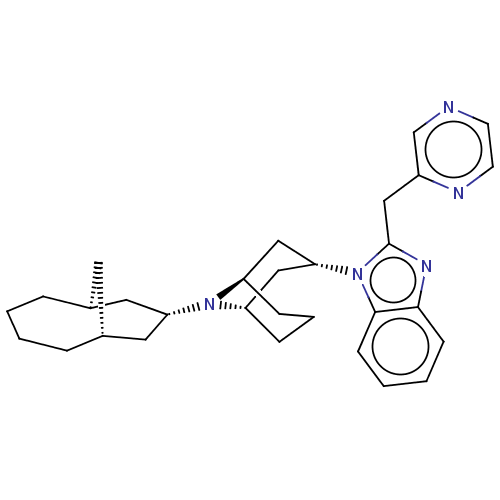 (US9090618, ZA60 | US9598411, Ref. No. ZA60) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18656 ((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd] where [L] is the concentration of labeled ligand a... | J Med Chem 49: 716-26 (2006) Article DOI: 10.1021/jm050293c BindingDB Entry DOI: 10.7270/Q2P84952 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583383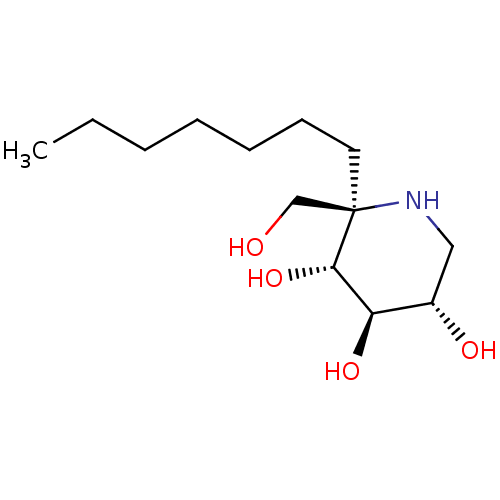 (CHEMBL5028005 | US20230339856, Compound (IIb3)) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50256377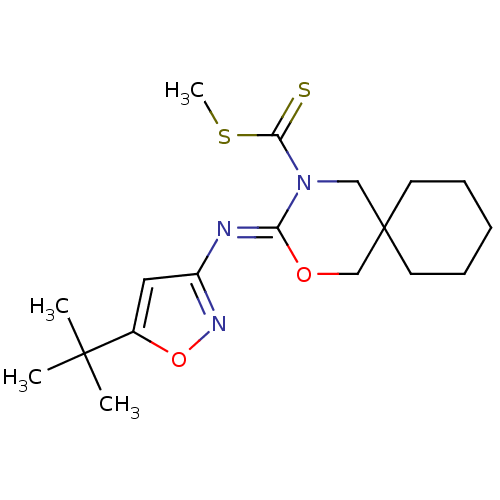 (CHEMBL449624 | methyl 3-(5-tert-butylisoxazol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172380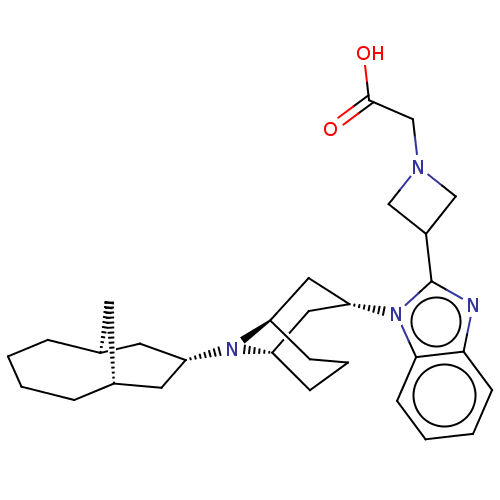 (US9090618, H4d | US9598411, Ref. No. H4d) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172446 (US9090618, ZA69 | US9598411, Ref. No. ZA69) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50256378 (CHEMBL482355 | methyl 3-(4-fluoronaphthalen-1-ylim...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50256430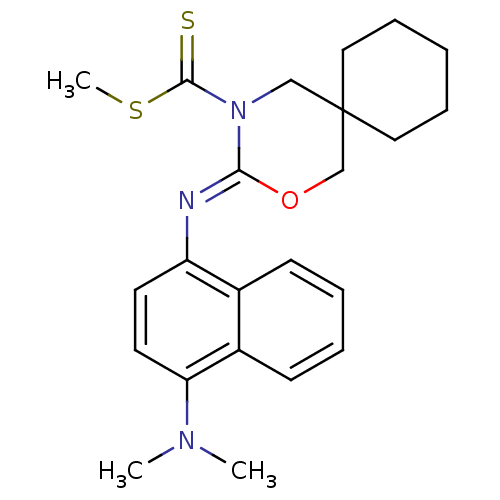 (CHEMBL481716 | methyl 3-(4-(dimethylamino)naphthal...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172416 (US9090618, ZA39 | US9598411, Ref. No. ZA39) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50284817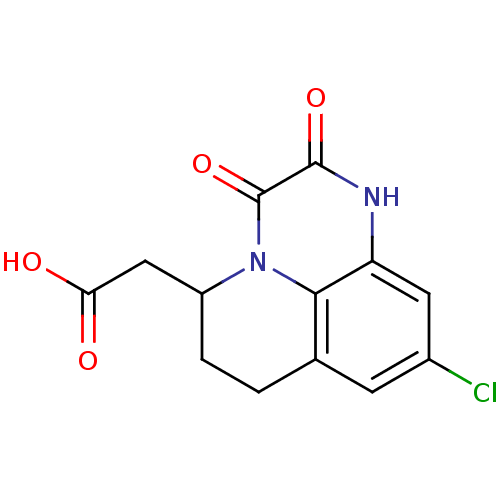 ((9-Chloro-2,3-dioxo-2,3,6,7-tetrahydro-1H,5H-pyrid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity for the glycine binding site of the NMDA receptor using [3H]- 5,7- dichloro -kynurenic acid as radio-ligand | Bioorg Med Chem Lett 5: 1533-1536 (1995) Article DOI: 10.1016/0960-894X(95)00244-N BindingDB Entry DOI: 10.7270/Q2FB52WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172432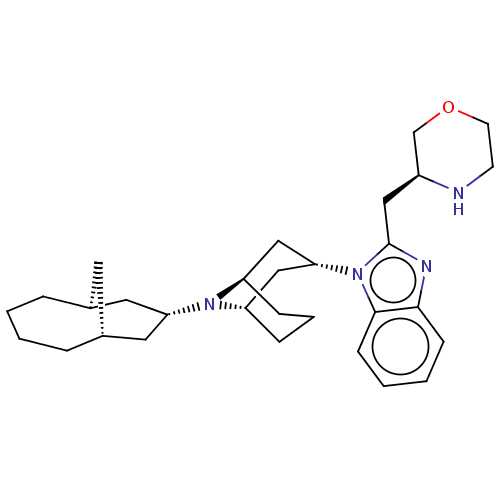 (US9090618, ZA55 | US9598411, Ref. No. ZA55) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50256432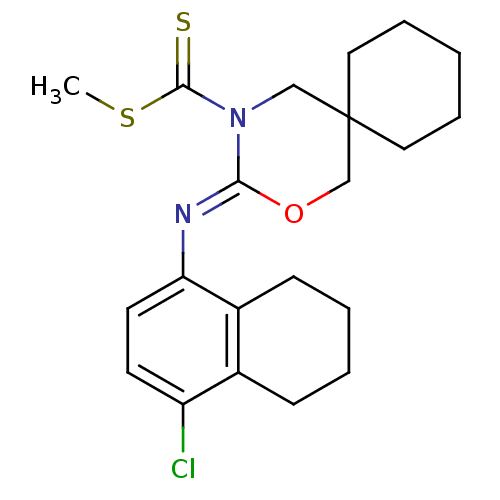 (CHEMBL480579 | methyl 3-(4-chloro-5,6,7,8-tetrahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 18: 6444-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.070 BindingDB Entry DOI: 10.7270/Q20V8CNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2939 total ) | Next | Last >> |