

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50446130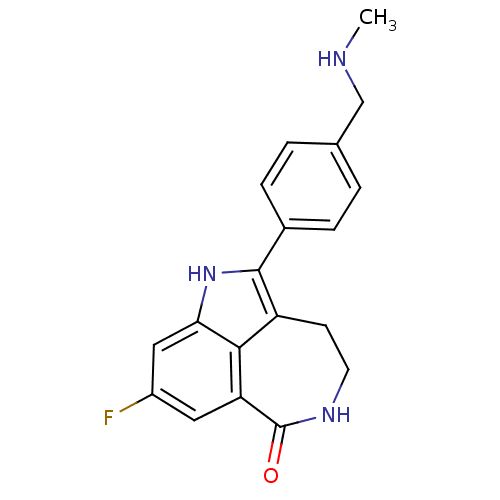 (AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human PARP2 (2 to 583 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H2A and H2B) a... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50446130 (AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human PARP2 (2 to 583 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H2A and H2B) a... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198665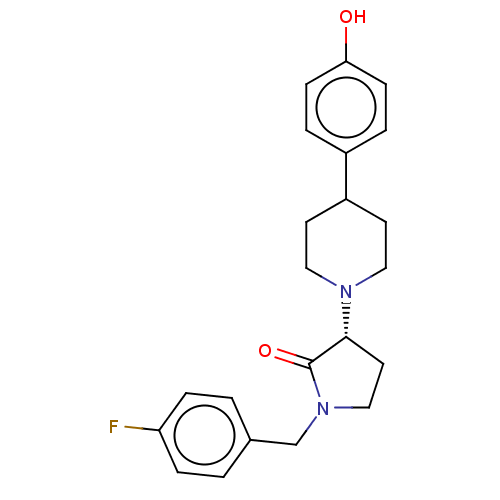 (US9221796, 2b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198694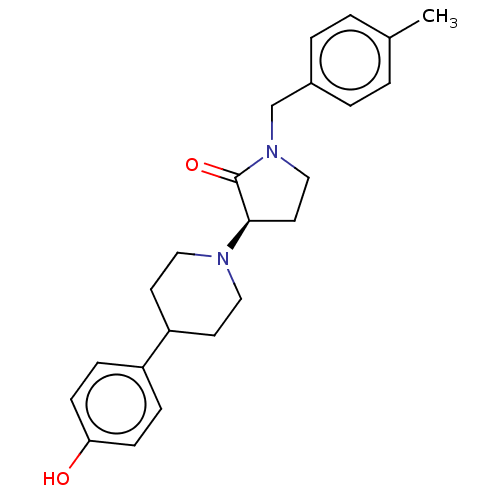 (US9221796, 23b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50446130 (AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50446130 (AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198728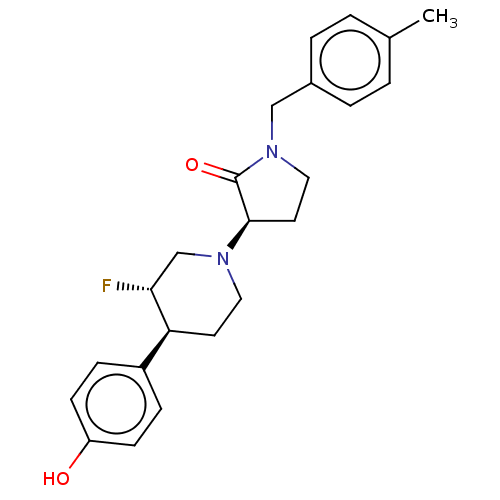 (US9221796, 46, P-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198726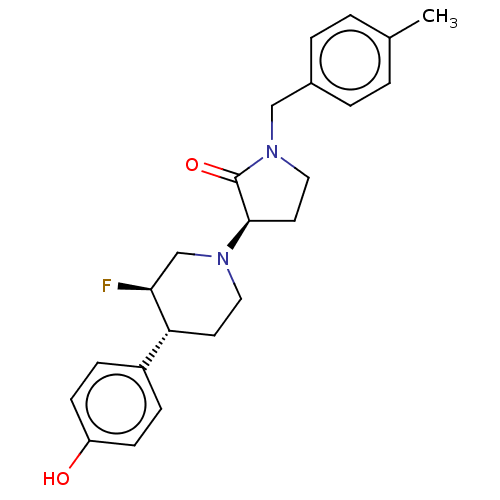 (US9221796, 46, P-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50330324 (CHEMBL4170867) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM198728 (US9221796, 46, P-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to GluN2B receptor in human cortex | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50330409 (CHEMBL4168402) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50330410 (CHEMBL4161899) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198735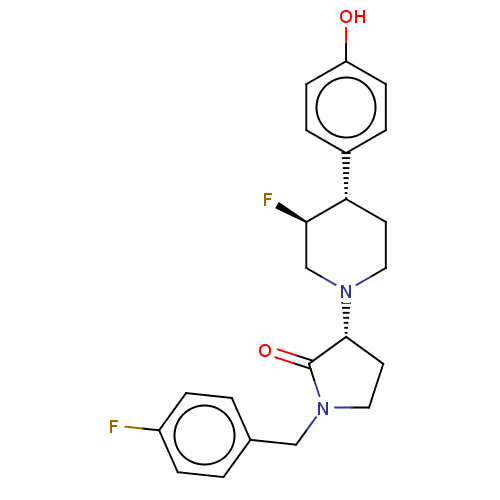 (US9221796, 48, P-3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272095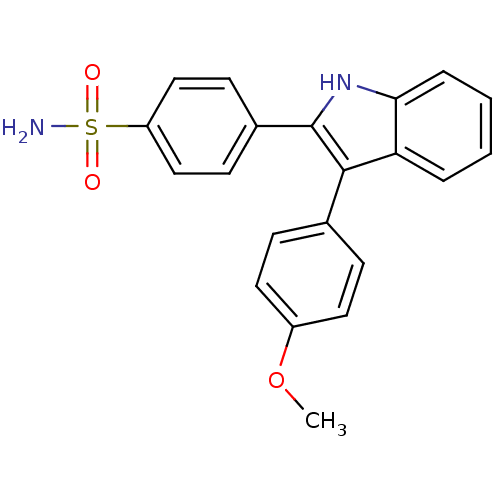 (4-(3-(4-methoxyphenyl)-1H-indol-2-yl)benzenesulfon...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272096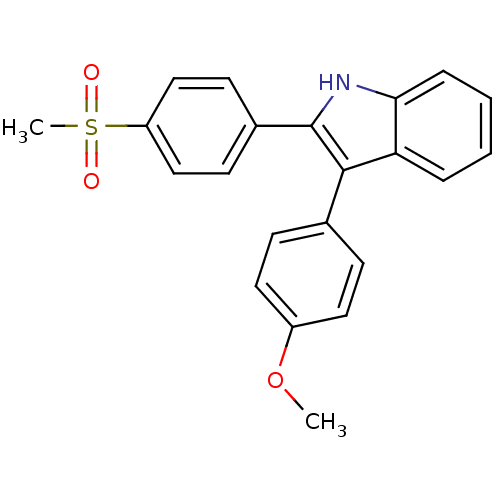 (3-(4-methoxyphenyl)-2-(4-(methylsulfonyl)phenyl)-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272129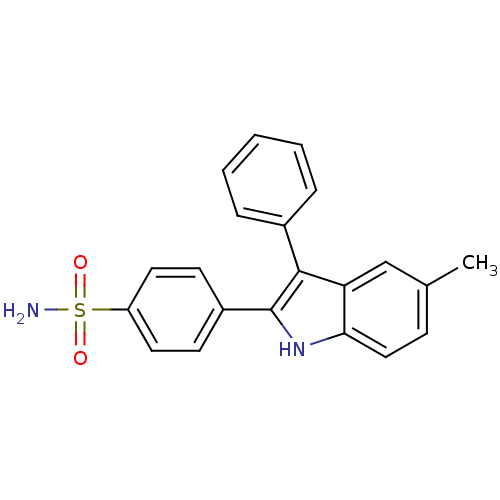 (4-(5-methyl-3-phenyl-1H-indol-2-yl)benzenesulfonam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272131 (3-(4-fluorophenyl)-2-(4-(methylsulfonyl)phenyl)-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272125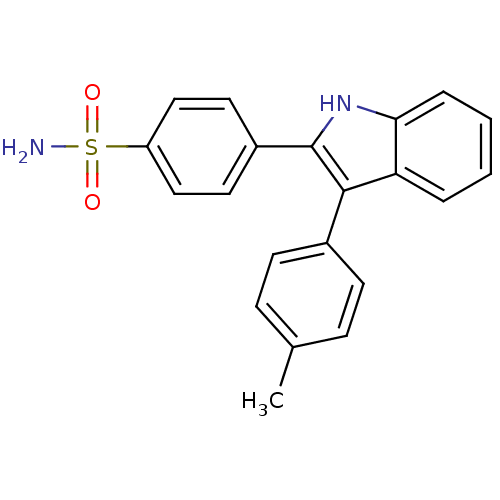 (4-(3-p-tolyl-1H-indol-2-yl)benzenesulfonamide | CH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272126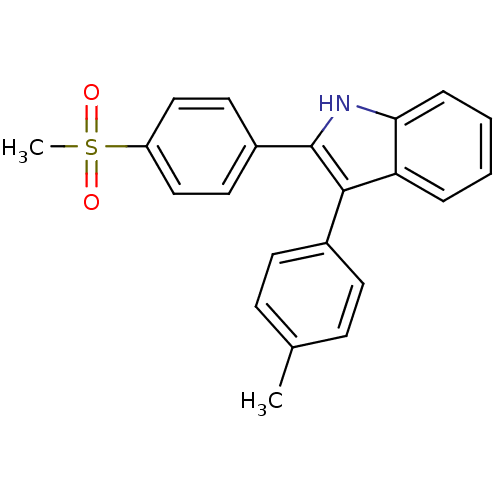 (2-(4-(methylsulfonyl)phenyl)-3-p-tolyl-1H-indole |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272105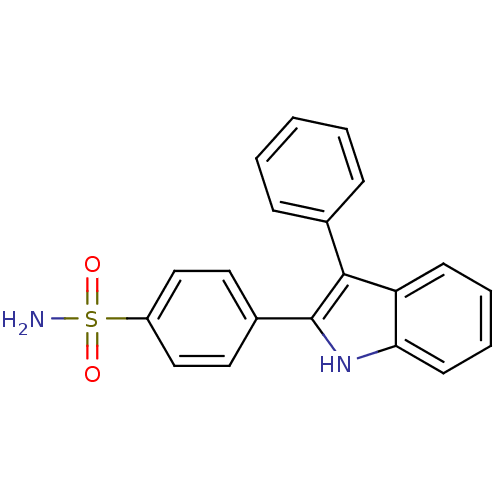 (4-(3-phenyl-1H-indol-2-yl)benzenesulfonamide | CHE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM371616 ((R)-4-(2-(6-Fluoropyridin-3-yl)-4-(trifluoromethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272110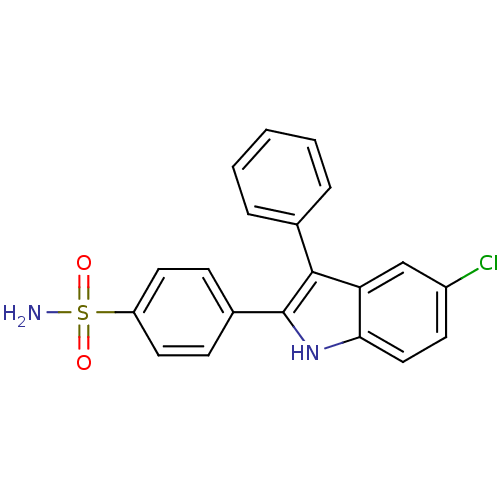 (4-(5-chloro-3-phenyl-1H-indol-2-yl)benzenesulfonam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272097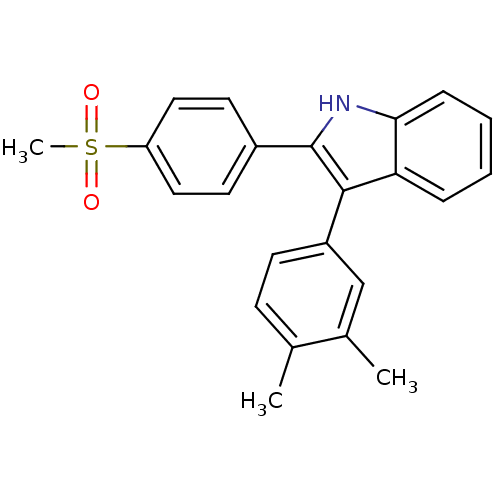 (3-(3,4-dimethylphenyl)-2-(4-(methylsulfonyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272128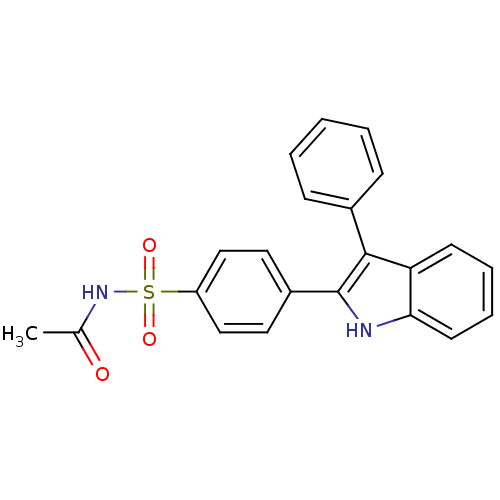 (CHEMBL500943 | N-(4-(3-phenyl-1H-indol-2-yl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272130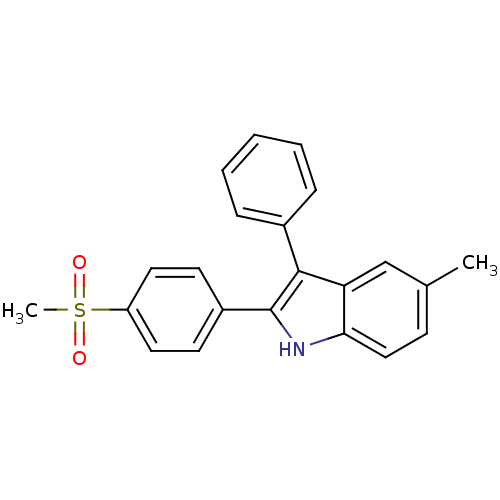 (5-methyl-2-(4-(methylsulfonyl)phenyl)-3-phenyl-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272111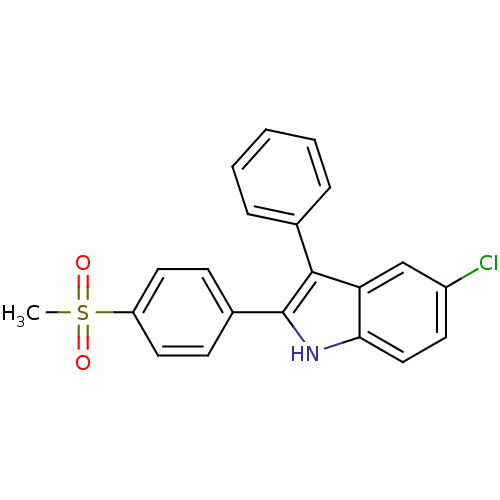 (5-chloro-2-(4-(methylsulfonyl)phenyl)-3-phenyl-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272124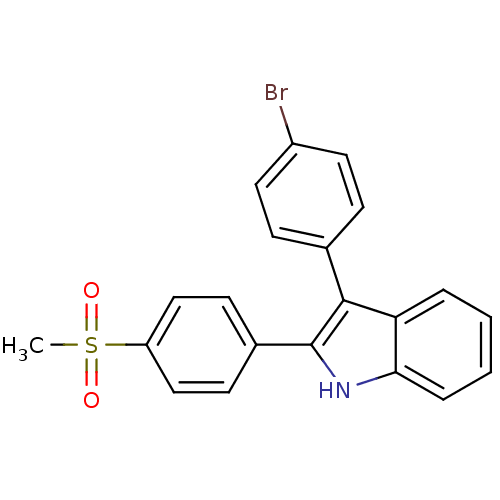 (3-(4-bromophenyl)-2-(4-(methylsulfonyl)phenyl)-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50548675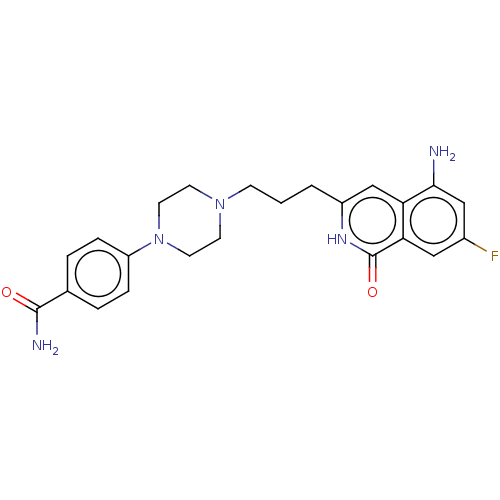 (CHEMBL4800042) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50548675 (CHEMBL4800042) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50545560 (CHEMBL4634421) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50545552 (CHEMBL4646742) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50545563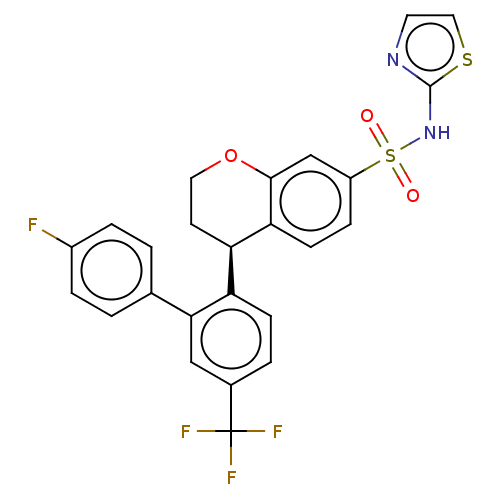 (CHEMBL4636838) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM27566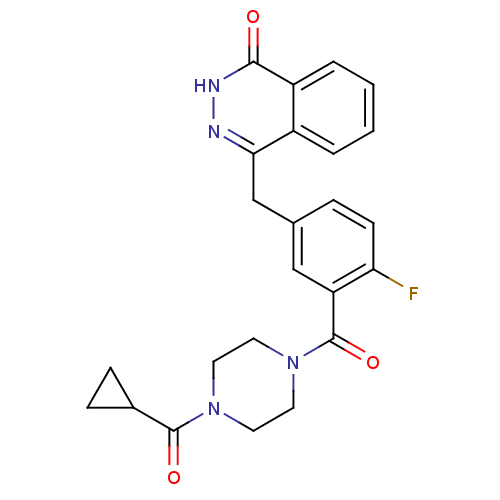 (4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human PARP2 (2 to 583 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H2A and H2B) a... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM27566 (4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human PARP2 (2 to 583 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H2A and H2B) a... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50545558 (CHEMBL4642748) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272090 (4-(5-chloro-3-(4-chlorophenyl)-1H-indol-2-yl)benze...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50548673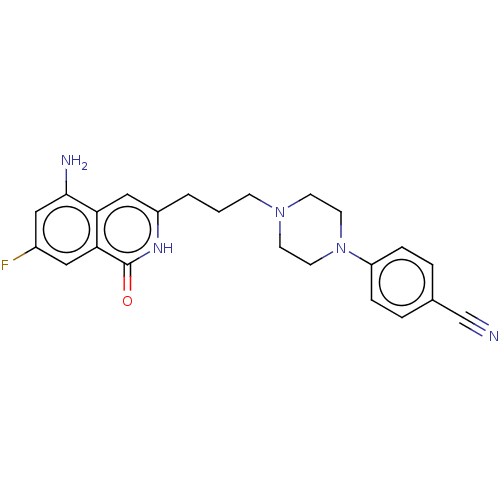 (CHEMBL4748390) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50548673 (CHEMBL4748390) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM371523 ((R)-N-(6-Fluoropyridin-2-yl)-4-(2-(1-methyl-1H-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) Article DOI: 10.1021/acs.jmedchem.0c00361 BindingDB Entry DOI: 10.7270/Q2V69P5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50084621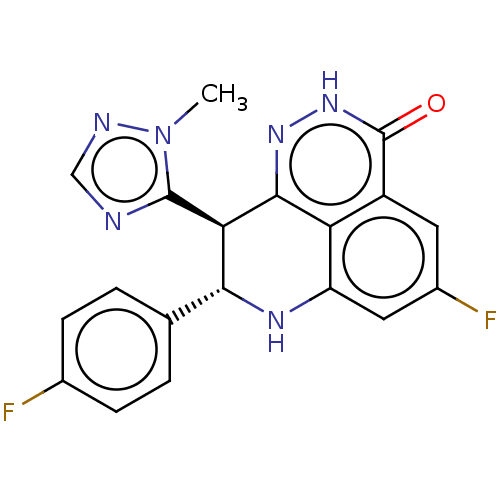 (BMN 673 | Talazoparib) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human PARP2 (2 to 583 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H2A and H2B) a... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50084621 (BMN 673 | Talazoparib) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human PARP2 (2 to 583 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H2A and H2B) a... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50548679 (CHEMBL4747106) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50548679 (CHEMBL4747106) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50548674 (CHEMBL4787850) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50548674 (CHEMBL4787850) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50272106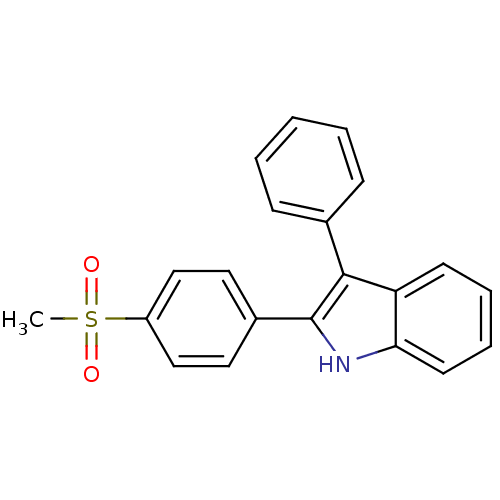 (2-(4-(methylsulfonyl)phenyl)-3-phenyl-1H-indole | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 43: 1297-303 (2008) Article DOI: 10.1016/j.ejmech.2007.06.022 BindingDB Entry DOI: 10.7270/Q2CC10F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50446130 (AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50446130 (AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50446130 (AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human PARP2 (2 to 583 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H2A and H2B) a... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 332 total ) | Next | Last >> |