

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511450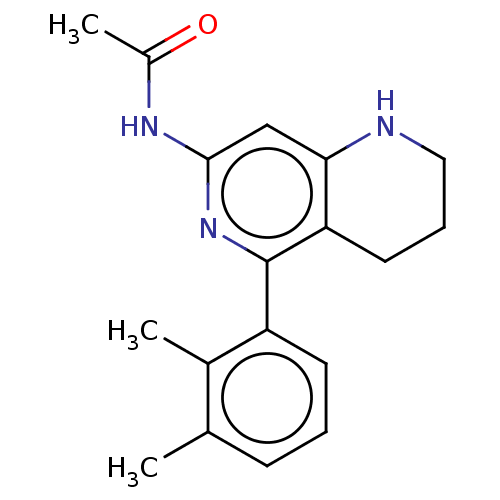 (CHEMBL4436749) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511450 (CHEMBL4436749) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177016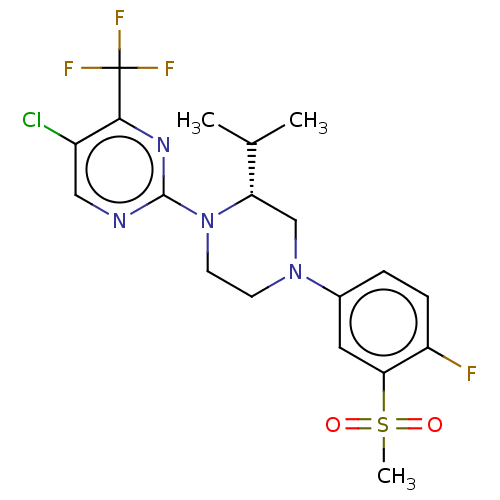 (CHEMBL3814501) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177012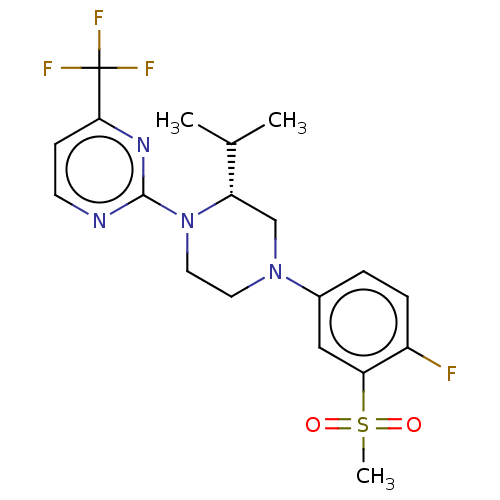 (CHEMBL3814153 | US10144715, Compound 7-32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111939 (US8618107, 105) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length His-tagged BTK expressed in baculovirus expression system by Z-LYTE assay | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177010 (CHEMBL3814006) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177015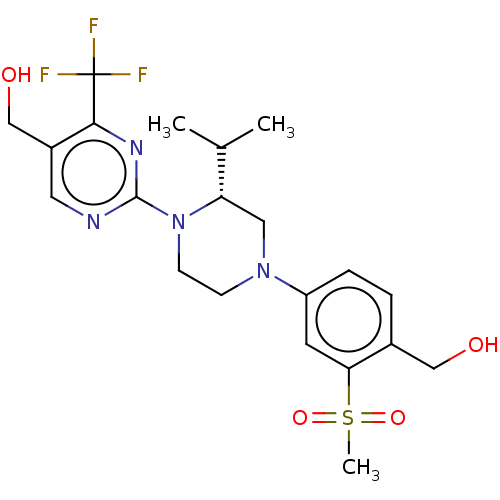 (CHEMBL3814206 | US10144715, Compound 19-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177016 (CHEMBL3814501) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177011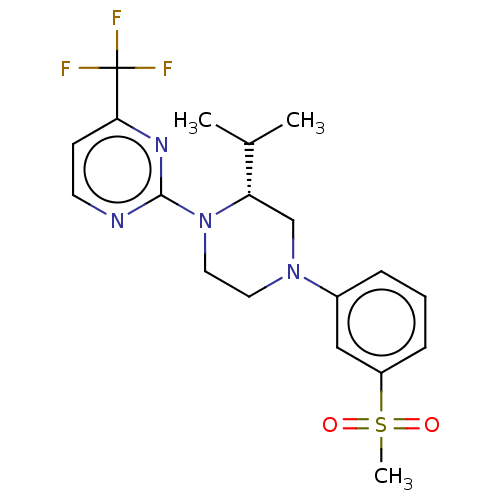 (CHEMBL3815014 | US10144715, Compound 7-13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177018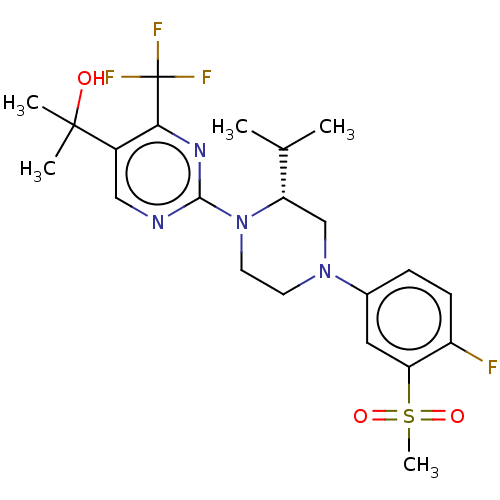 (CHEMBL3814478 | US10144715, Compound 14-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177017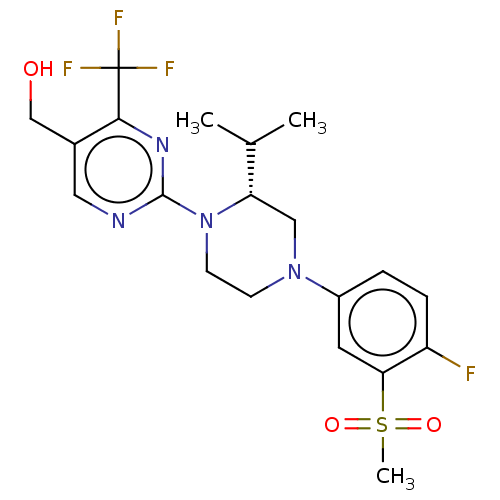 (CHEMBL3815001 | US10144715, Compound 11-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177010 (CHEMBL3814006) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177012 (CHEMBL3814153 | US10144715, Compound 7-32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177011 (CHEMBL3815014 | US10144715, Compound 7-13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177015 (CHEMBL3814206 | US10144715, Compound 19-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177014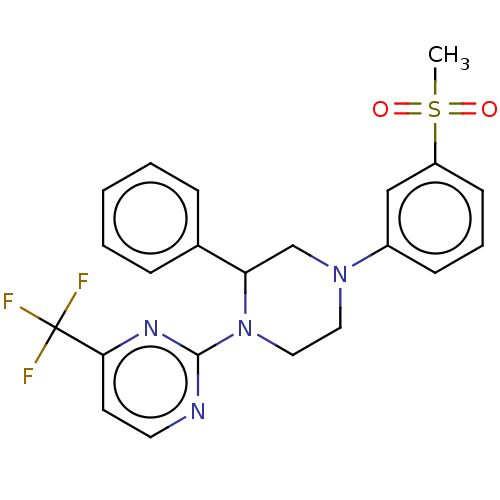 (CHEMBL3813875) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177014 (CHEMBL3813875) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177017 (CHEMBL3815001 | US10144715, Compound 11-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177018 (CHEMBL3814478 | US10144715, Compound 14-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177019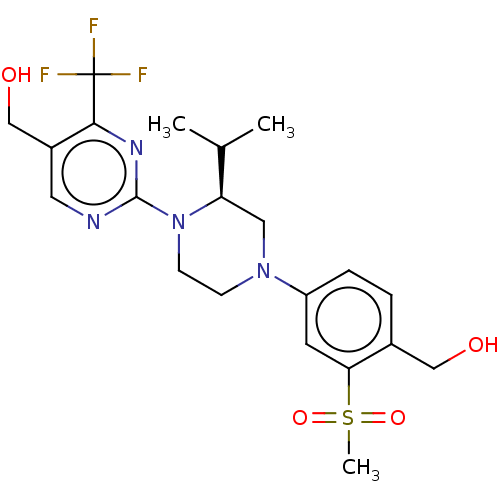 (CHEMBL3813863 | US10144715, Compound 19-2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177019 (CHEMBL3813863 | US10144715, Compound 19-2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177014 (CHEMBL3813875) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177014 (CHEMBL3813875) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177013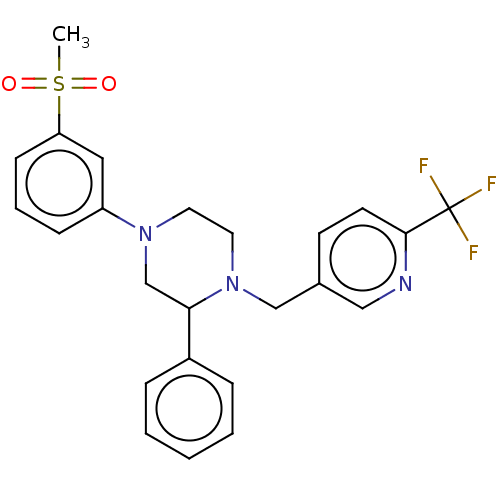 (CHEMBL3814372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177013 (CHEMBL3814372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177013 (CHEMBL3814372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177013 (CHEMBL3814372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177013 (CHEMBL3814372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177013 (CHEMBL3814372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511450 (CHEMBL4436749) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511430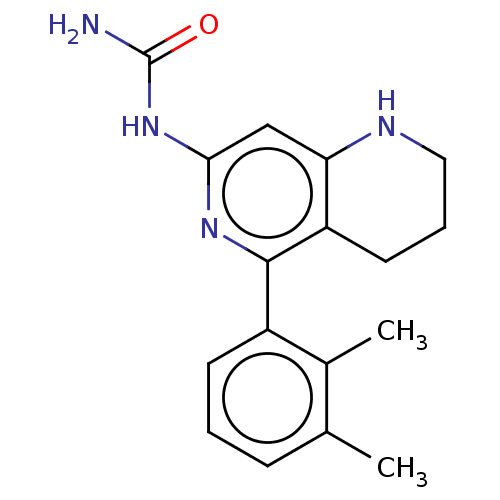 (CHEMBL4455353) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511453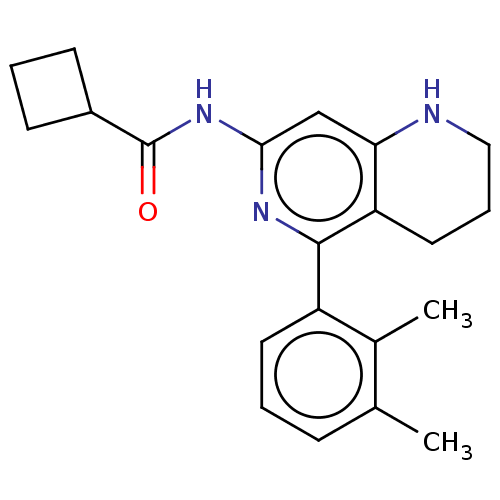 (CHEMBL4451394) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511431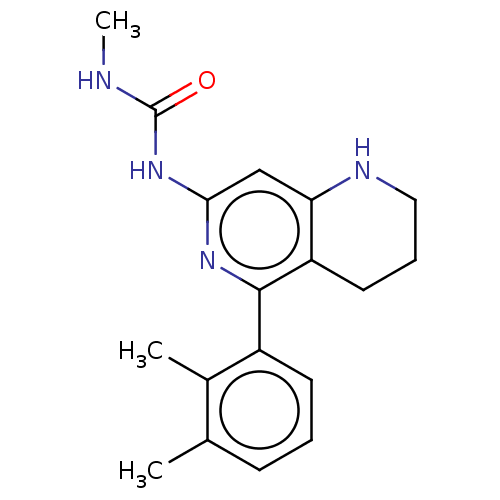 (CHEMBL4468099) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511452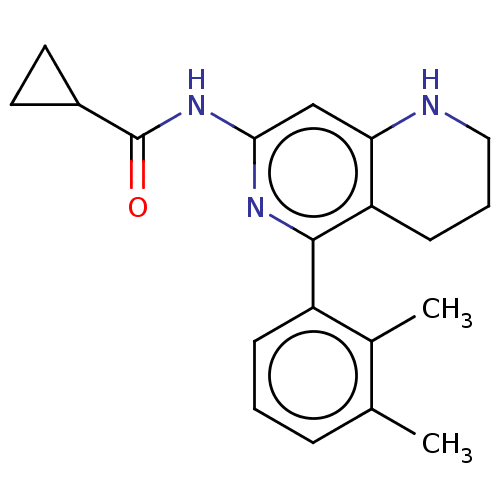 (CHEMBL4570266) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511454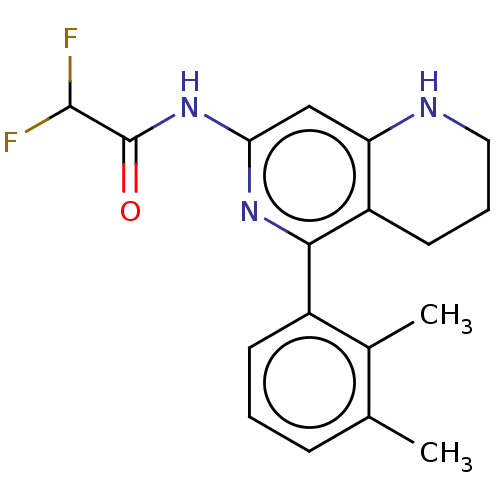 (CHEMBL4460228) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511432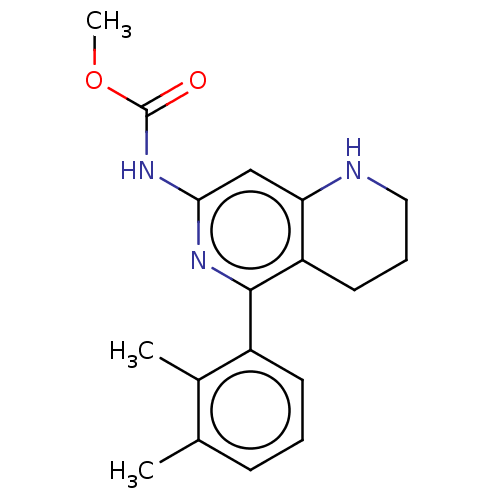 (CHEMBL4435945) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511466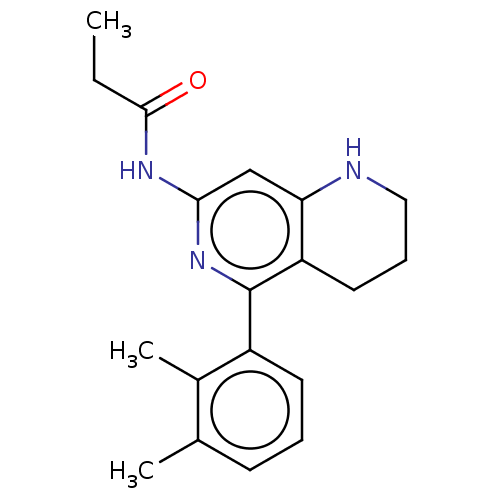 (CHEMBL4529335) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50152144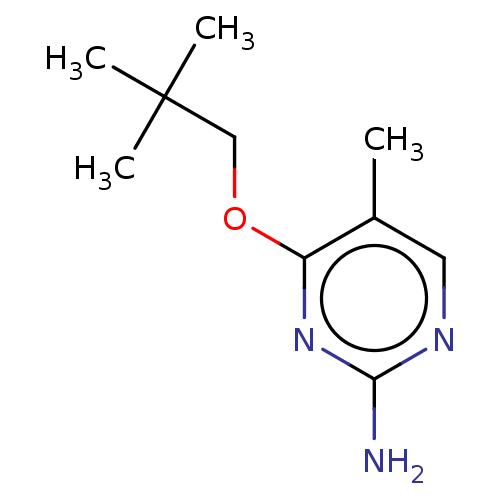 (CHEMBL3781661) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511448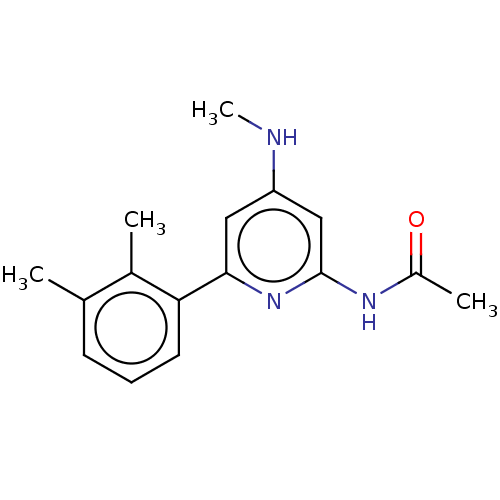 (CHEMBL4460446) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50329315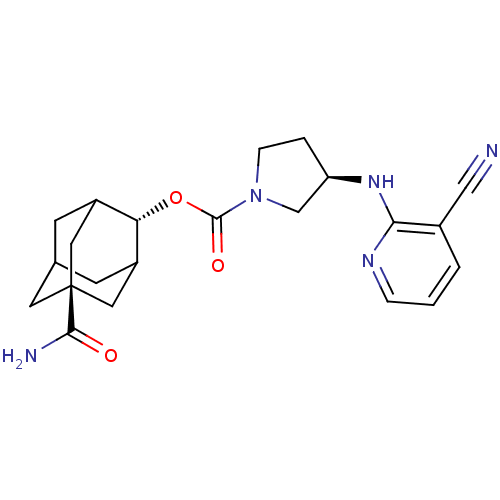 ((R)-3-(3-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader | Bioorg Med Chem Lett 20: 6725-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.142 BindingDB Entry DOI: 10.7270/Q2X92BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253406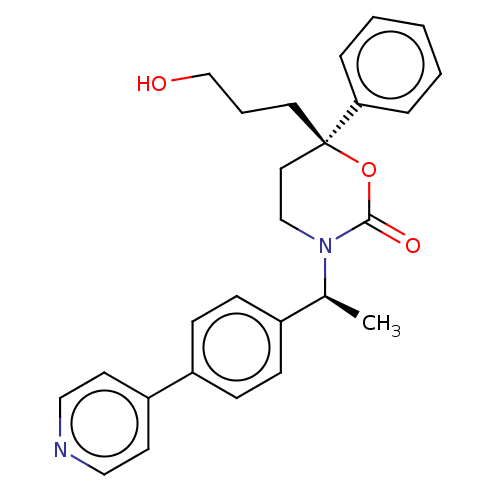 (CHEMBL4101787) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353392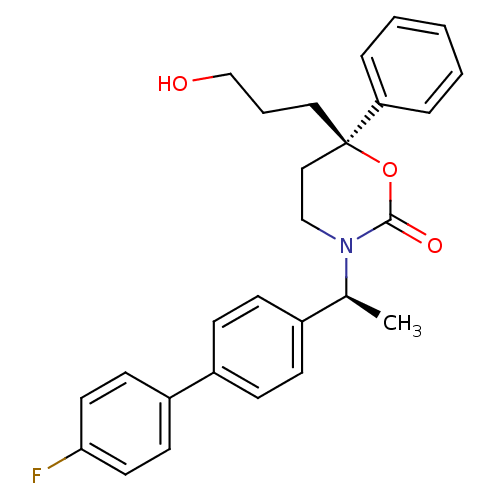 (CHEMBL1829761 | US8575157, 197 | US8592410, Compar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of 11 beta-HSD1 in differentiated human adipocytes assessed as conversion of [3H]-cortisone to [3H]-cortisol after 10 mins by HPLC | J Med Chem 54: 6050-62 (2011) Article DOI: 10.1021/jm2005354 BindingDB Entry DOI: 10.7270/Q2P26ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015453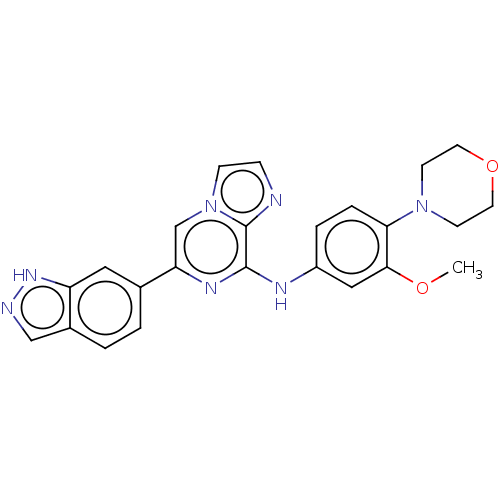 (CHEMBL3265037) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511428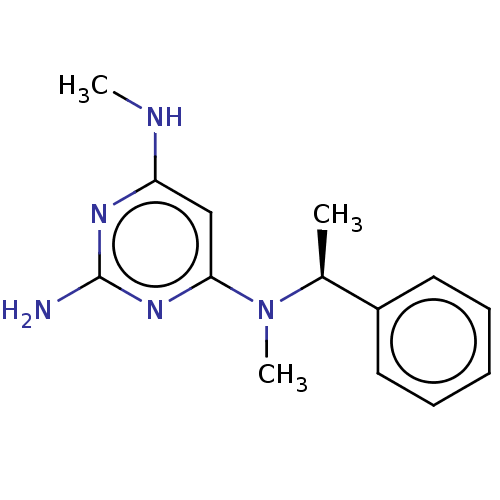 (CHEMBL4435983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50329313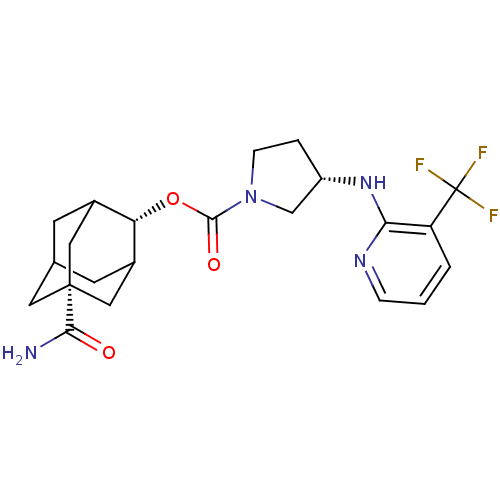 ((S)-3-(3-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader | Bioorg Med Chem Lett 20: 6725-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.142 BindingDB Entry DOI: 10.7270/Q2X92BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253514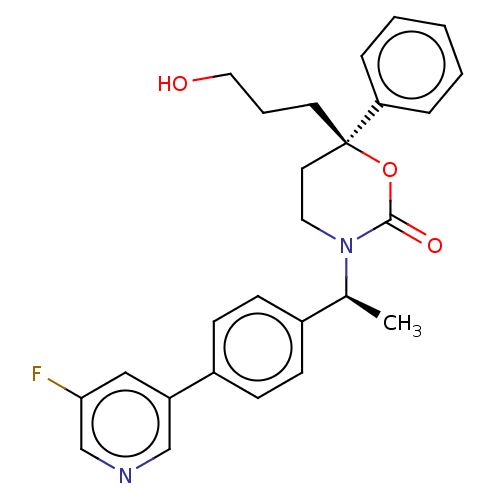 (CHEMBL4075869) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHO cell microsomes using [3H]cortisone as substrate preincubated with substrate for 10 mins followed by... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253514 (CHEMBL4075869) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50306433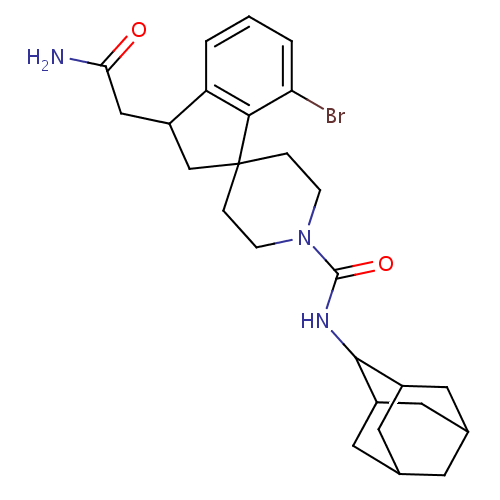 (CHEMBL601211 | N-(adamantan-2-yl)-7-bromo-3-(carba...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in CHO cells assessed as conversion of [3H]cortisone to [3H]cortisol by SPA | Bioorg Med Chem Lett 20: 881-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.082 BindingDB Entry DOI: 10.7270/Q2KK9BWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353407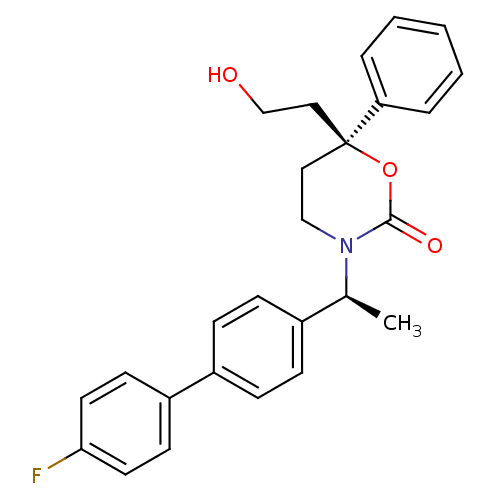 (CHEMBL1829760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human 11 beta-HSD1 expressed in CHO cells assessed as conversion of [3H]cortisone to [3H]cortisol after 1 hr by scintillation proximity... | J Med Chem 54: 6050-62 (2011) Article DOI: 10.1021/jm2005354 BindingDB Entry DOI: 10.7270/Q2P26ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353390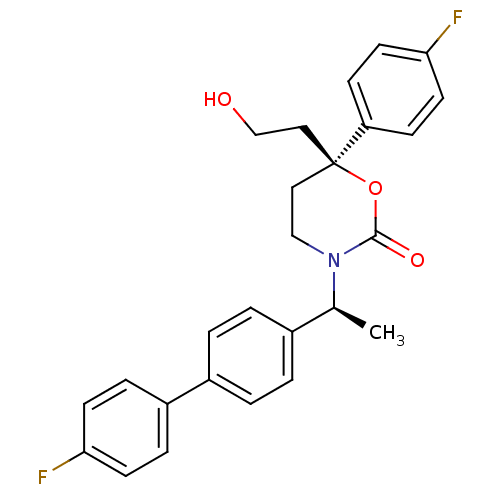 (CHEMBL1829768 | US8575157, 193 | US8592410, Compar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human 11 beta-HSD1 expressed in CHO cells assessed as conversion of [3H]cortisone to [3H]cortisol after 1 hr by scintillation proximity... | J Med Chem 54: 6050-62 (2011) Article DOI: 10.1021/jm2005354 BindingDB Entry DOI: 10.7270/Q2P26ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1588 total ) | Next | Last >> |