

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24621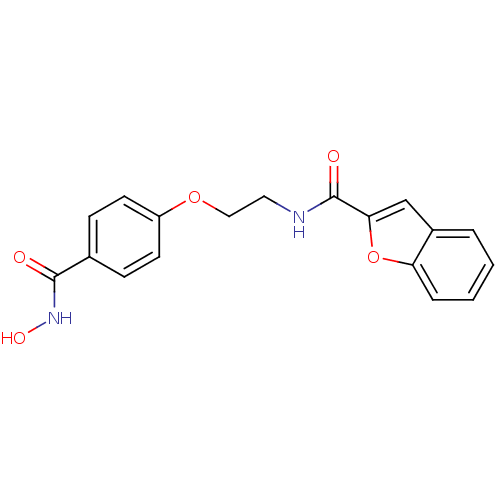 (CG-003 | N-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24732 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -48.8 | 32 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24620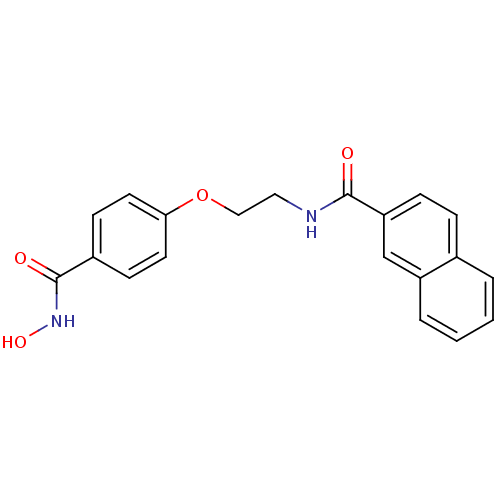 (CG-002 | N-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | -46.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24731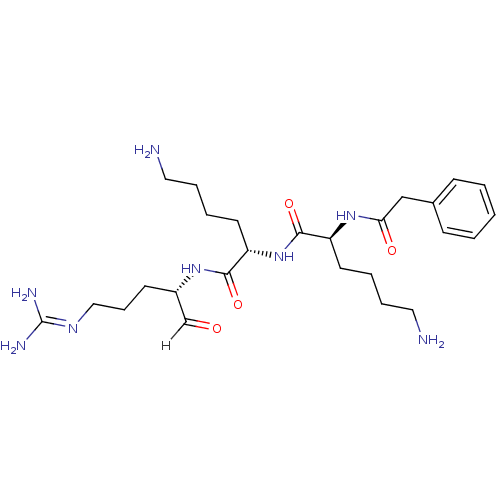 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | -47.8 | 51 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24734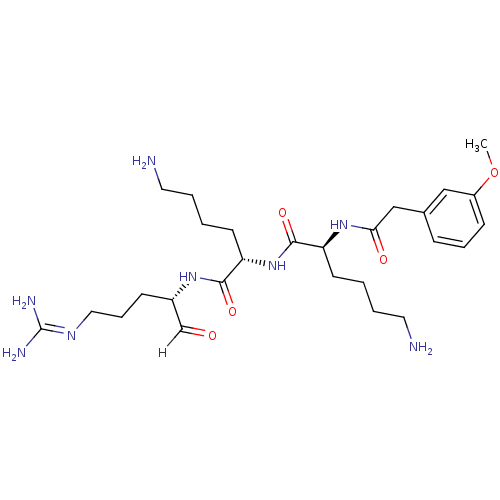 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -47.3 | 60 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24746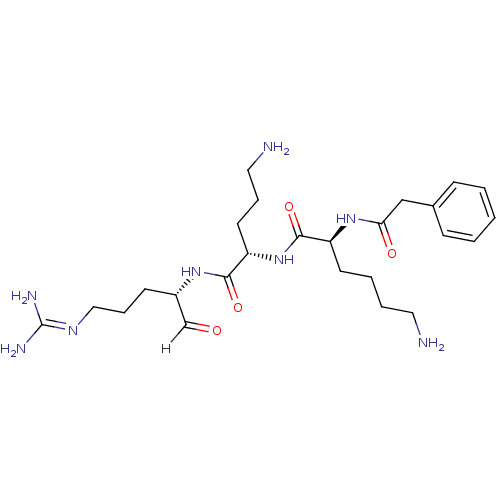 ((2S)-6-amino-N-[(1S)-4-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -46.8 | 73 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24739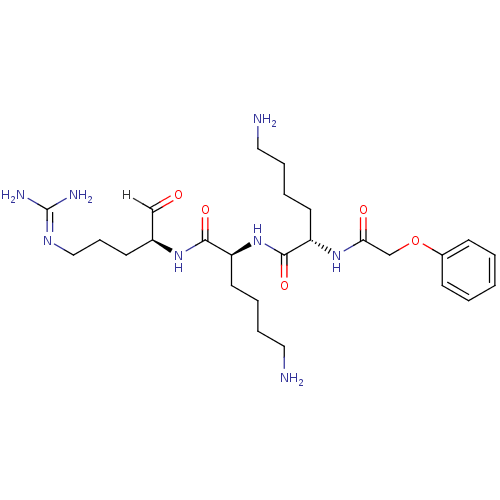 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | -45.8 | 107 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24733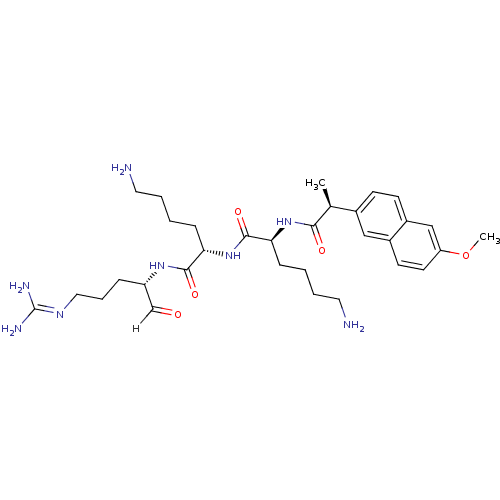 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -45.7 | 112 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24735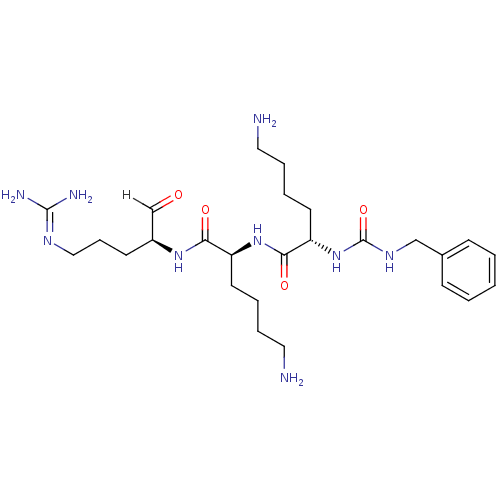 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -45.0 | 146 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24741 ((2S)-6-amino-N-[(2S)-5-carbamimidamido-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28 | -44.8 | 154 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24736 (Capped tripeptide aldehyde inhibitor, 24 | benzyl ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | -43.9 | 222 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24728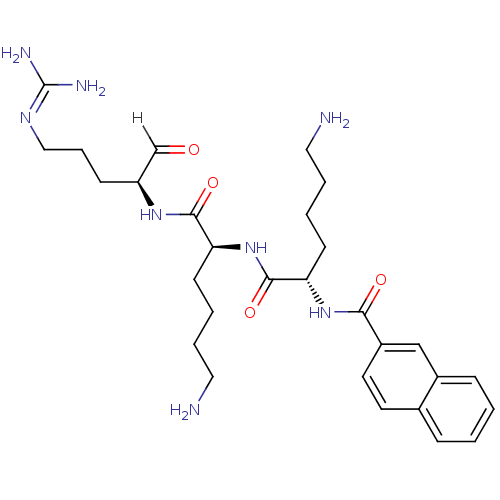 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 41 | -43.9 | 231 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24618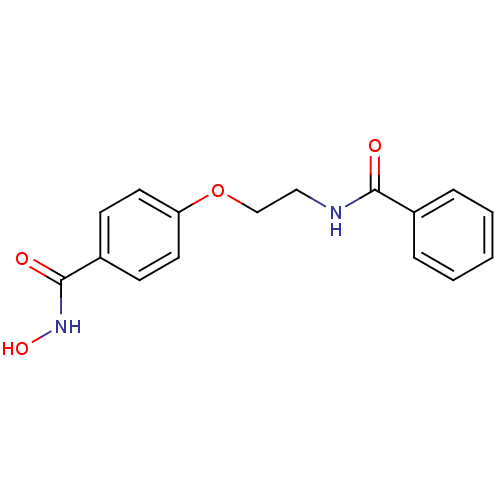 (CG-001 | N-hydroxy-4-[2-(phenylformamido)ethoxy]be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | -41.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24744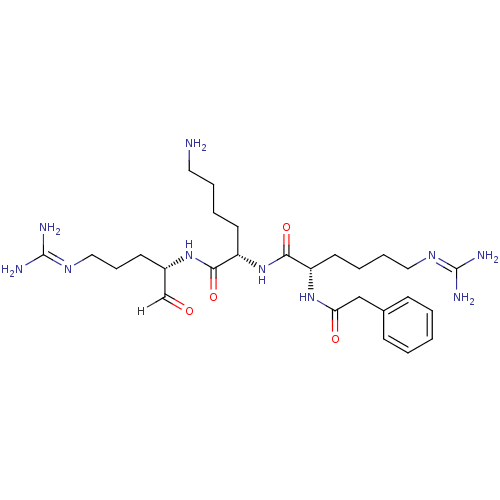 ((2S)-N-[(1S)-5-amino-1-{[(2S)-5-carbamimidamido-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 44 | -43.7 | 245 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24742 ((2S)-6-amino-2-[(2S)-5-amino-2-(1-phenylacetamido)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 46 | -43.6 | 255 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24737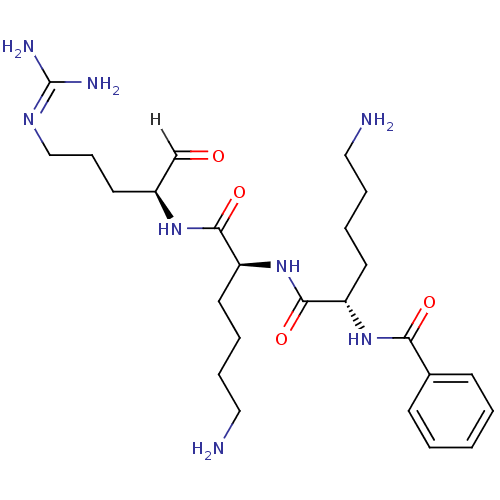 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | -43.4 | 271 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24748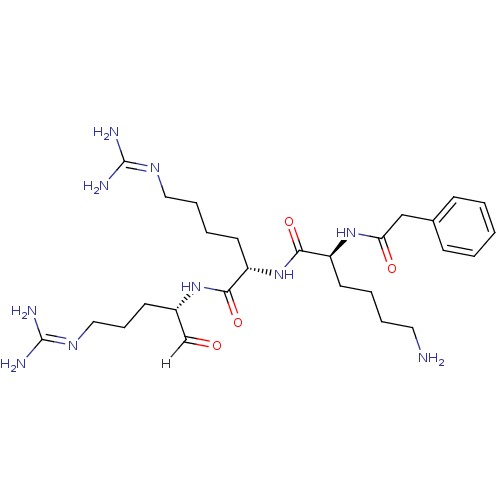 ((2S)-2-[(2S)-6-amino-2-(1-phenylacetamido)hexanami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | -43.2 | 297 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24745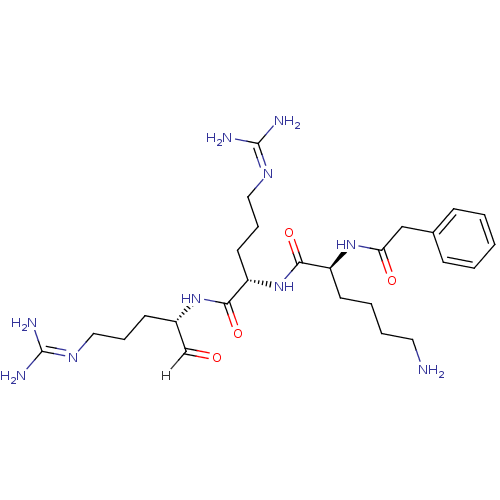 ((2S)-6-amino-N-[(1S)-4-carbamimidamido-1-{[(2S)-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 58 | -43.0 | 325 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24738 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 81 | -42.1 | 454 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24730 ((2S)-6-amino-2-[(2S)-6-amino-2-[(2E)-3-phenylprop-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 104 | -41.5 | 580 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24743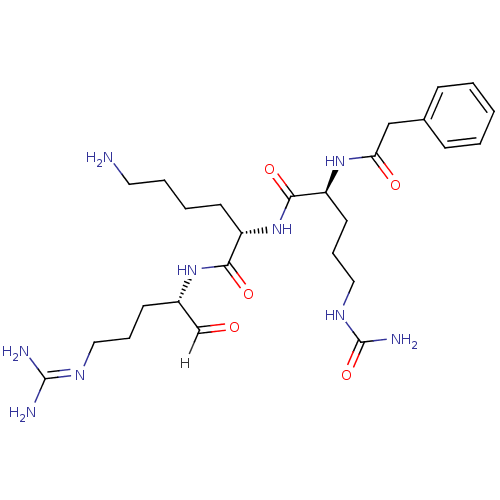 ((2S)-6-amino-N-[(2S)-5-carbamimidamido-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 111 | -41.3 | 619 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24740 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | -40.3 | 891 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 280 | -37.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24747 ((2S)-6-amino-N-[(1S)-1-{[(2S)-5-carbamimidamido-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.07E+3 | -33.7 | 1.16E+4 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24749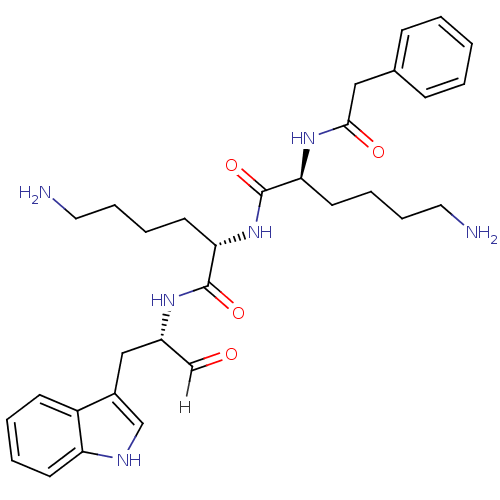 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-1-(1H-indol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | -31.7 | 2.57E+4 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24623 (4-[2-({3-[(dimethylamino)methyl]-1-benzofuran-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | >-24.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50463896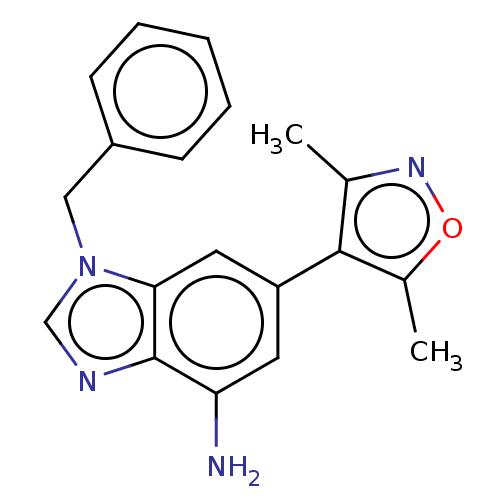 (CHEMBL4244412) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Zenith Epigenetics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged BRD4 (BD1) (unknown origin) using biotinylated tetra-acetylated histone H4 peptide after 1 hr by alpha-screen ass... | J Med Chem 61: 8202-8211 (2018) Article DOI: 10.1021/acs.jmedchem.8b00666 BindingDB Entry DOI: 10.7270/Q2F76G6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50463896 (CHEMBL4244412) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zenith Epigenetics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged BRD4 (BD2) (unknown origin) using biotinylated tetra-acetylated histone H4 peptide after 30 mins by alpha-screen ... | J Med Chem 61: 8202-8211 (2018) Article DOI: 10.1021/acs.jmedchem.8b00666 BindingDB Entry DOI: 10.7270/Q2F76G6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50463897 (CHEMBL4241205) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Zenith Epigenetics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged BRD4 (BD1) (unknown origin) using biotinylated tetra-acetylated histone H4 peptide after 4 hrs by alpha-screen as... | J Med Chem 61: 8202-8211 (2018) Article DOI: 10.1021/acs.jmedchem.8b00666 BindingDB Entry DOI: 10.7270/Q2F76G6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50463896 (CHEMBL4244412) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Zenith Epigenetics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged BRD4 (BD1) (unknown origin) using biotinylated tetra-acetylated histone H4 peptide after 5 mins by alpha-screen a... | J Med Chem 61: 8202-8211 (2018) Article DOI: 10.1021/acs.jmedchem.8b00666 BindingDB Entry DOI: 10.7270/Q2F76G6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15239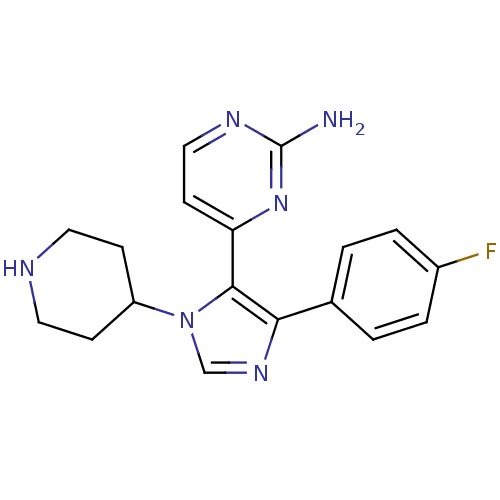 (4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Texas Southwestern Medical Center at Dallas | Assay Description Kinase activity was assayed in reaction buffer containing substrate, enzyme, and inhibitor in the presence of 0.17 mM ATP/[gamma-32P] ATP. 32P incorp... | Structure 6: 1117-28 (1998) Article DOI: 10.1016/s0969-2126(98)00113-0 BindingDB Entry DOI: 10.7270/Q2XP7351 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50463896 (CHEMBL4244412) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Zenith Epigenetics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged BRD4 (BD1) (unknown origin) using biotinylated tetra-acetylated histone H4 peptide after 4 hrs by alpha-screen as... | J Med Chem 61: 8202-8211 (2018) Article DOI: 10.1021/acs.jmedchem.8b00666 BindingDB Entry DOI: 10.7270/Q2F76G6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zenith Epigenetics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged BRD4 (BD2) (unknown origin) using biotinylated tetra-acetylated histone H4 peptide after 30 mins by alpha-screen ... | J Med Chem 61: 8202-8211 (2018) Article DOI: 10.1021/acs.jmedchem.8b00666 BindingDB Entry DOI: 10.7270/Q2F76G6T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50463896 (CHEMBL4244412) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zenith Epigenetics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged BRD4 (BD1) (unknown origin) using biotinylated tetra-acetylated histone H4 peptide after 30 mins by alpha-screen ... | J Med Chem 61: 8202-8211 (2018) Article DOI: 10.1021/acs.jmedchem.8b00666 BindingDB Entry DOI: 10.7270/Q2F76G6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15238 (4-[1-(cyclopropylmethyl)-4-(4-fluorophenyl)-1H-imi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Texas Southwestern Medical Center at Dallas | Assay Description Kinase activity was assayed in reaction buffer containing substrate, enzyme, and inhibitor in the presence of 0.17 mM ATP/[gamma-32P] ATP. 32P incorp... | Structure 6: 1117-28 (1998) Article DOI: 10.1016/s0969-2126(98)00113-0 BindingDB Entry DOI: 10.7270/Q2XP7351 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50463898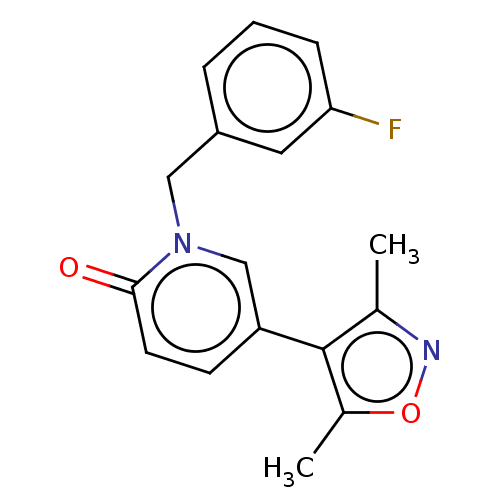 (CHEMBL4241469) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Zenith Epigenetics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged BRD4 (BD2) (unknown origin) using biotinylated tetra-acetylated histone H4 peptide after 30 mins by alpha-screen ... | J Med Chem 61: 8202-8211 (2018) Article DOI: 10.1021/acs.jmedchem.8b00666 BindingDB Entry DOI: 10.7270/Q2F76G6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zenith Epigenetics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged BRD4 (BD1/BD2) (unknown origin) using biotinylated tetra-acetylated histone H4 peptide after 30 mins by alpha-scr... | J Med Chem 61: 8202-8211 (2018) Article DOI: 10.1021/acs.jmedchem.8b00666 BindingDB Entry DOI: 10.7270/Q2F76G6T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zenith Epigenetics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged BRD4 (BD1) (unknown origin) using biotinylated tetra-acetylated histone H4 peptide after 30 mins by alpha-screen ... | J Med Chem 61: 8202-8211 (2018) Article DOI: 10.1021/acs.jmedchem.8b00666 BindingDB Entry DOI: 10.7270/Q2F76G6T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zenith Epigenetics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged BRD4 (BD2) (unknown origin) using biotinylated tetra-acetylated histone H4 peptide after 30 mins by alpha-screen ... | J Med Chem 61: 8202-8211 (2018) Article DOI: 10.1021/acs.jmedchem.8b00666 BindingDB Entry DOI: 10.7270/Q2F76G6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM13336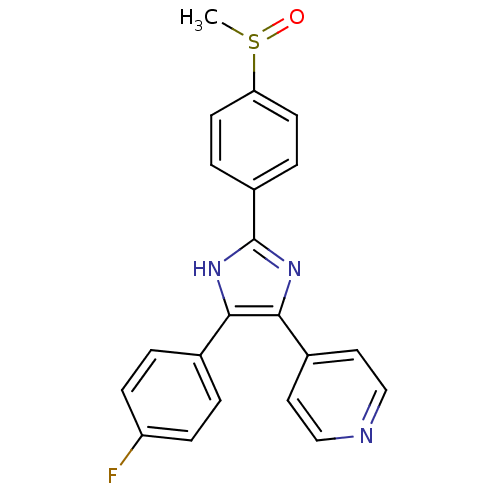 (4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Texas Southwestern Medical Center at Dallas | Assay Description Kinase activity was assayed in reaction buffer containing substrate, enzyme, and inhibitor in the presence of 0.17 mM ATP/[gamma-32P] ATP. 32P incorp... | Structure 6: 1117-28 (1998) Article DOI: 10.1016/s0969-2126(98)00113-0 BindingDB Entry DOI: 10.7270/Q2XP7351 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50463897 (CHEMBL4241205) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Zenith Epigenetics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged BRD4 (BD2) (unknown origin) using biotinylated tetra-acetylated histone H4 peptide after 30 mins by alpha-screen ... | J Med Chem 61: 8202-8211 (2018) Article DOI: 10.1021/acs.jmedchem.8b00666 BindingDB Entry DOI: 10.7270/Q2F76G6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Zenith Epigenetics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged BRD4 (BD1/BD2) (unknown origin) using biotinylated tetra-acetylated histone H4 peptide after 30 mins by alpha-scr... | J Med Chem 61: 8202-8211 (2018) Article DOI: 10.1021/acs.jmedchem.8b00666 BindingDB Entry DOI: 10.7270/Q2F76G6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50369261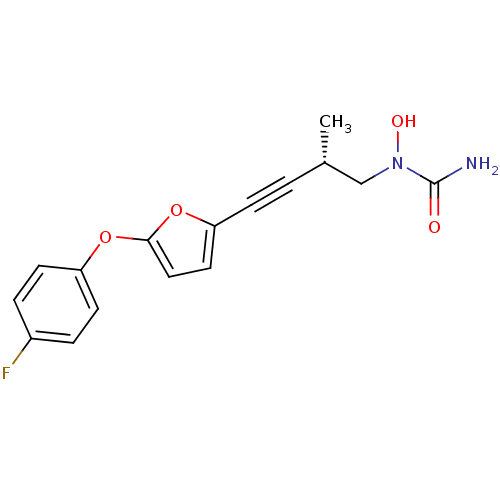 (CHEMBL1907812) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase (5-LO) measured as LTB4 production in human whole blood stimulated with calcium ionophore (A23187). | J Med Chem 40: 1955-68 (1997) Article DOI: 10.1021/jm9700474 BindingDB Entry DOI: 10.7270/Q2NV9JWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50463897 (CHEMBL4241205) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Zenith Epigenetics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged BRD4 (BD1) (unknown origin) using biotinylated tetra-acetylated histone H4 peptide after 30 mins by alpha-screen ... | J Med Chem 61: 8202-8211 (2018) Article DOI: 10.1021/acs.jmedchem.8b00666 BindingDB Entry DOI: 10.7270/Q2F76G6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50463896 (CHEMBL4244412) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Zenith Epigenetics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged BRD4 (BD1/BD2) (unknown origin) using biotinylated tetra-acetylated histone H4 peptide after 30 mins by alpha-scr... | J Med Chem 61: 8202-8211 (2018) Article DOI: 10.1021/acs.jmedchem.8b00666 BindingDB Entry DOI: 10.7270/Q2F76G6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 488 total ) | Next | Last >> |