

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118030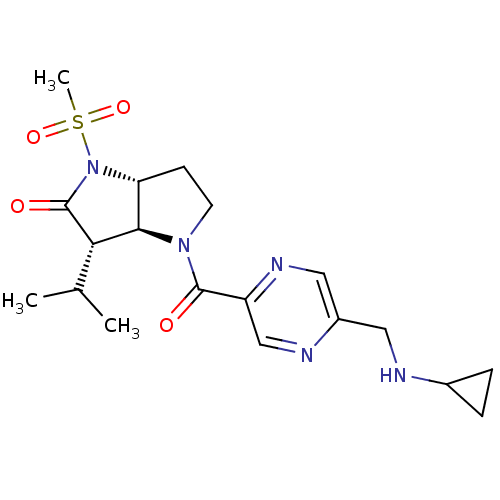 (4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060602 (2-[3-((2R,8aS)-1,4-Dioxo-hexahydro-pyrrolo[1,2-a]p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in presence of Gpp(NH)p (Pre treated with 1 nM) calculated for the high affinity components of the [3H]spiroperidol b... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50074092 (6'-oxo-1-[(2S)-tetrahydro-1H-2-pyrrolylcarbonyl]-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17428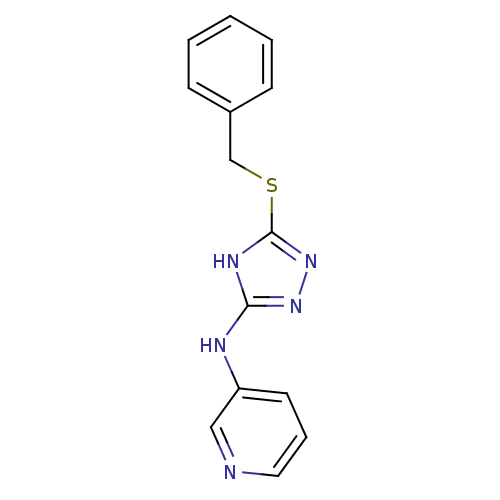 (1,2,4-Triazole Compound, 86 | N-[5-(benzylsulfanyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50060601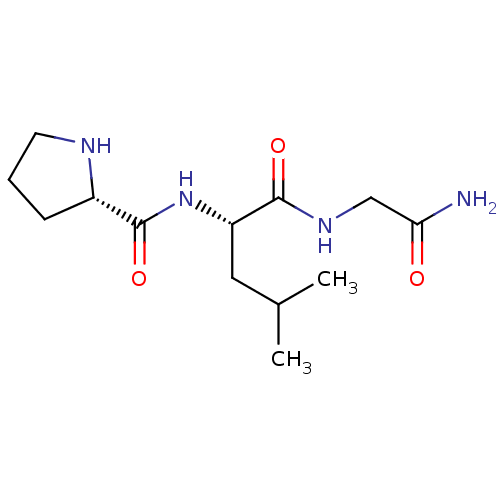 ((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060601 ((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in presence of Gpp(NH)p (Pre treated with 1 uM) calculated for the high affinity components of the [3H]spiroperidol b... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17355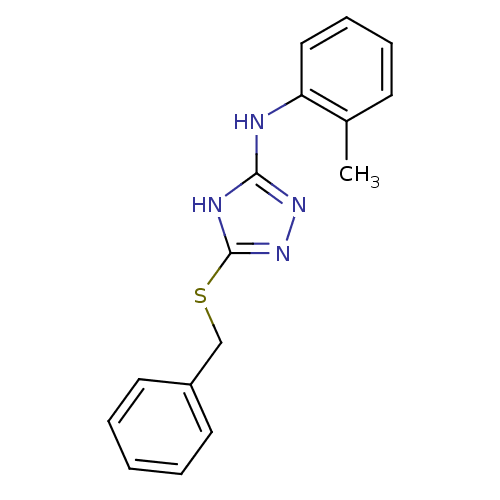 (1,2,4-Triazole Compound, 13 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17388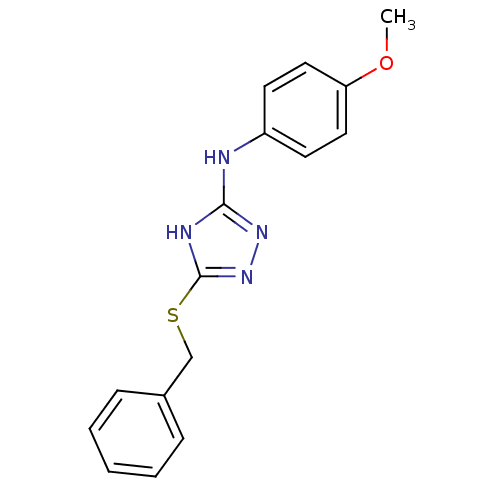 (1,2,4-Triazole Compound, 46 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50074093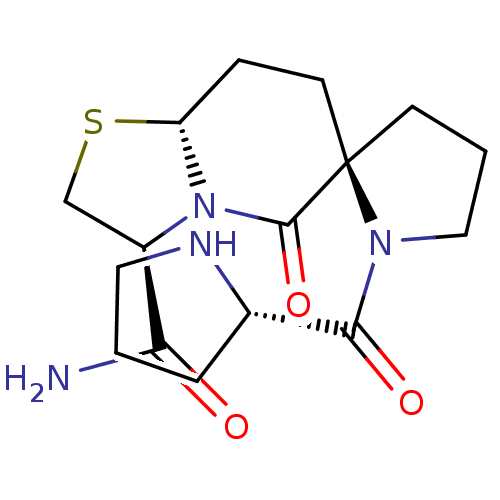 ((2R,3'S,8a'R)-5'-oxo-1-((S)-pyrrolidine-2-carbonyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50060601 ((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50074092 (6'-oxo-1-[(2S)-tetrahydro-1H-2-pyrrolylcarbonyl]-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060603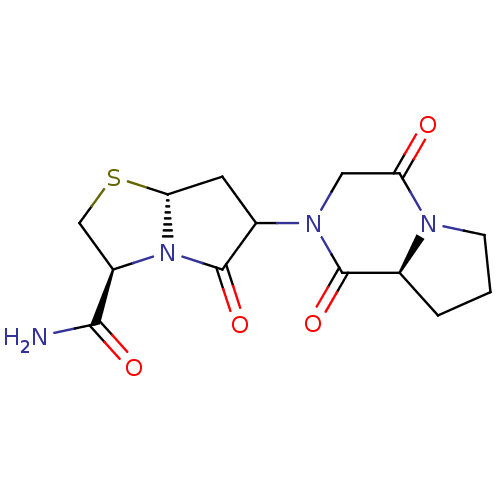 ((3S,7aR)-6-((2R,8aS)-1,4-Dioxo-hexahydro-pyrrolo[1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Percentage of receptor in the low affinity form for the compound to Dopamine receptor D2 in absence of Gpp(NH)p (pre treated with 100 nM) | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060603 ((3S,7aR)-6-((2R,8aS)-1,4-Dioxo-hexahydro-pyrrolo[1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in presence of Gpp(NH)p (Pre treated with 100 nM) calculated for the high affinity components of the [3H]spiroperidol... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50074093 ((2R,3'S,8a'R)-5'-oxo-1-((S)-pyrrolidine-2-carbonyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060600 ((S)-2-((S)-1,4-Dioxo-hexahydro-pyrrolo[1,2-a]pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Percentage of receptor in the high affinity form for the compound to Dopamine receptor D2 in presence of Gpp(NH)p (pretreated with 100 nM) | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17365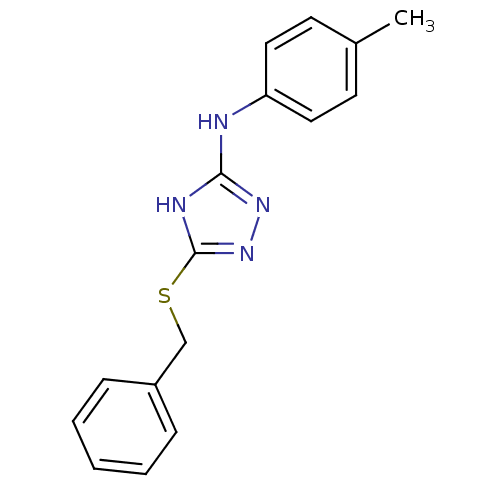 (1,2,4-Triazole Compound, 23 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | -57.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50074092 (6'-oxo-1-[(2S)-tetrahydro-1H-2-pyrrolylcarbonyl]-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060600 ((S)-2-((S)-1,4-Dioxo-hexahydro-pyrrolo[1,2-a]pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in presence of Gpp(NH)p (Pre treated with 100 nM) calculated for the high affinity components of the [3H]spiroperidol... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060600 ((S)-2-((S)-1,4-Dioxo-hexahydro-pyrrolo[1,2-a]pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in absence of Gpp(NH)p calculated for the high affinity components of the [3H]spiroperidol binding to Dopamine recept... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060602 (2-[3-((2R,8aS)-1,4-Dioxo-hexahydro-pyrrolo[1,2-a]p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in absence of Gpp(NH)p calculated for the high affinity components of the [3H]spiroperidol binding to Dopamine recept... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060601 ((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in absence of Gpp(NH)p calculated for the high affinity components of the [3H]spiroperidol binding to Dopamine recept... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50060601 ((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50074093 ((2R,3'S,8a'R)-5'-oxo-1-((S)-pyrrolidine-2-carbonyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060603 ((3S,7aR)-6-((2R,8aS)-1,4-Dioxo-hexahydro-pyrrolo[1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in absence of Gpp(NH)p calculated for the high affinity components of the [3H]spiroperidol binding to Dopamine recept... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060600 ((S)-2-((S)-1,4-Dioxo-hexahydro-pyrrolo[1,2-a]pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in presence of Gpp(NH)p calculated for the high affinity components of the [3H]spiroperidol binding to Dopamine recep... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164899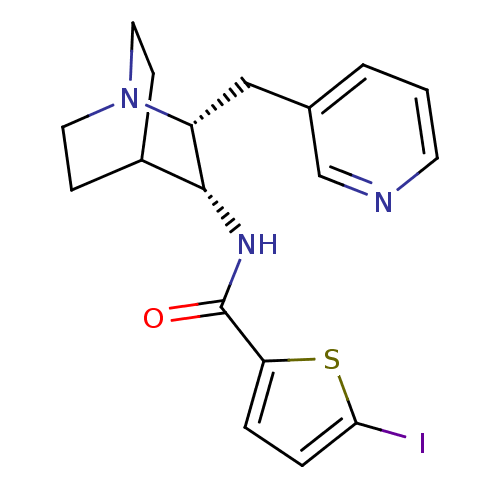 (5-Iodo-thiophene-2-carboxylic acid ((2R,3R)-2-pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against [3H]methyllycaconitine binding towards Nicotinic acetylcholine receptor alpha-7 of rat brain hippocampus | Bioorg Med Chem Lett 15: 2073-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.045 BindingDB Entry DOI: 10.7270/Q2GT5NX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50074092 (6'-oxo-1-[(2S)-tetrahydro-1H-2-pyrrolylcarbonyl]-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50060601 ((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17390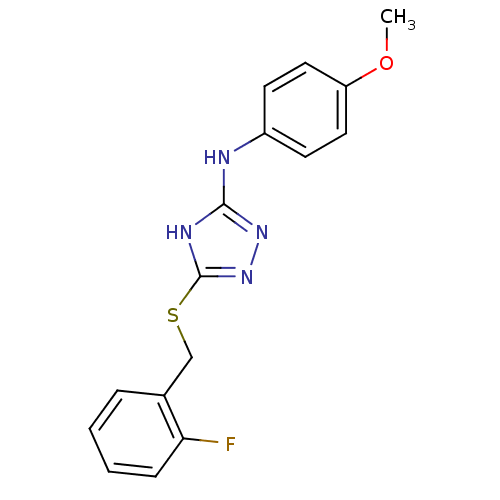 (1,2,4-Triazole Compound, 48 | 5-{[(2-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060601 ((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in presence of Gpp(NH)p calculated for the high affinity components of the [3H]spiroperidol binding to Dopamine recep... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060603 ((3S,7aR)-6-((2R,8aS)-1,4-Dioxo-hexahydro-pyrrolo[1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in presence of Gpp(NH)p calculated for the high affinity components of the [3H]spiroperidol binding to Dopamine recep... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17362 (1,2,4-Triazole Compound, 20 | 5-[(3-methylbut-2-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118028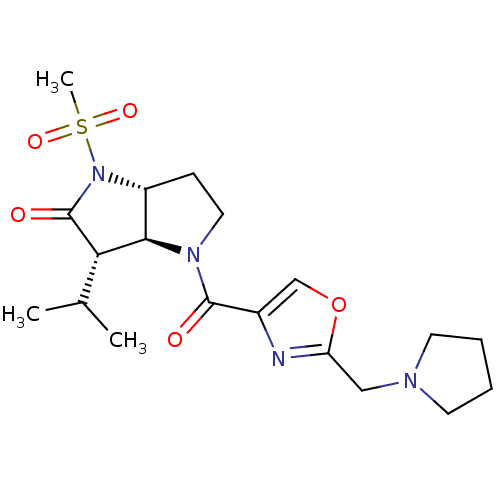 (3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.160 | n/a | n/a | n/a | n/a | 4.90 | 3.05E+4 | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118028 (3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305851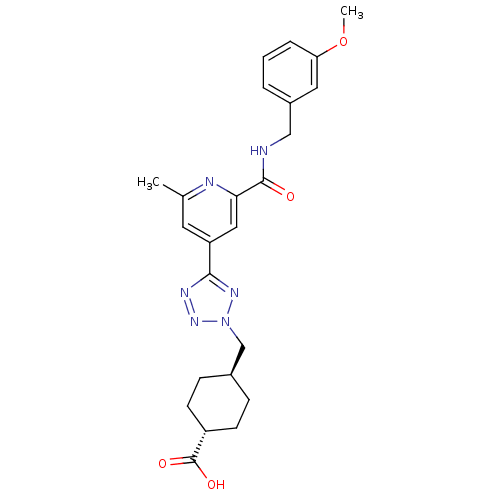 (CHEMBL596273 | trans-4-((5-(2-(3-methoxybenzylcarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17395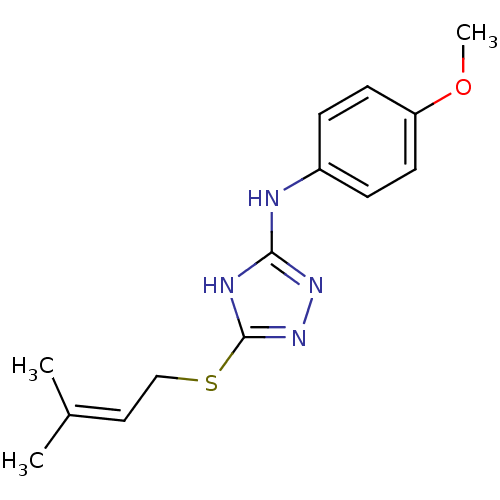 (1,2,4-Triazole Compound, 53 | N-(4-methoxyphenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305844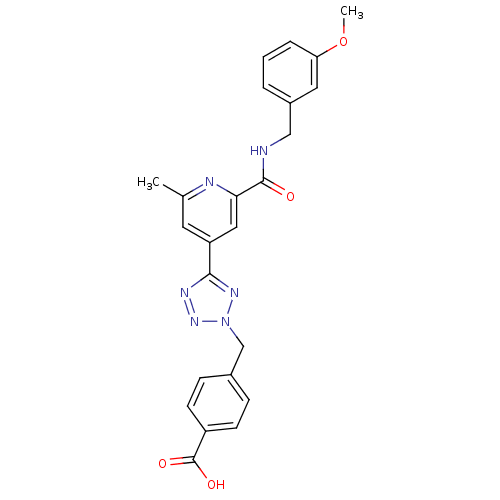 (4-((5-(2-(3-methoxybenzylcarbamoyl)-6-methylpyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060602 (2-[3-((2R,8aS)-1,4-Dioxo-hexahydro-pyrrolo[1,2-a]p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in presence of Gpp(NH)p calculated for the high affinity components of the [3H]spiroperidol binding to Dopamine recep... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50074093 ((2R,3'S,8a'R)-5'-oxo-1-((S)-pyrrolidine-2-carbonyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305858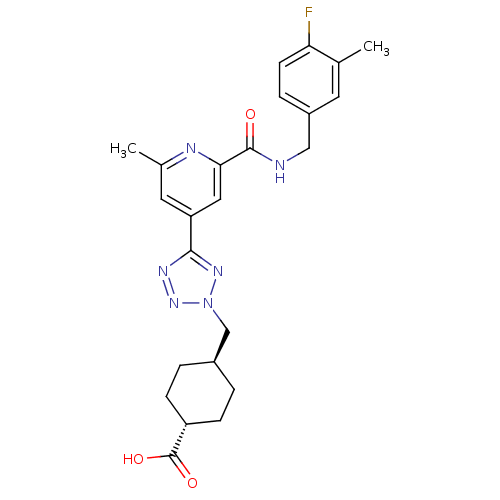 (CHEMBL605928 | trans-4-((5-(2-(4-fluoro-3-methylbe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine synthetase (Homo sapiens (Human)) | BDBM85288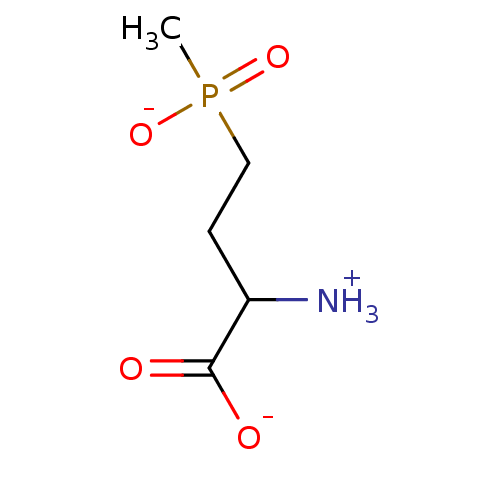 (Phosphinothricin analog, 1 (L Isomer)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a | |
Chevron Chemical Company | Assay Description The enzyme was assayed at pH 7.0 using the pyruvate kinase/lactate dehydroenase coupling system. | Bioorg Chem 18: 154-9 (1990) BindingDB Entry DOI: 10.7270/Q24J0CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17358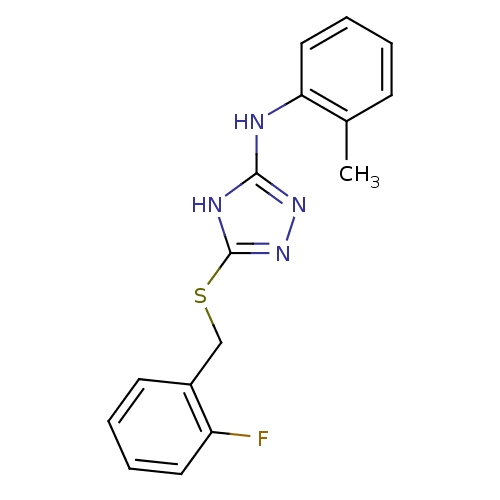 (1,2,4-Triazole Compound, 16 | 5-{[(2-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.230 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305856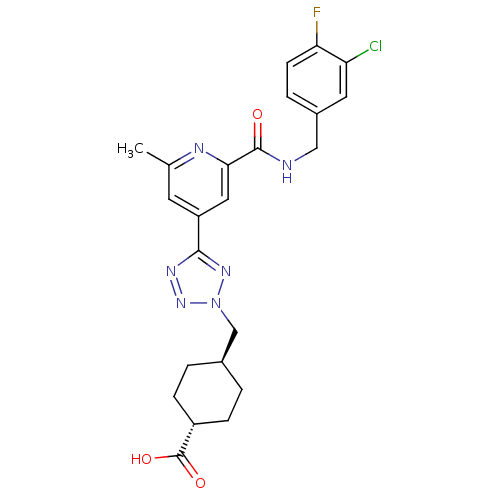 (CHEMBL603206 | trans-4-((5-(2-(3-chloro-4-fluorobe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305855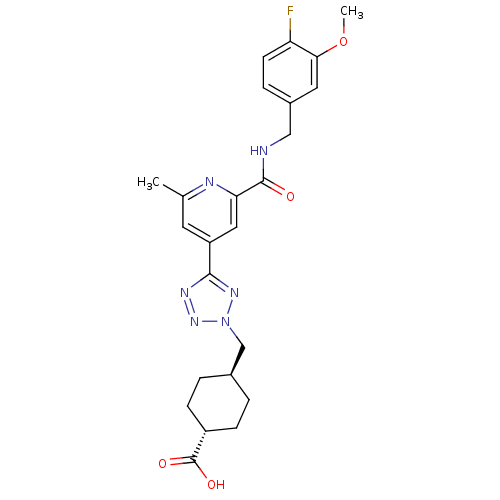 (CHEMBL595156 | trans-4-((5-(2-(4-fluoro-3-methoxyb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17430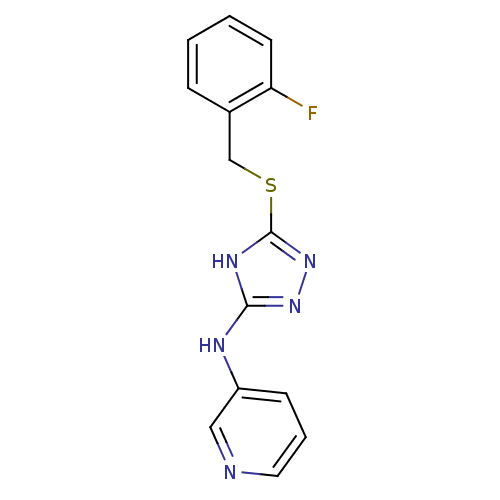 (1,2,4-Triazole Compound, 88 | N-(5-{[(2-fluorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | 0.00000207 | 6.63E+3 | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50244490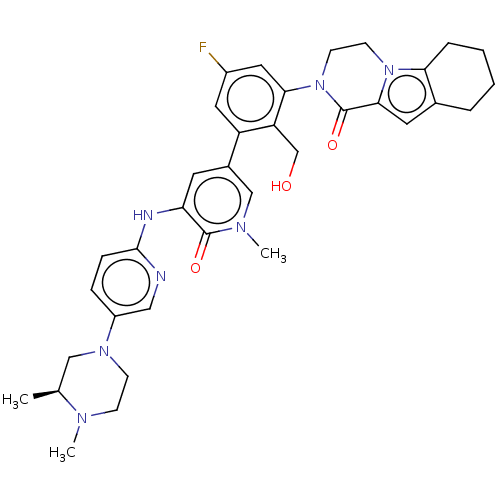 (CHEMBL4102992) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... | J Med Chem 61: 2227-2245 (2018) Article DOI: 10.1021/acs.jmedchem.7b01712 BindingDB Entry DOI: 10.7270/Q2H134F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111951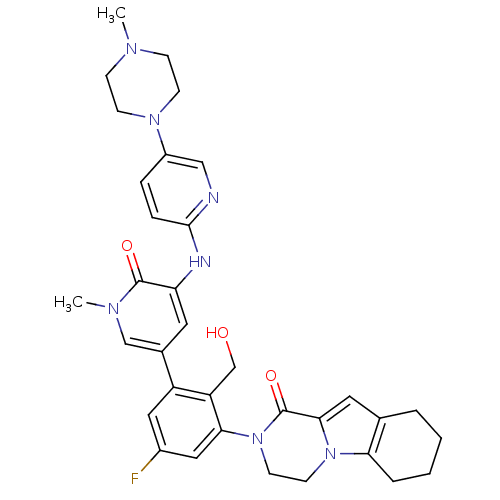 (US8618107, 197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... | J Med Chem 61: 2227-2245 (2018) Article DOI: 10.1021/acs.jmedchem.7b01712 BindingDB Entry DOI: 10.7270/Q2H134F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50244489 (CHEMBL4095379) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... | J Med Chem 61: 2227-2245 (2018) Article DOI: 10.1021/acs.jmedchem.7b01712 BindingDB Entry DOI: 10.7270/Q2H134F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5716 total ) | Next | Last >> |