

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50260444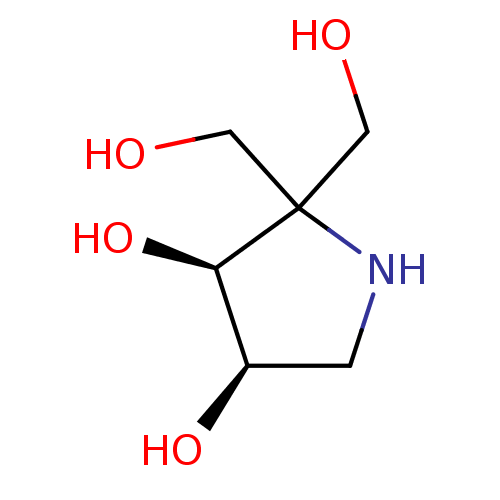 (CHEMBL4073111) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Savitribai Phule Pune University (Formerly University of Pune) Curated by ChEMBL | Assay Description Competitive inhibition of coffee beans alpha-galactosidase pre-incubated for 1 hr followed by p-nitrophenyl-alpha-D-galactopyranoside substrate addit... | Bioorg Med Chem 25: 5148-5159 (2017) Article DOI: 10.1016/j.bmc.2017.07.026 BindingDB Entry DOI: 10.7270/Q23T9KP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50260443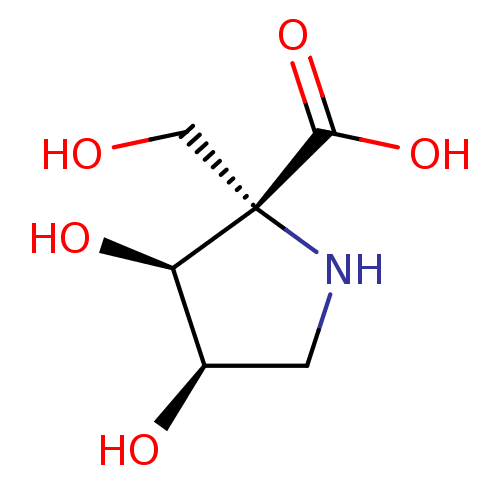 (CHEMBL4096086) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Savitribai Phule Pune University (Formerly University of Pune) Curated by ChEMBL | Assay Description Competitive inhibition of coffee beans alpha-galactosidase pre-incubated for 1 hr followed by p-nitrophenyl-alpha-D-galactopyranoside substrate addit... | Bioorg Med Chem 25: 5148-5159 (2017) Article DOI: 10.1016/j.bmc.2017.07.026 BindingDB Entry DOI: 10.7270/Q23T9KP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3965 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3130 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM50168995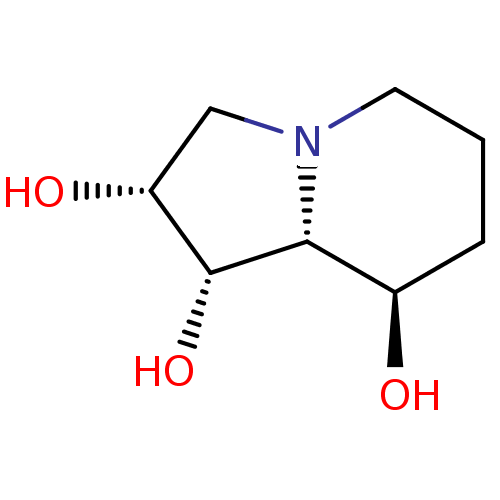 ((-)-swainsonine | (1S,2R,8R,8aR)-Octahydro-indoliz...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3990 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM50168995 ((-)-swainsonine | (1S,2R,8R,8aR)-Octahydro-indoliz...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2199 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3994 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3991 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2948 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2199 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3990 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM50482721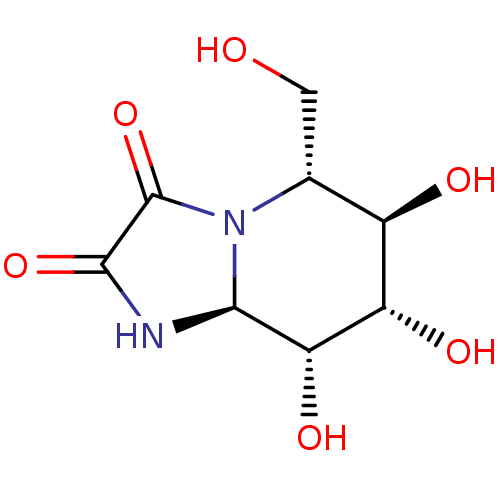 (Kifunensine) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3990 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM50482721 (Kifunensine) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2199 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3858 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3962 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 4073 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50260445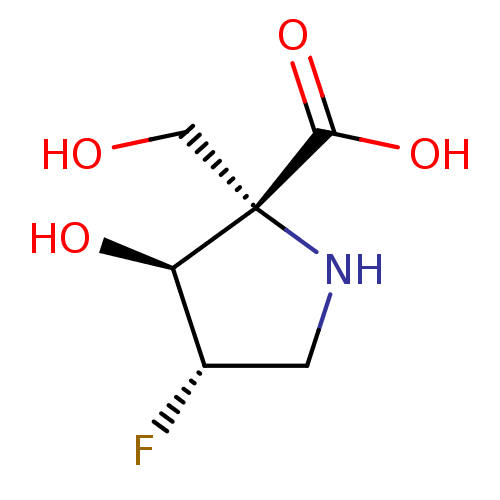 (CHEMBL4064761) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Savitribai Phule Pune University (Formerly University of Pune) Curated by ChEMBL | Assay Description Inhibition of coffee beans alpha-galactosidase pre-incubated for 1 hr followed by p-nitrophenyl-alpha-D-galactopyranoside substrate addition by fluor... | Bioorg Med Chem 25: 5148-5159 (2017) Article DOI: 10.1016/j.bmc.2017.07.026 BindingDB Entry DOI: 10.7270/Q23T9KP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase/Alpha-1,2-mannosidase family protein/Alpha-1,2-mannosidase, putative/Glycoside hydrolase family 92/Putative alpha-1,2-mannosidase (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 1032 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 7.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3963 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 8.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 4093 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 4092 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 1878 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM36373 ((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 5.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3784 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM50065259 ((2R,3R,4R,5R)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3990 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,2-mannosidase, putative (Bacteroides thetaiotaomicron ) | BDBM50065259 ((2R,3R,4R,5R)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2199 assessed as reduction of mannose release using 4NP-mannopyranoside substrate | Nat Chem Biol 6: 125-32 (2010) Article DOI: 10.1038/nchembio.278 BindingDB Entry DOI: 10.7270/Q2ZW1PQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50315887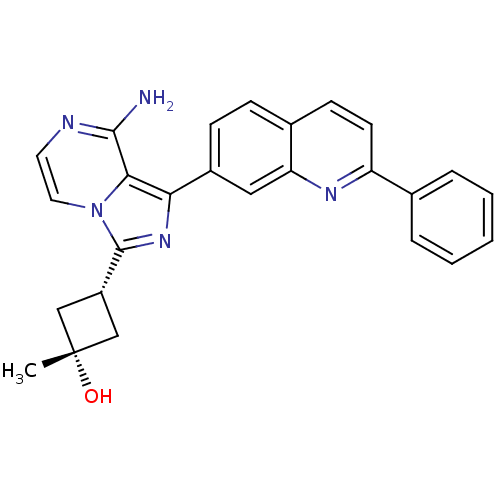 ((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM248094 (VX-497 (2)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Nycomed | Assay Description The assay was performed in a 100 μL final volume in 96-well UV plates (Costar, 3635) with a reaction buffer composed of 50 mM Tris-HCl (pH 8.0), 1... | J Enzyme Inhib Med Chem 29: 408-19 (2014) Article DOI: 10.3109/14756366.2013.793184 BindingDB Entry DOI: 10.7270/Q28P5ZDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071033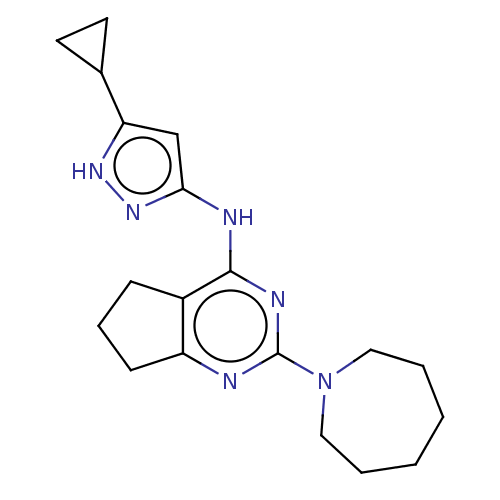 (CHEMBL3409721) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50260444 (CHEMBL4073111) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Savitribai Phule Pune University (Formerly University of Pune) Curated by ChEMBL | Assay Description Competitive inhibition of coffee beans alpha-galactosidase pre-incubated for 1 hr followed by p-nitrophenyl-alpha-D-galactopyranoside substrate addit... | Bioorg Med Chem 25: 5148-5159 (2017) Article DOI: 10.1016/j.bmc.2017.07.026 BindingDB Entry DOI: 10.7270/Q23T9KP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071014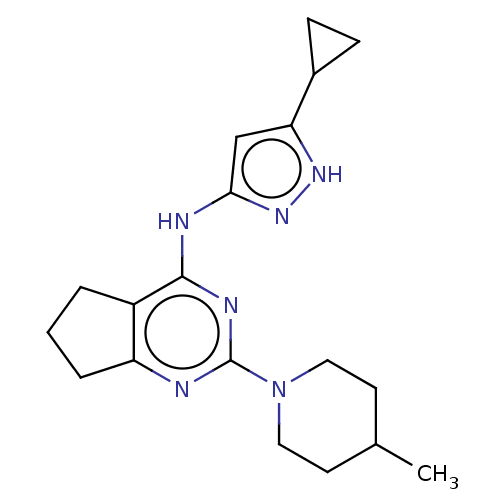 (CHEMBL3409716) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071057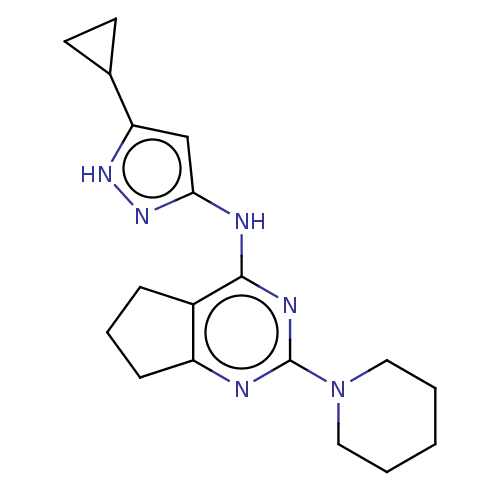 (CHEMBL3409713) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071036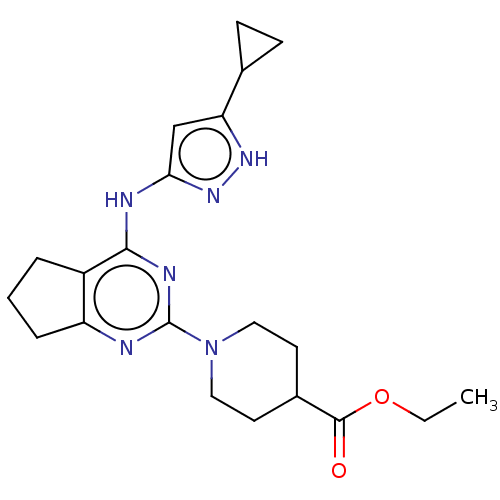 (CHEMBL3409718) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071031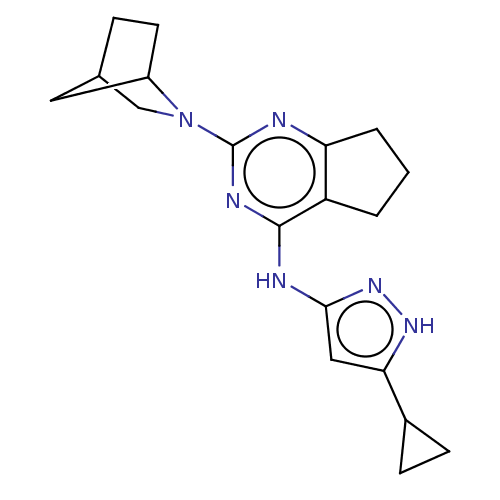 (CHEMBL3409723) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071032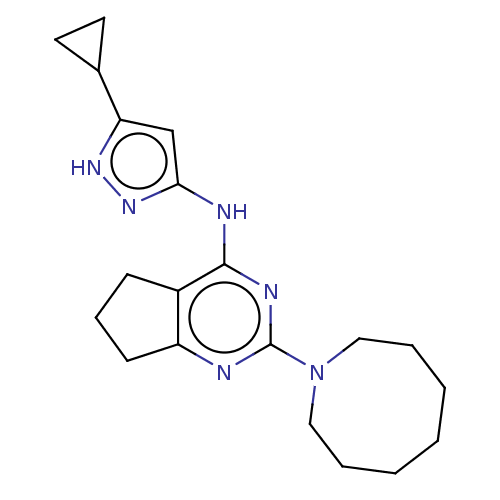 (CHEMBL3409722) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50260443 (CHEMBL4096086) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Savitribai Phule Pune University (Formerly University of Pune) Curated by ChEMBL | Assay Description Competitive inhibition of coffee beans alpha-galactosidase pre-incubated for 1 hr followed by p-nitrophenyl-alpha-D-galactopyranoside substrate addit... | Bioorg Med Chem 25: 5148-5159 (2017) Article DOI: 10.1016/j.bmc.2017.07.026 BindingDB Entry DOI: 10.7270/Q23T9KP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071056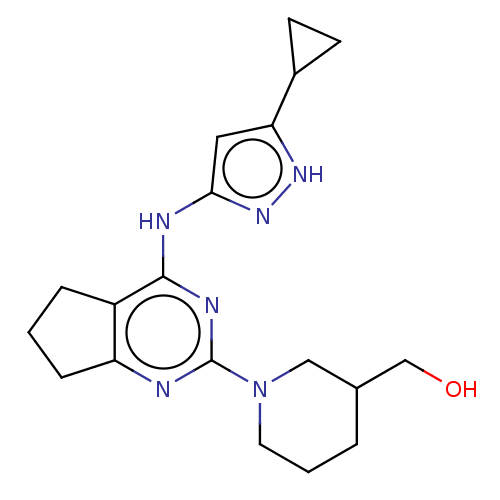 (CHEMBL3409714) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071026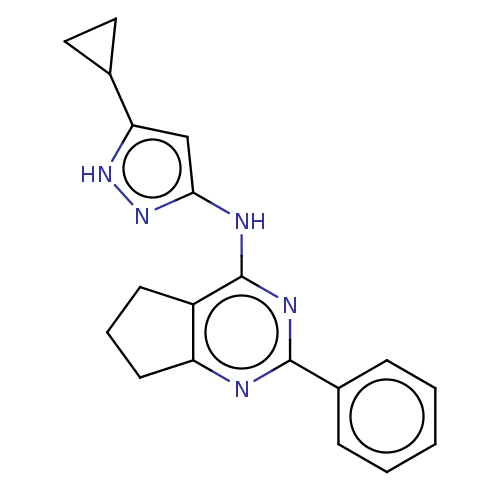 (CHEMBL3409728) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071019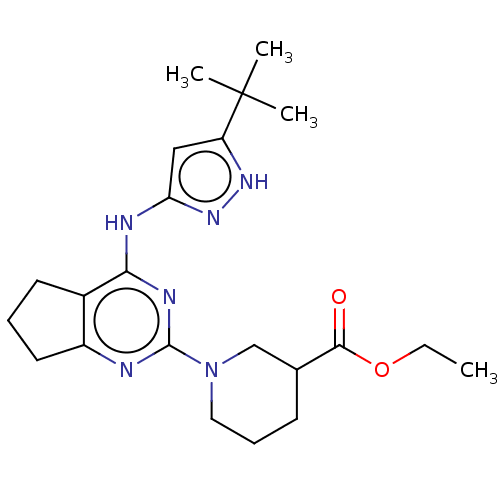 (CHEMBL3409735) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50071014 (CHEMBL3409716) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IR (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolved f... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071035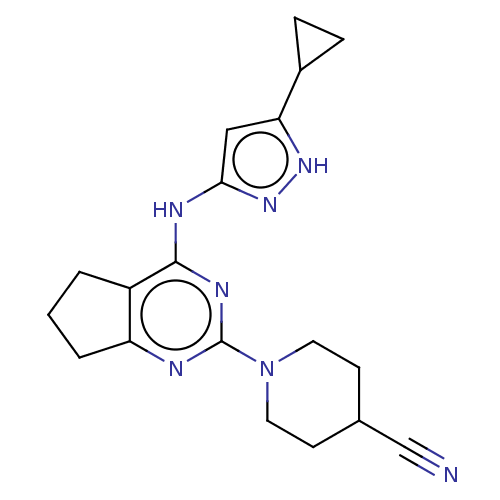 (CHEMBL3409719) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Nycomed | Assay Description The assay was performed in a 100 μL final volume in 96-well UV plates (Costar, 3635) with a reaction buffer composed of 50 mM Tris-HCl (pH 8.0), 1... | J Enzyme Inhib Med Chem 29: 408-19 (2014) Article DOI: 10.3109/14756366.2013.793184 BindingDB Entry DOI: 10.7270/Q28P5ZDV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071029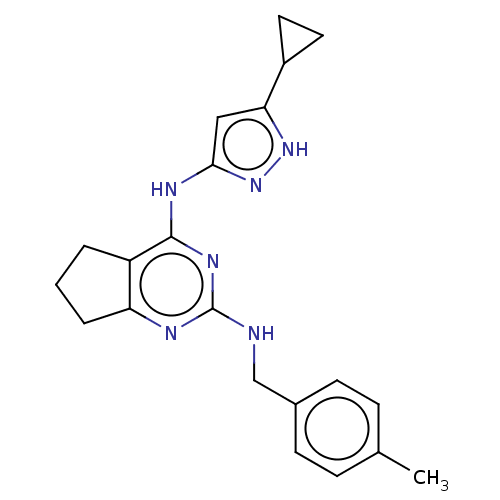 (CHEMBL3409725) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071038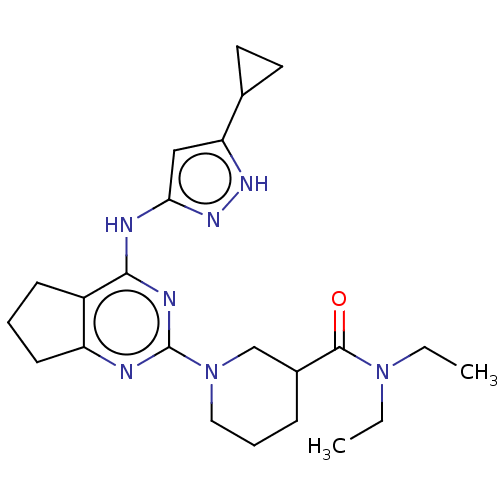 (CHEMBL3409715) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 184 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071037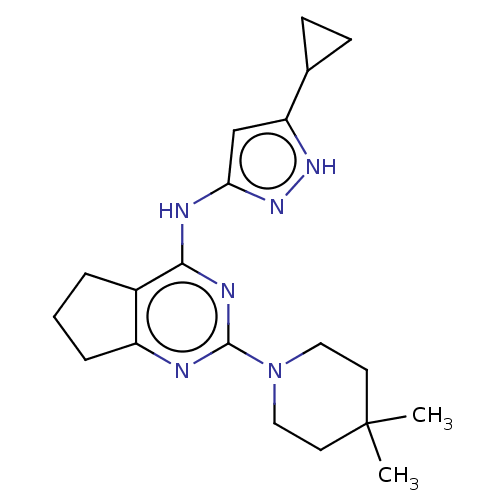 (CHEMBL3409717) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM248096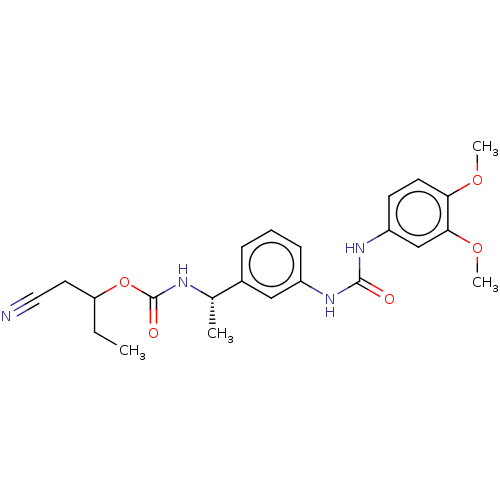 (IMPDH II inhibitor, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Nycomed | Assay Description The assay was performed in a 100 μL final volume in 96-well UV plates (Costar, 3635) with a reaction buffer composed of 50 mM Tris-HCl (pH 8.0), 1... | J Enzyme Inhib Med Chem 29: 408-19 (2014) Article DOI: 10.3109/14756366.2013.793184 BindingDB Entry DOI: 10.7270/Q28P5ZDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071022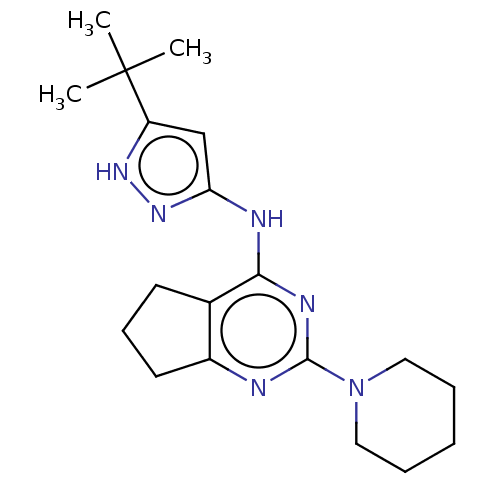 (CHEMBL3409732) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071030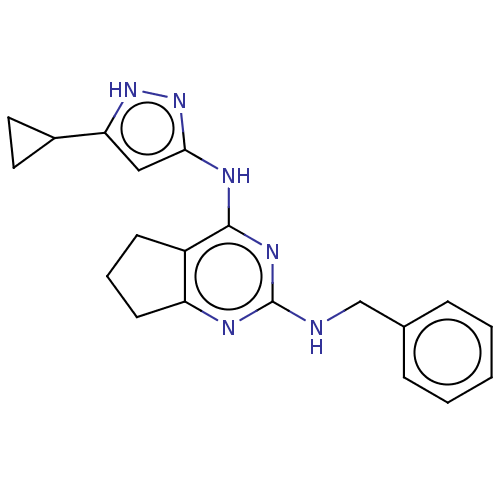 (CHEMBL3409724) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071021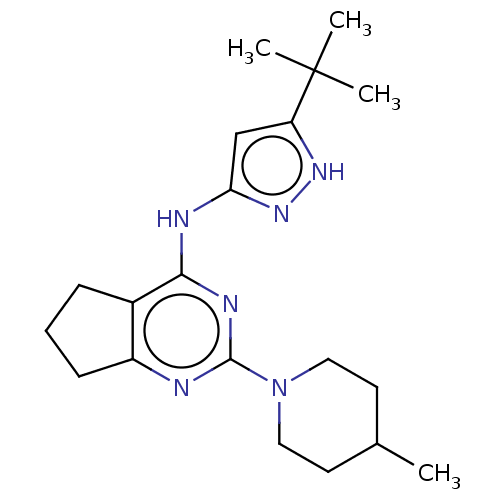 (CHEMBL3409733) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM248095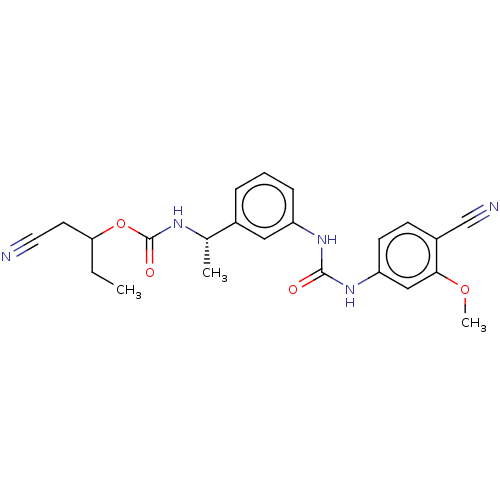 (VX-148 (3)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Nycomed | Assay Description The assay was performed in a 100 μL final volume in 96-well UV plates (Costar, 3635) with a reaction buffer composed of 50 mM Tris-HCl (pH 8.0), 1... | J Enzyme Inhib Med Chem 29: 408-19 (2014) Article DOI: 10.3109/14756366.2013.793184 BindingDB Entry DOI: 10.7270/Q28P5ZDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50071025 (CHEMBL3409729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 442 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... | Eur J Med Chem 92: 246-56 (2015) Article DOI: 10.1016/j.ejmech.2014.12.053 BindingDB Entry DOI: 10.7270/Q2639RFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 108 total ) | Next | Last >> |