 Found 493 hits with Last Name = 'symanowicz' and Initial = 'pt'
Found 493 hits with Last Name = 'symanowicz' and Initial = 'pt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239500
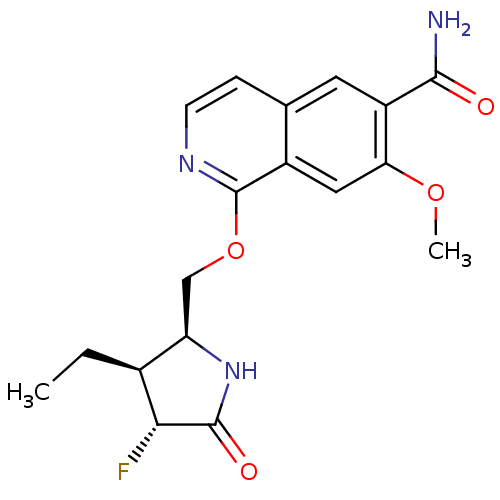
(CHEMBL4066705 | US10329302, Example 337 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239498
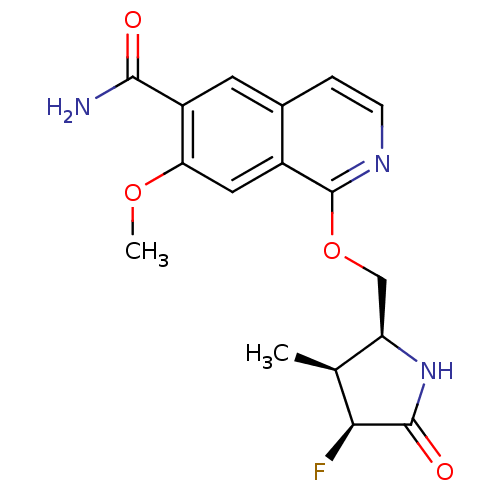
(CHEMBL4093120 | US10329302, Example 189 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@@H](F)[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H18FN3O4/c1-8-12(21-16(23)14(8)18)7-25-17-10-6-13(24-2)11(15(19)22)5-9(10)3-4-20-17/h3-6,8,12,14H,7H2,1-2H3,(H2,19,22)(H,21,23)/t8-,12+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239507
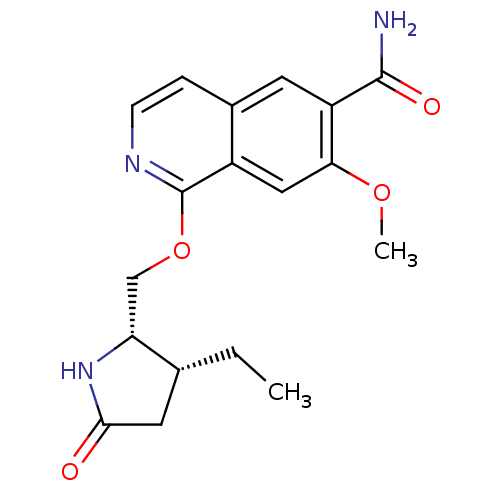
(CHEMBL4091434 | US10329302, Example 246 | US107935...)Show SMILES CC[C@@H]1CC(=O)N[C@@H]1COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C18H21N3O4/c1-3-10-7-16(22)21-14(10)9-25-18-12-8-15(24-2)13(17(19)23)6-11(12)4-5-20-18/h4-6,8,10,14H,3,7,9H2,1-2H3,(H2,19,23)(H,21,22)/t10-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239508
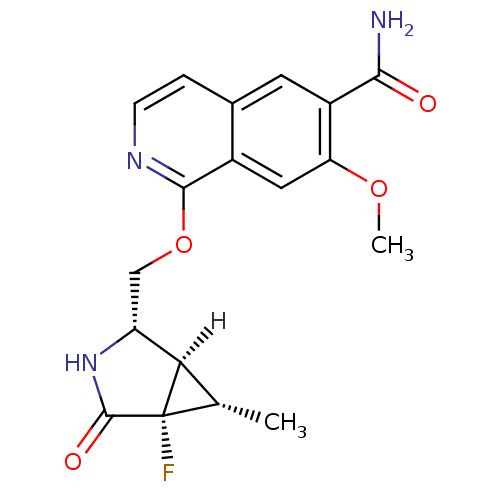
(CHEMBL4085199 | US10329302, Example 309 | US107935...)Show SMILES [H][C@]12[C@H](C)[C@@]1(F)C(=O)N[C@@H]2COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C18H18FN3O4/c1-8-14-12(22-17(24)18(8,14)19)7-26-16-10-6-13(25-2)11(15(20)23)5-9(10)3-4-21-16/h3-6,8,12,14H,7H2,1-2H3,(H2,20,23)(H,22,24)/t8-,12+,14+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239493
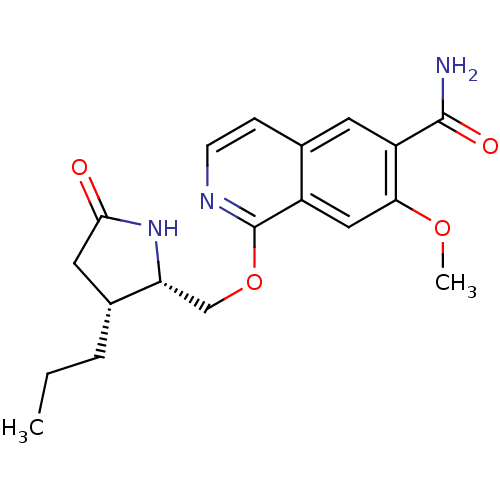
(CHEMBL4103497 | US10329302, Example 312 | US107935...)Show SMILES CCC[C@@H]1CC(=O)N[C@@H]1COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C19H23N3O4/c1-3-4-12-8-17(23)22-15(12)10-26-19-13-9-16(25-2)14(18(20)24)7-11(13)5-6-21-19/h5-7,9,12,15H,3-4,8,10H2,1-2H3,(H2,20,24)(H,22,23)/t12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239491
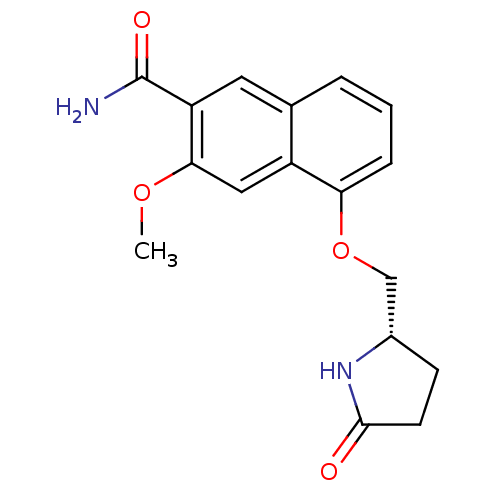
(CHEMBL4083655 | US10329302, Example 173 | US107935...)Show SMILES COc1cc2c(OC[C@@H]3CCC(=O)N3)cccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H18N2O4/c1-22-15-8-12-10(7-13(15)17(18)21)3-2-4-14(12)23-9-11-5-6-16(20)19-11/h2-4,7-8,11H,5-6,9H2,1H3,(H2,18,21)(H,19,20)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239506

(CHEMBL4071526 | US10329302, Example 188 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@H](F)[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H18FN3O4/c1-8-12(21-16(23)14(8)18)7-25-17-10-6-13(24-2)11(15(19)22)5-9(10)3-4-20-17/h3-6,8,12,14H,7H2,1-2H3,(H2,19,22)(H,21,23)/t8-,12+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human whole blood assessed as reduction R848-induced IL-6 secretion by measuring plasma protein binding corrected IC50 preincu... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production by measuring plasma protein binding corrected IC50 af... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239497
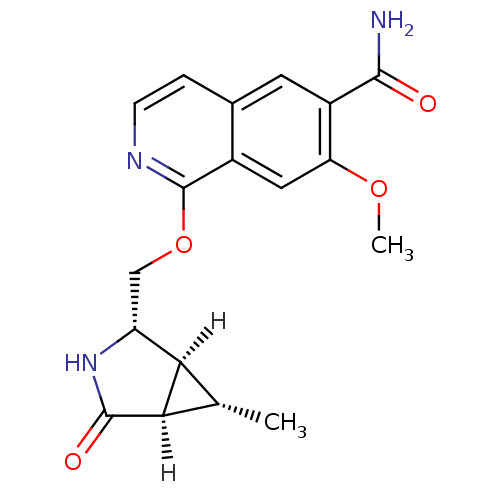
(CHEMBL4084228 | US10329302, Example 271 | US107935...)Show SMILES [H][C@]12[C@H](C)[C@@]1([H])C(=O)N[C@@H]2COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C18H19N3O4/c1-8-14-12(21-17(23)15(8)14)7-25-18-10-6-13(24-2)11(16(19)22)5-9(10)3-4-20-18/h3-6,8,12,14-15H,7H2,1-2H3,(H2,19,22)(H,21,23)/t8-,12+,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239492

(CHEMBL4070515 | US10329302, Example 211 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)C[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H19N3O4/c1-9-5-15(21)20-13(9)8-24-17-11-7-14(23-2)12(16(18)22)6-10(11)3-4-19-17/h3-4,6-7,9,13H,5,8H2,1-2H3,(H2,18,22)(H,20,21)/t9-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50243852
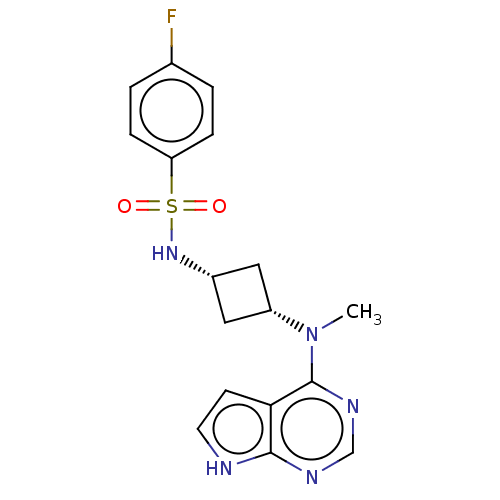
(CHEMBL4103698)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)c1ccc(F)cc1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(6.1,-10.71,;7.44,-11.48,;8.77,-10.7,;9.16,-9.21,;10.65,-9.6,;10.25,-11.09,;11.98,-8.83,;13.3,-9.59,;14.06,-10.92,;12.53,-10.91,;14.64,-8.82,;15.97,-9.6,;17.31,-8.83,;17.31,-7.29,;18.64,-6.52,;15.97,-6.52,;14.64,-7.29,;7.44,-13.01,;6.12,-13.78,;6.11,-15.33,;7.45,-16.1,;8.79,-15.32,;10.25,-15.78,;11.15,-14.53,;10.24,-13.29,;8.78,-13.78,)| Show InChI InChI=1S/C17H18FN5O2S/c1-23(17-15-6-7-19-16(15)20-10-21-17)13-8-12(9-13)22-26(24,25)14-4-2-11(18)3-5-14/h2-7,10,12-13,22H,8-9H2,1H3,(H,19,20,21)/t12-,13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239505
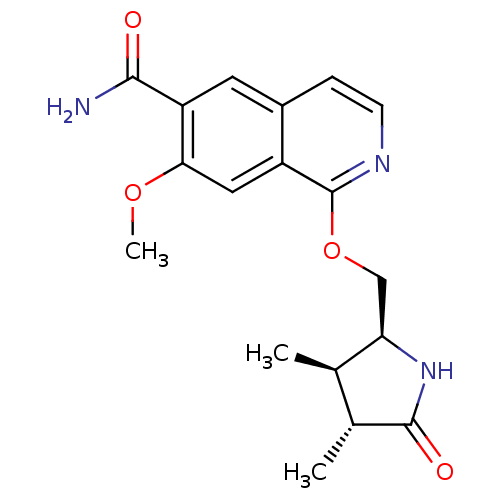
(CHEMBL4061801 | US10329302, Example 248 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@H](C)[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C18H21N3O4/c1-9-10(2)17(23)21-14(9)8-25-18-12-7-15(24-3)13(16(19)22)6-11(12)4-5-20-18/h4-7,9-10,14H,8H2,1-3H3,(H2,19,22)(H,21,23)/t9-,10-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50021656
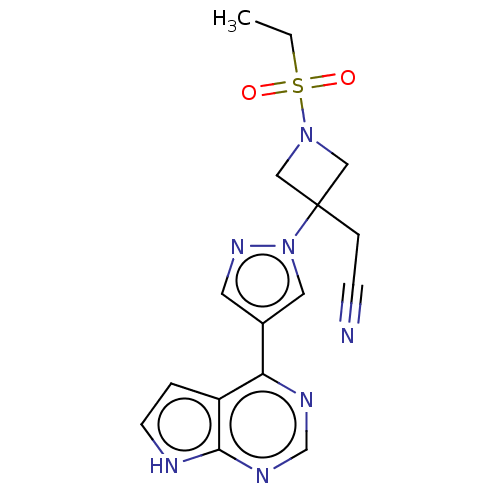
(BARICITINIB | INCB-028050 | LY-3009104 | US1011290...)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1ncnc2[nH]ccc12 Show InChI InChI=1S/C16H17N7O2S/c1-2-26(24,25)22-9-16(10-22,4-5-17)23-8-12(7-21-23)14-13-3-6-18-15(13)20-11-19-14/h3,6-8,11H,2,4,9-10H2,1H3,(H,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239488
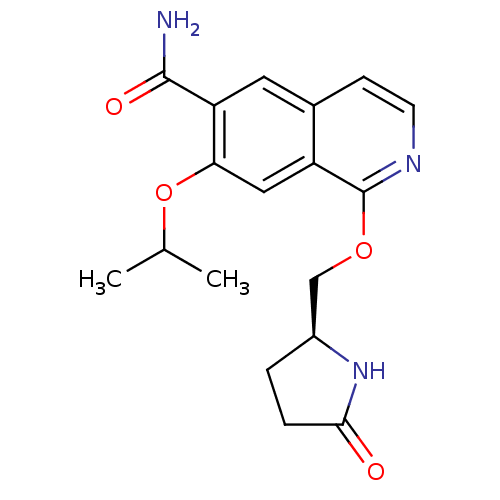
(CHEMBL4092338 | US10329302, Example 26 | US1079357...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CCC(=O)N3)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C18H21N3O4/c1-10(2)25-15-8-13-11(7-14(15)17(19)23)5-6-20-18(13)24-9-12-3-4-16(22)21-12/h5-8,10,12H,3-4,9H2,1-2H3,(H2,19,23)(H,21,22)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239496

(CHEMBL4075552 | US10329302, Example 264 | US107935...)Show SMILES [H][C@]12C[C@@]1([H])C(=O)N[C@@H]2COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C17H17N3O4/c1-23-14-6-9-8(4-12(14)15(18)21)2-3-19-17(9)24-7-13-10-5-11(10)16(22)20-13/h2-4,6,10-11,13H,5,7H2,1H3,(H2,18,21)(H,20,22)/t10-,11+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50243810
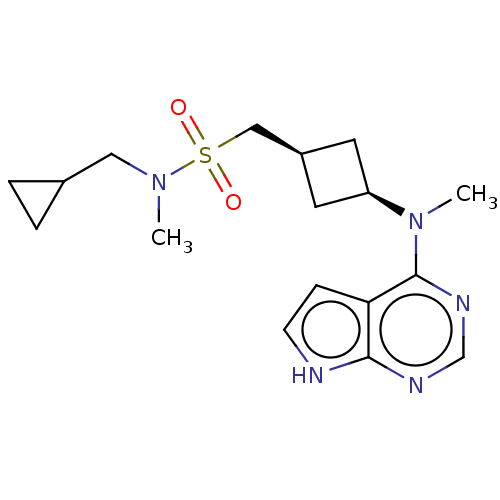
(CHEMBL4093955)Show SMILES CN(CC1CC1)S(=O)(=O)C[C@H]1C[C@H](C1)N(C)c1ncnc2[nH]ccc12 |r,wD:12.15,10.10,(14.93,-10.76,;14.93,-12.3,;16.27,-13.08,;17.6,-12.32,;19.14,-12.32,;18.38,-10.98,;13.59,-13.07,;14.35,-14.4,;12.82,-14.39,;12.26,-12.31,;10.93,-13.08,;9.44,-12.69,;9.05,-14.18,;10.53,-14.57,;7.72,-14.96,;6.38,-14.19,;7.72,-16.5,;6.39,-17.27,;6.39,-18.81,;7.72,-19.58,;9.06,-18.8,;10.53,-19.27,;11.43,-18.02,;10.52,-16.78,;9.06,-17.26,)| Show InChI InChI=1S/C17H25N5O2S/c1-21(9-12-3-4-12)25(23,24)10-13-7-14(8-13)22(2)17-15-5-6-18-16(15)19-11-20-17/h5-6,11-14H,3-4,7-10H2,1-2H3,(H,18,19,20)/t13-,14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human AchE (Acetylcholinesterase) |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239504

(CHEMBL4061890 | US10329302, Example 262 | US107935...)Show SMILES COc1cc2c(OC[C@@H]3CCC(=O)N3)ccnc2cc1C(N)=O |r| Show InChI InChI=1S/C16H17N3O4/c1-22-14-7-10-12(6-11(14)16(17)21)18-5-4-13(10)23-8-9-2-3-15(20)19-9/h4-7,9H,2-3,8H2,1H3,(H2,17,21)(H,19,20)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50243869
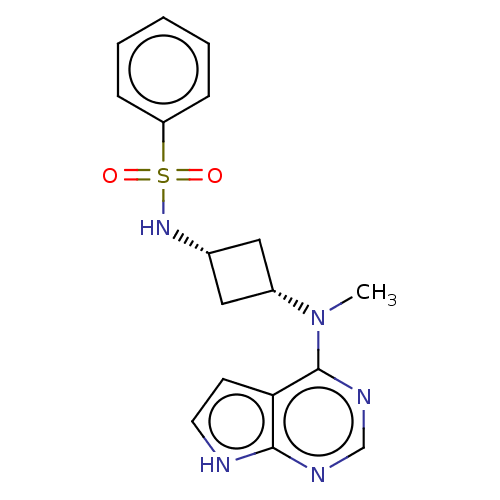
(CHEMBL4101374)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)c1ccccc1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(4.65,-10.97,;5.99,-11.74,;7.32,-10.96,;7.71,-9.46,;9.2,-9.86,;8.81,-11.35,;10.53,-9.08,;11.86,-9.85,;12.62,-11.18,;11.09,-11.17,;13.2,-9.08,;14.53,-9.85,;15.86,-9.08,;15.86,-7.53,;14.51,-6.77,;13.19,-7.55,;5.99,-13.28,;4.66,-14.05,;4.66,-15.59,;6,-16.36,;7.34,-15.58,;8.81,-16.05,;9.71,-14.8,;8.79,-13.55,;7.33,-14.04,)| Show InChI InChI=1S/C17H19N5O2S/c1-22(17-15-7-8-18-16(15)19-11-20-17)13-9-12(10-13)21-25(23,24)14-5-3-2-4-6-14/h2-8,11-13,21H,9-10H2,1H3,(H,18,19,20)/t12-,13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239500

(CHEMBL4066705 | US10329302, Example 337 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50021656

(BARICITINIB | INCB-028050 | LY-3009104 | US1011290...)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1ncnc2[nH]ccc12 Show InChI InChI=1S/C16H17N7O2S/c1-2-26(24,25)22-9-16(10-22,4-5-17)23-8-12(7-21-23)14-13-3-6-18-15(13)20-11-19-14/h3,6-8,11H,2,4,9-10H2,1H3,(H,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK2 using FITC-KGGEEEEYFELVKK as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239489
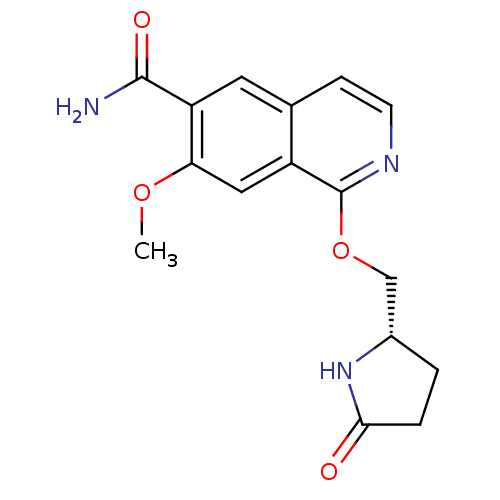
(CHEMBL4100091 | US10329302, Example 121 | US107935...)Show SMILES COc1cc2c(OC[C@@H]3CCC(=O)N3)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C16H17N3O4/c1-22-13-7-11-9(6-12(13)15(17)21)4-5-18-16(11)23-8-10-2-3-14(20)19-10/h4-7,10H,2-3,8H2,1H3,(H2,17,21)(H,19,20)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50243837
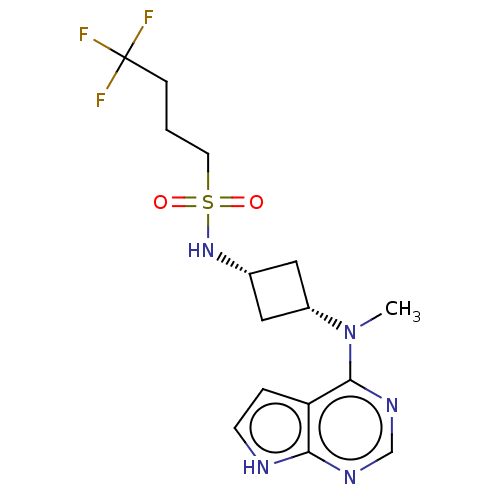
(CHEMBL4079179)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)CCCC(F)(F)F)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(6.04,-12.03,;7.37,-12.79,;8.7,-12.02,;9.1,-10.52,;10.58,-10.92,;10.19,-12.41,;11.92,-10.14,;13.24,-10.9,;14.01,-12.23,;12.47,-12.23,;14.58,-10.13,;15.92,-10.91,;17.25,-10.14,;18.58,-10.91,;19.92,-10.14,;18.58,-12.45,;19.91,-11.68,;7.38,-14.33,;6.05,-15.1,;6.05,-16.65,;7.38,-17.42,;8.72,-16.64,;10.19,-17.11,;11.09,-15.85,;10.18,-14.61,;8.71,-15.1,)| Show InChI InChI=1S/C15H20F3N5O2S/c1-23(14-12-3-5-19-13(12)20-9-21-14)11-7-10(8-11)22-26(24,25)6-2-4-15(16,17)18/h3,5,9-11,22H,2,4,6-8H2,1H3,(H,19,20,21)/t10-,11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239507

(CHEMBL4091434 | US10329302, Example 246 | US107935...)Show SMILES CC[C@@H]1CC(=O)N[C@@H]1COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C18H21N3O4/c1-3-10-7-16(22)21-14(10)9-25-18-12-8-15(24-2)13(17(19)23)6-11(12)4-5-20-18/h4-6,8,10,14H,3,7,9H2,1-2H3,(H2,19,23)(H,21,22)/t10-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159779
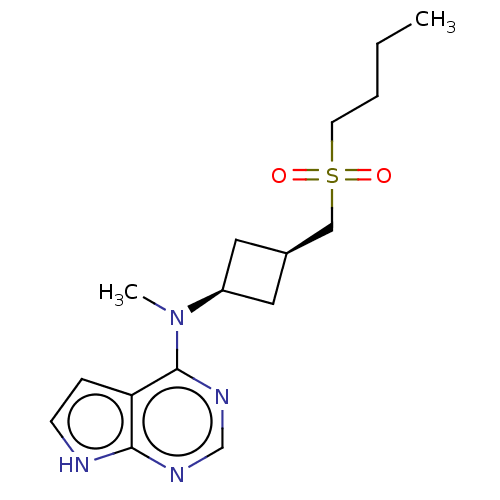
(US10966980, Example 30 | US9035074, 30)Show SMILES CCCCS(=O)(=O)C[C@H]1C[C@H](C1)N(C)c1ncnc2[nH]ccc12 |r,wD:10.12,8.7,(5.98,3.24,;4.65,4.01,;3.32,3.24,;1.98,4.01,;.65,3.24,;-.12,1.91,;1.74,2.15,;-.69,4.01,;-1.78,2.92,;-3.32,2.92,;-3.32,1.38,;-1.78,1.38,;-4.65,.61,;-5.98,1.38,;-4.65,-.93,;-5.98,-1.7,;-5.98,-3.24,;-4.65,-4.01,;-3.32,-3.24,;-1.85,-3.72,;-.95,-2.47,;-1.85,-1.22,;-3.32,-1.7,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK2 using FITC-KGGEEEEYFELVKK as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50243813
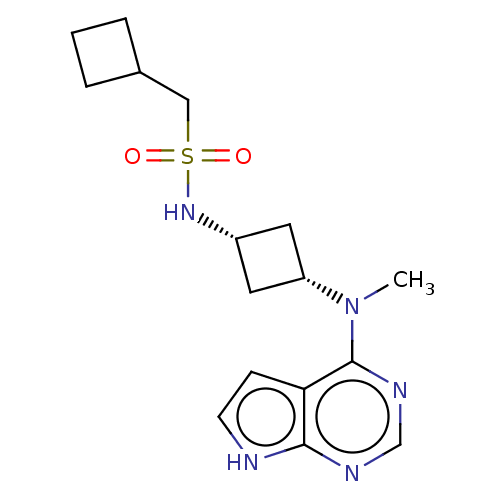
(CHEMBL4068319)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)CC1CCC1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(5.93,-12.31,;7.26,-13.07,;8.6,-12.3,;8.99,-10.8,;10.48,-11.2,;10.08,-12.69,;11.81,-10.42,;13.14,-11.19,;13.9,-12.52,;12.37,-12.51,;14.48,-10.42,;15.81,-11.19,;16.2,-12.67,;17.69,-12.28,;17.29,-10.79,;7.27,-14.61,;5.94,-15.39,;5.94,-16.93,;7.27,-17.7,;8.61,-16.92,;10.08,-17.39,;10.98,-16.14,;10.07,-14.89,;8.6,-15.38,)| Show InChI InChI=1S/C16H23N5O2S/c1-21(16-14-5-6-17-15(14)18-10-19-16)13-7-12(8-13)20-24(22,23)9-11-3-2-4-11/h5-6,10-13,20H,2-4,7-9H2,1H3,(H,17,18,19)/t12-,13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the Compound was tested against HIV protease enzyme. |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50243908
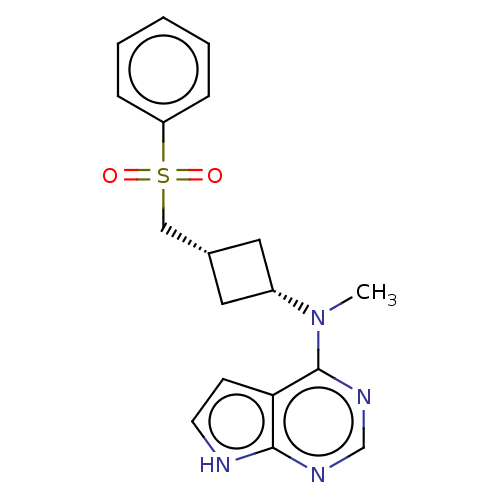
(CHEMBL4103586 | US10966980, Example 221)Show SMILES CN([C@@H]1C[C@H](CS(=O)(=O)c2ccccc2)C1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.4,(6.34,-12.52,;7.67,-13.29,;9,-12.51,;9.39,-11.02,;10.88,-11.41,;12.21,-10.64,;13.54,-11.4,;14.3,-12.73,;12.77,-12.72,;14.87,-10.63,;16.2,-11.41,;17.54,-10.64,;17.54,-9.1,;16.2,-8.33,;14.87,-9.1,;10.49,-12.9,;7.68,-14.82,;6.35,-15.59,;6.35,-17.14,;7.68,-17.91,;9.02,-17.13,;10.49,-17.6,;11.39,-16.34,;10.47,-15.1,;9.01,-15.59,)| Show InChI InChI=1S/C18H20N4O2S/c1-22(18-16-7-8-19-17(16)20-12-21-18)14-9-13(10-14)11-25(23,24)15-5-3-2-4-6-15/h2-8,12-14H,9-11H2,1H3,(H,19,20,21)/t13-,14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50243909
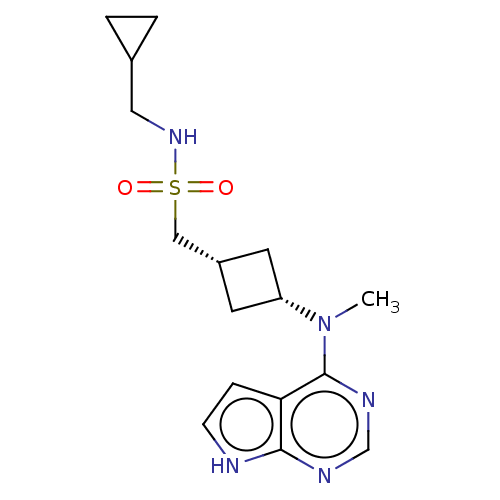
(CHEMBL4066876)Show SMILES CN([C@@H]1C[C@H](CS(=O)(=O)NCC2CC2)C1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.4,(6.35,-12.54,;7.68,-13.31,;9.01,-12.53,;9.41,-11.04,;10.9,-11.43,;12.23,-10.66,;13.56,-11.42,;14.32,-12.75,;12.79,-12.74,;14.89,-10.65,;16.23,-11.42,;17.56,-10.65,;19.1,-10.65,;18.33,-9.31,;10.5,-12.92,;7.69,-14.85,;6.36,-15.62,;6.36,-17.16,;7.69,-17.93,;9.03,-17.15,;10.5,-17.62,;11.4,-16.37,;10.49,-15.13,;9.02,-15.61,)| Show InChI InChI=1S/C16H23N5O2S/c1-21(16-14-4-5-17-15(14)18-10-19-16)13-6-12(7-13)9-24(22,23)20-8-11-2-3-11/h4-5,10-13,20H,2-3,6-9H2,1H3,(H,17,18,19)/t12-,13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50243904
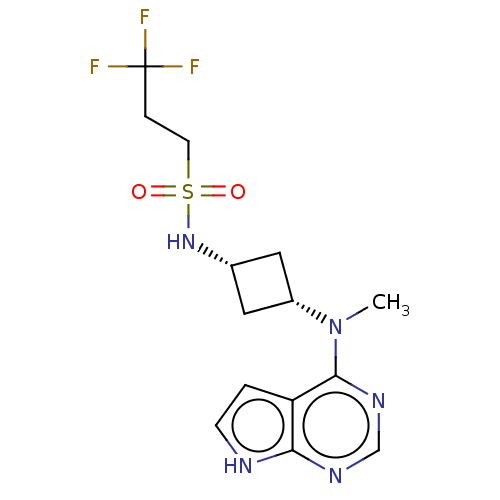
(CHEMBL4103435)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)CCC(F)(F)F)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(7.09,-13.36,;8.42,-14.12,;9.75,-13.35,;10.15,-11.85,;11.64,-12.25,;11.24,-13.74,;12.97,-11.47,;14.3,-12.23,;15.06,-13.56,;13.52,-13.56,;15.63,-11.46,;16.97,-12.24,;18.3,-11.47,;19.63,-12.24,;18.3,-9.93,;19.63,-10.69,;8.43,-15.66,;7.1,-16.43,;7.1,-17.98,;8.43,-18.75,;9.77,-17.97,;11.24,-18.44,;12.14,-17.18,;11.23,-15.94,;9.76,-16.43,)| Show InChI InChI=1S/C14H18F3N5O2S/c1-22(13-11-2-4-18-12(11)19-8-20-13)10-6-9(7-10)21-25(23,24)5-3-14(15,16)17/h2,4,8-10,21H,3,5-7H2,1H3,(H,18,19,20)/t9-,10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50243811
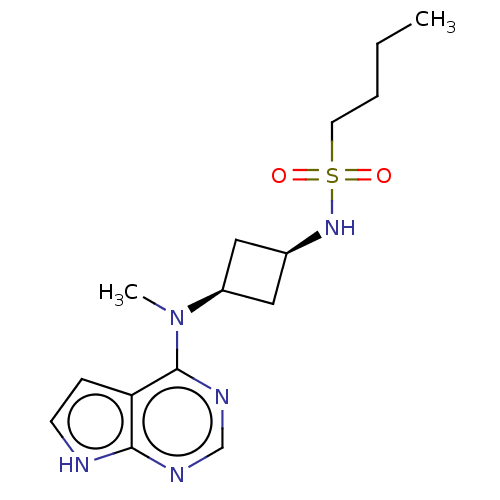
(CHEMBL4087961)Show SMILES CCCCS(=O)(=O)N[C@H]1C[C@H](C1)N(C)c1ncnc2[nH]ccc12 |r,wD:10.12,8.7,(18.14,-11.53,;16.81,-10.76,;15.47,-11.53,;14.14,-10.76,;12.8,-11.53,;13.56,-12.86,;12.03,-12.85,;11.47,-10.77,;10.14,-11.54,;8.65,-11.14,;8.26,-12.64,;9.75,-13.03,;6.93,-13.42,;5.59,-12.65,;6.93,-14.96,;5.61,-15.73,;5.6,-17.27,;6.94,-18.04,;8.28,-17.26,;9.75,-17.73,;10.65,-16.48,;9.73,-15.23,;8.27,-15.72,)| Show InChI InChI=1S/C15H23N5O2S/c1-3-4-7-23(21,22)19-11-8-12(9-11)20(2)15-13-5-6-16-14(13)17-10-18-15/h5-6,10-12,19H,3-4,7-9H2,1-2H3,(H,16,17,18)/t11-,12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239494

(CHEMBL4079243 | US10329302, Example 340 | US107935...)Show SMILES COC[C@@H]1CC(=O)N[C@@H]1COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C18H21N3O5/c1-24-8-11-6-16(22)21-14(11)9-26-18-12-7-15(25-2)13(17(19)23)5-10(12)3-4-20-18/h3-5,7,11,14H,6,8-9H2,1-2H3,(H2,19,23)(H,21,22)/t11-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM330002

(US10463675, Example 85 | US10980815, Ex. No. 85 | ...)Show SMILES Cn1cc(Nc2nccc(n2)N2C[C@@H]3CC[C@H](C2)N3C(=O)NCC#N)cn1 |r,THB:19:18:11.12.17:15.14| Show InChI InChI=1S/C17H21N9O/c1-24-9-12(8-21-24)22-16-19-6-4-15(23-16)25-10-13-2-3-14(11-25)26(13)17(27)20-7-5-18/h4,6,8-9,13-14H,2-3,7,10-11H2,1H3,(H,20,27)(H,19,22,23)/t13-,14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human JAK1 using fluorescence labeled peptide as substrate and using 1 mM ATP by microfluidic based mobility shift assay |
J Med Chem 61: 8597-8612 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00917
BindingDB Entry DOI: 10.7270/Q20C507F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM330002

(US10463675, Example 85 | US10980815, Ex. No. 85 | ...)Show SMILES Cn1cc(Nc2nccc(n2)N2C[C@@H]3CC[C@H](C2)N3C(=O)NCC#N)cn1 |r,THB:19:18:11.12.17:15.14| Show InChI InChI=1S/C17H21N9O/c1-24-9-12(8-21-24)22-16-19-6-4-15(23-16)25-10-13-2-3-14(11-25)26(13)17(27)20-7-5-18/h4,6,8-9,13-14H,2-3,7,10-11H2,1H3,(H,20,27)(H,19,22,23)/t13-,14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human JAK1 using fluorescence labeled peptide as substrate and using 1 mM ATP by microfluidic based mobility shift assay |
J Med Chem 61: 8597-8612 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00917
BindingDB Entry DOI: 10.7270/Q20C507F |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239498

(CHEMBL4093120 | US10329302, Example 189 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@@H](F)[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H18FN3O4/c1-8-12(21-16(23)14(8)18)7-25-17-10-6-13(24-2)11(15(19)22)5-9(10)3-4-20-17/h3-6,8,12,14H,7H2,1-2H3,(H2,19,22)(H,21,23)/t8-,12+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50243841
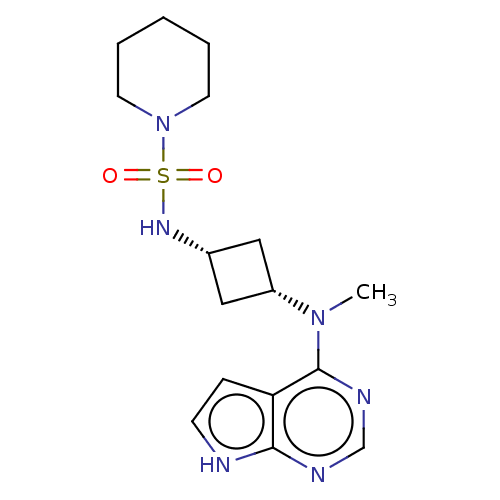
(CHEMBL4077614)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)N1CCCCC1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(6.14,-13.71,;7.47,-14.47,;8.8,-13.7,;9.19,-12.21,;10.68,-12.6,;10.28,-14.09,;12.01,-11.83,;13.33,-12.59,;14.09,-13.91,;12.56,-13.91,;14.67,-11.82,;16.01,-12.6,;17.34,-11.84,;17.35,-10.3,;16.02,-9.52,;14.67,-10.28,;7.47,-16.01,;6.15,-16.78,;6.15,-18.32,;7.48,-19.09,;8.82,-18.31,;10.28,-18.78,;11.18,-17.53,;10.27,-16.29,;8.81,-16.77,)| Show InChI InChI=1S/C16H24N6O2S/c1-21(16-14-5-6-17-15(14)18-11-19-16)13-9-12(10-13)20-25(23,24)22-7-3-2-4-8-22/h5-6,11-13,20H,2-4,7-10H2,1H3,(H,17,18,19)/t12-,13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50529618
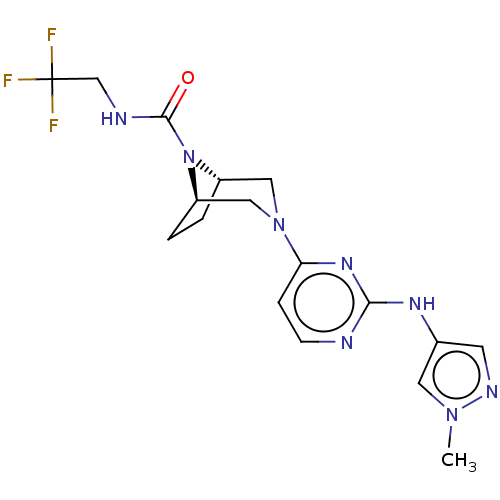
(CHEMBL4526032)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1ccnc(Nc3cnn(C)c3)n1)N2C(=O)NCC(F)(F)F |r| Show InChI InChI=1S/C17H21F3N8O/c1-26-7-11(6-23-26)24-15-21-5-4-14(25-15)27-8-12-2-3-13(9-27)28(12)16(29)22-10-17(18,19)20/h4-7,12-13H,2-3,8-10H2,1H3,(H,22,29)(H,21,24,25)/t12-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human JAK1 using fluorescence labeled peptide as substrate and using 1 mM ATP by microfluidic based mobility shift assay |
J Med Chem 61: 8597-8612 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00917
BindingDB Entry DOI: 10.7270/Q20C507F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159749
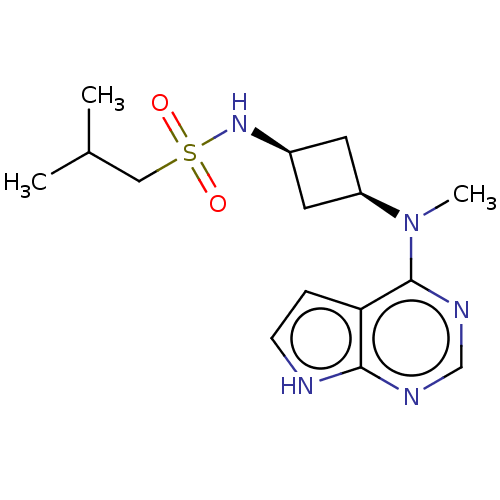
(US10966980, Example 3 | US9035074, 3)Show SMILES CC(C)CS(=O)(=O)N[C@H]1C[C@H](C1)N(C)c1ncnc2[nH]ccc12 |r,wD:10.12,8.7,(5.44,2.67,;3.95,2.27,;3.55,.79,;2.86,3.36,;1.37,2.96,;1.37,4.5,;-.11,3.36,;.97,1.48,;-.51,1.08,;-1.85,1.85,;-2.62,.51,;-1.28,-.26,;-4.1,.12,;-5.19,1.2,;-4.1,-1.42,;-5.44,-2.19,;-5.44,-3.73,;-4.1,-4.5,;-2.77,-3.73,;-1.31,-4.21,;-.4,-2.96,;-1.31,-1.72,;-2.77,-2.19,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50529618

(CHEMBL4526032)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1ccnc(Nc3cnn(C)c3)n1)N2C(=O)NCC(F)(F)F |r| Show InChI InChI=1S/C17H21F3N8O/c1-26-7-11(6-23-26)24-15-21-5-4-14(25-15)27-8-12-2-3-13(9-27)28(12)16(29)22-10-17(18,19)20/h4-7,12-13H,2-3,8-10H2,1H3,(H,22,29)(H,21,24,25)/t12-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human JAK1 using fluorescence labeled peptide as substrate and using 1 mM ATP by microfluidic based mobility shift assay |
J Med Chem 61: 8597-8612 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00917
BindingDB Entry DOI: 10.7270/Q20C507F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human JAK1 using fluorescence labeled peptide as substrate and using 1 mM ATP by microfluidic based mobility shift assay |
J Med Chem 61: 8597-8612 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00917
BindingDB Entry DOI: 10.7270/Q20C507F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human JAK1 using fluorescence labeled peptide as substrate and using 1 mM ATP by microfluidic based mobility shift assay |
J Med Chem 61: 8597-8612 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00917
BindingDB Entry DOI: 10.7270/Q20C507F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50243839

(CHEMBL4099048)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)CCCC#N)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(6.15,-11.79,;7.49,-12.56,;8.82,-11.79,;9.21,-10.29,;10.7,-10.68,;10.31,-12.17,;12.03,-9.91,;13.36,-10.67,;14.12,-12,;12.59,-12,;14.7,-9.9,;16.03,-10.67,;17.37,-9.91,;18.7,-10.68,;20.03,-11.45,;7.49,-14.1,;6.17,-14.87,;6.16,-16.42,;7.5,-17.19,;8.84,-16.41,;10.31,-16.87,;11.21,-15.62,;10.29,-14.38,;8.83,-14.86,)| Show InChI InChI=1S/C15H20N6O2S/c1-21(15-13-4-6-17-14(13)18-10-19-15)12-8-11(9-12)20-24(22,23)7-3-2-5-16/h4,6,10-12,20H,2-3,7-9H2,1H3,(H,17,18,19)/t11-,12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the Compound was tested against HIV protease enzyme. |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM330002

(US10463675, Example 85 | US10980815, Ex. No. 85 | ...)Show SMILES Cn1cc(Nc2nccc(n2)N2C[C@@H]3CC[C@H](C2)N3C(=O)NCC#N)cn1 |r,THB:19:18:11.12.17:15.14| Show InChI InChI=1S/C17H21N9O/c1-24-9-12(8-21-24)22-16-19-6-4-15(23-16)25-10-13-2-3-14(11-25)26(13)17(27)20-7-5-18/h4,6,8-9,13-14H,2-3,7,10-11H2,1H3,(H,20,27)(H,19,22,23)/t13-,14+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TYK2 using fluorescence labeled peptide as substrate and using 1 mM ATP level by microfluidic based mobility shift assay |
J Med Chem 61: 8597-8612 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00917
BindingDB Entry DOI: 10.7270/Q20C507F |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM330002

(US10463675, Example 85 | US10980815, Ex. No. 85 | ...)Show SMILES Cn1cc(Nc2nccc(n2)N2C[C@@H]3CC[C@H](C2)N3C(=O)NCC#N)cn1 |r,THB:19:18:11.12.17:15.14| Show InChI InChI=1S/C17H21N9O/c1-24-9-12(8-21-24)22-16-19-6-4-15(23-16)25-10-13-2-3-14(11-25)26(13)17(27)20-7-5-18/h4,6,8-9,13-14H,2-3,7,10-11H2,1H3,(H,20,27)(H,19,22,23)/t13-,14+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TYK2 using fluorescence labeled peptide as substrate and using 1 mM ATP level by microfluidic based mobility shift assay |
J Med Chem 61: 8597-8612 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00917
BindingDB Entry DOI: 10.7270/Q20C507F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM329924

(US10463675, Example 7 | US10980815, Ex. No. 7 | US...)Show SMILES Cn1cc(Nc2nccc(n2)N2C[C@@H]3CC[C@H](C2)N3C(=O)[C@@H]2CC2(F)F)cn1 |r,THB:19:18:11.12.17:15.14| Show InChI InChI=1S/C18H21F2N7O/c1-25-8-11(7-22-25)23-17-21-5-4-15(24-17)26-9-12-2-3-13(10-26)27(12)16(28)14-6-18(14,19)20/h4-5,7-8,12-14H,2-3,6,9-10H2,1H3,(H,21,23,24)/t12-,13+,14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human JAK1 using fluorescence labeled peptide as substrate and using 1 mM ATP by microfluidic based mobility shift assay |
J Med Chem 61: 8597-8612 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00917
BindingDB Entry DOI: 10.7270/Q20C507F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50243906
(CHEMBL4063642)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)N(C)C1CC1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(5.8,-11.92,;7.14,-12.68,;8.47,-11.91,;8.86,-10.41,;10.35,-10.81,;9.96,-12.3,;11.68,-10.03,;13.01,-10.8,;13.77,-12.13,;12.24,-12.12,;14.35,-10.03,;15.68,-10.8,;14.35,-8.49,;15.13,-7.16,;13.59,-7.16,;7.14,-14.22,;5.81,-15,;5.81,-16.54,;7.15,-17.31,;8.49,-16.53,;9.96,-17,;10.86,-15.75,;9.94,-14.5,;8.48,-14.99,)| Show InChI InChI=1S/C15H22N6O2S/c1-20(15-13-5-6-16-14(13)17-9-18-15)12-7-10(8-12)19-24(22,23)21(2)11-3-4-11/h5-6,9-12,19H,3-4,7-8H2,1-2H3,(H,16,17,18)/t10-,12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data