 Found 986 hits with Last Name = 'conde' and Initial = 's'
Found 986 hits with Last Name = 'conde' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50322767
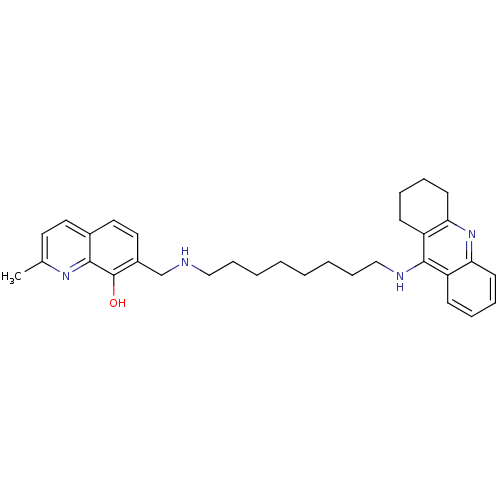
(2-Methyl-7-{[8-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Cc1ccc2ccc(CNCCCCCCCCNc3c4CCCCc4nc4ccccc34)c(O)c2n1 Show InChI InChI=1S/C32H40N4O/c1-23-16-17-24-18-19-25(32(37)30(24)35-23)22-33-20-10-4-2-3-5-11-21-34-31-26-12-6-8-14-28(26)36-29-15-9-7-13-27(29)31/h6,8,12,14,16-19,33,37H,2-5,7,9-11,13,15,20-22H2,1H3,(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31592
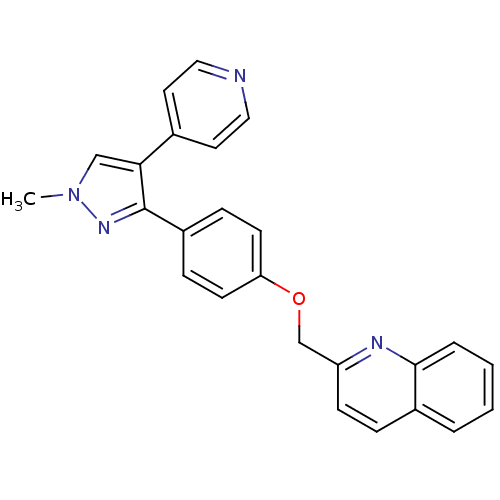
(PF-2545920 | US9138494, MP-10 | substituted pyraz...)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C25H20N4O/c1-29-16-23(18-12-14-26-15-13-18)25(28-29)20-7-10-22(11-8-20)30-17-21-9-6-19-4-2-3-5-24(19)27-21/h2-16H,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.646 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant PDE10A expressed in baculovirus infected Sf9 cells using [3H]cAMP as substrate after 60 mins by TopCount scintillation ... |
J Med Chem 57: 4196-212 (2014)
Article DOI: 10.1021/jm500073h
BindingDB Entry DOI: 10.7270/Q2X068K6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50322768
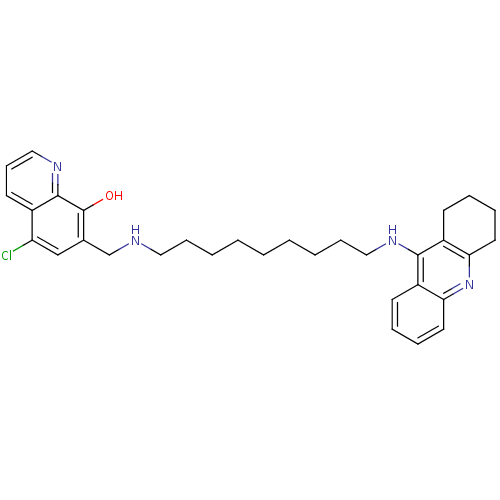
(5-Chloro-7-{[9-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(Cl)c2cccnc12 Show InChI InChI=1S/C32H39ClN4O/c33-27-21-23(32(38)31-24(27)15-12-20-36-31)22-34-18-10-4-2-1-3-5-11-19-35-30-25-13-6-8-16-28(25)37-29-17-9-7-14-26(29)30/h6,8,12-13,15-16,20-21,34,38H,1-5,7,9-11,14,17-19,22H2,(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322767

(2-Methyl-7-{[8-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Cc1ccc2ccc(CNCCCCCCCCNc3c4CCCCc4nc4ccccc34)c(O)c2n1 Show InChI InChI=1S/C32H40N4O/c1-23-16-17-24-18-19-25(32(37)30(24)35-23)22-33-20-10-4-2-3-5-11-21-34-31-26-12-6-8-14-28(26)36-29-15-9-7-13-27(29)31/h6,8,12,14,16-19,33,37H,2-5,7,9-11,13,15,20-22H2,1H3,(H,34,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50347535
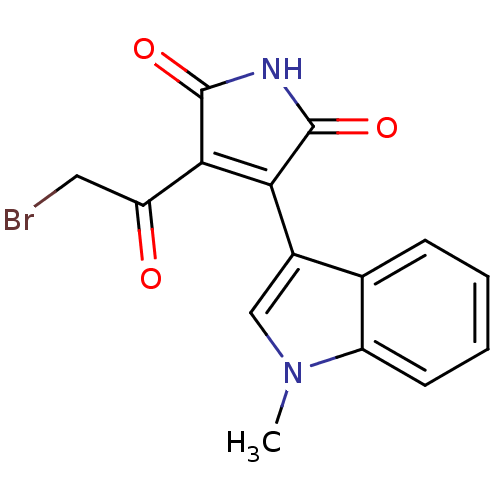
(CHEMBL1801637)Show SMILES Cn1cc(C2=C(C(=O)CBr)C(=O)NC2=O)c2ccccc12 |c:4| Show InChI InChI=1S/C15H11BrN2O3/c1-18-7-9(8-4-2-3-5-10(8)18)12-13(11(19)6-16)15(21)17-14(12)20/h2-5,7H,6H2,1H3,(H,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qui£?mica Medica-CSIC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using prephosphorylated polypeptide substrate after 30 mins by Glow-type luminescence assay in presence of 1... |
J Med Chem 54: 4042-56 (2011)
Article DOI: 10.1021/jm1016279
BindingDB Entry DOI: 10.7270/Q29887C1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322766
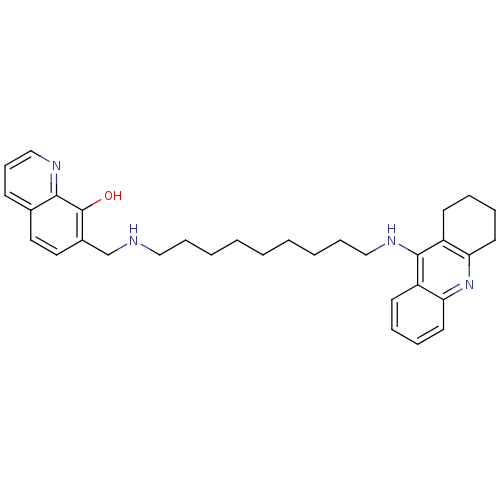
(7-{[9-(1,2,3,4-Tetrahydroacridin-9-ylamino)nonylam...)Show SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C32H40N4O/c37-32-25(19-18-24-13-12-22-34-30(24)32)23-33-20-10-4-2-1-3-5-11-21-35-31-26-14-6-8-16-28(26)36-29-17-9-7-15-27(29)31/h6,8,12-14,16,18-19,22,33,37H,1-5,7,9-11,15,17,20-21,23H2,(H,35,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50322766

(7-{[9-(1,2,3,4-Tetrahydroacridin-9-ylamino)nonylam...)Show SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C32H40N4O/c37-32-25(19-18-24-13-12-22-34-30(24)32)23-33-20-10-4-2-1-3-5-11-21-35-31-26-14-6-8-16-28(26)36-29-17-9-7-15-27(29)31/h6,8,12-14,16,18-19,22,33,37H,1-5,7,9-11,15,17,20-21,23H2,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50322767

(2-Methyl-7-{[8-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Cc1ccc2ccc(CNCCCCCCCCNc3c4CCCCc4nc4ccccc34)c(O)c2n1 Show InChI InChI=1S/C32H40N4O/c1-23-16-17-24-18-19-25(32(37)30(24)35-23)22-33-20-10-4-2-3-5-11-21-34-31-26-12-6-8-14-28(26)36-29-15-9-7-13-27(29)31/h6,8,12,14,16-19,33,37H,2-5,7,9-11,13,15,20-22H2,1H3,(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322768

(5-Chloro-7-{[9-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(Cl)c2cccnc12 Show InChI InChI=1S/C32H39ClN4O/c33-27-21-23(32(38)31-24(27)15-12-20-36-31)22-34-18-10-4-2-1-3-5-11-19-35-30-25-13-6-8-16-28(25)37-29-17-9-7-14-26(29)30/h6,8,12-13,15-16,20-21,34,38H,1-5,7,9-11,14,17-19,22H2,(H,35,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322777
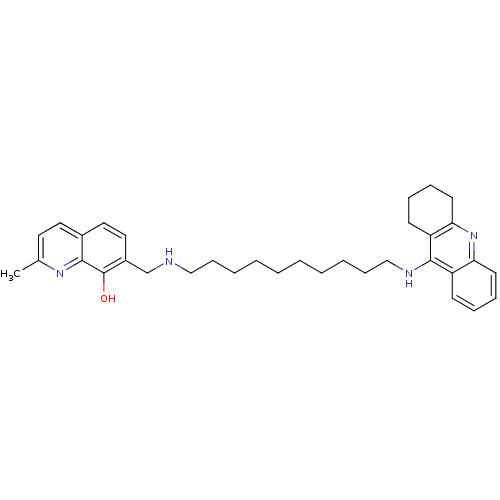
(2-Methyl-7-{[10-(1,2,3,4-tetrahydroacridin-9-ylami...)Show SMILES Cc1ccc2ccc(CNCCCCCCCCCCNc3c4CCCCc4nc4ccccc34)c(O)c2n1 Show InChI InChI=1S/C34H44N4O/c1-25-18-19-26-20-21-27(34(39)32(26)37-25)24-35-22-12-6-4-2-3-5-7-13-23-36-33-28-14-8-10-16-30(28)38-31-17-11-9-15-29(31)33/h8,10,14,16,18-21,35,39H,2-7,9,11-13,15,17,22-24H2,1H3,(H,36,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method |
Eur J Med Chem 46: 2224-35 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.003
BindingDB Entry DOI: 10.7270/Q2X34XWC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE by Ellman's method |
Eur J Med Chem 45: 6152-8 (2010)
Article DOI: 10.1016/j.ejmech.2010.09.039
BindingDB Entry DOI: 10.7270/Q2KW5G84 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BuChE using butyrylthiocholine as substrate by UV spectroscopic analysis |
Eur J Med Chem 81: 350-8 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.075
BindingDB Entry DOI: 10.7270/Q23F4R6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat... |
Eur J Med Chem 130: 60-72 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.034
BindingDB Entry DOI: 10.7270/Q2JD5086 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in horse serum by Ellman method |
J Med Chem 52: 7249-57 (2009)
Article DOI: 10.1021/jm900628z
BindingDB Entry DOI: 10.7270/Q2668DB1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50322766

(7-{[9-(1,2,3,4-Tetrahydroacridin-9-ylamino)nonylam...)Show SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C32H40N4O/c37-32-25(19-18-24-13-12-22-34-30(24)32)23-33-20-10-4-2-1-3-5-11-21-35-31-26-14-6-8-16-28(26)36-29-17-9-7-15-27(29)31/h6,8,12-14,16,18-19,22,33,37H,1-5,7,9-11,15,17,20-21,23H2,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322766

(7-{[9-(1,2,3,4-Tetrahydroacridin-9-ylamino)nonylam...)Show SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C32H40N4O/c37-32-25(19-18-24-13-12-22-34-30(24)32)23-33-20-10-4-2-1-3-5-11-21-35-31-26-14-6-8-16-28(26)36-29-17-9-7-15-27(29)31/h6,8,12-14,16,18-19,22,33,37H,1-5,7,9-11,15,17,20-21,23H2,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322770
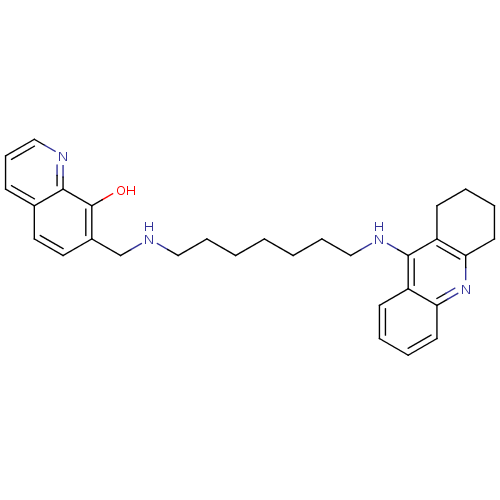
(7-{[7-(1,2,3,4-Tetrahydroacridin-9-ylamino)heptyla...)Show SMILES Oc1c(CNCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C30H36N4O/c35-30-23(17-16-22-11-10-20-32-28(22)30)21-31-18-8-2-1-3-9-19-33-29-24-12-4-6-14-26(24)34-27-15-7-5-13-25(27)29/h4,6,10-12,14,16-17,20,31,35H,1-3,5,7-9,13,15,18-19,21H2,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322782
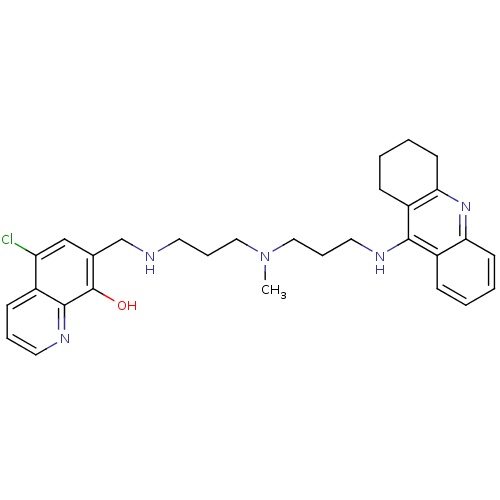
(5-Chloro-7-{{3-{methyl[3-(1,2,3,4-tetrahydroacridi...)Show SMILES CN(CCCNCc1cc(Cl)c2cccnc2c1O)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H36ClN5O/c1-36(17-7-14-32-20-21-19-25(31)22-11-6-15-34-29(22)30(21)37)18-8-16-33-28-23-9-2-4-12-26(23)35-27-13-5-3-10-24(27)28/h2,4,6,9,11-12,15,19,32,37H,3,5,7-8,10,13-14,16-18,20H2,1H3,(H,33,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322771
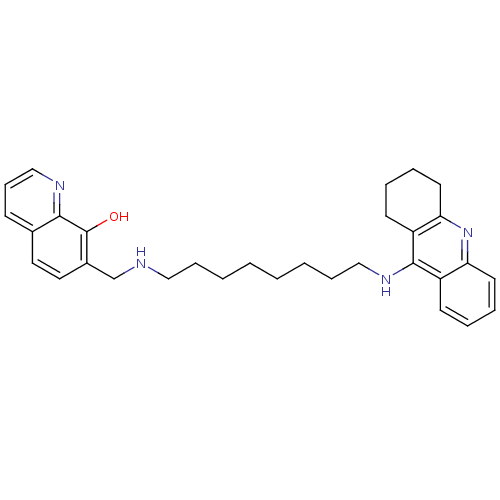
(7-{[8-(1,2,3,4-Tetrahydroacridin-9-ylamino)octylam...)Show SMILES Oc1c(CNCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C31H38N4O/c36-31-24(18-17-23-12-11-21-33-29(23)31)22-32-19-9-3-1-2-4-10-20-34-30-25-13-5-7-15-27(25)35-28-16-8-6-14-26(28)30/h5,7,11-13,15,17-18,21,32,36H,1-4,6,8-10,14,16,19-20,22H2,(H,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's method |
Eur J Med Chem 45: 6152-8 (2010)
Article DOI: 10.1016/j.ejmech.2010.09.039
BindingDB Entry DOI: 10.7270/Q2KW5G84 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE in serum |
J Med Chem 52: 7249-57 (2009)
Article DOI: 10.1021/jm900628z
BindingDB Entry DOI: 10.7270/Q2668DB1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of AChE in bovine erythrocyte by Ellman method |
J Med Chem 52: 7249-57 (2009)
Article DOI: 10.1021/jm900628z
BindingDB Entry DOI: 10.7270/Q2668DB1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of AChE in bovine erythrocytes using acetylthiocholine as substrate by Ellman method |
Eur J Med Chem 46: 2224-35 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.003
BindingDB Entry DOI: 10.7270/Q2X34XWC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50347531
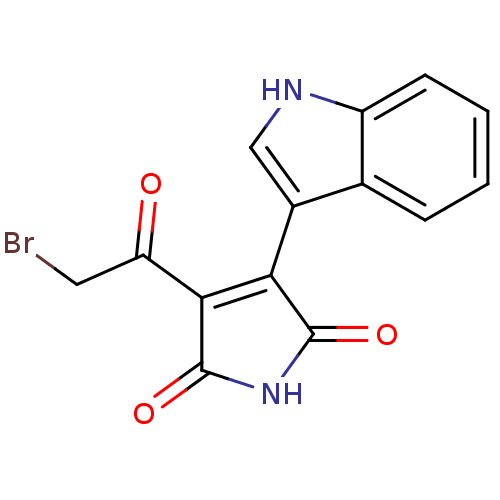
(CHEMBL1801633)Show SMILES BrCC(=O)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:4| Show InChI InChI=1S/C14H9BrN2O3/c15-5-10(18)12-11(13(19)17-14(12)20)8-6-16-9-4-2-1-3-7(8)9/h1-4,6,16H,5H2,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qui£?mica Medica-CSIC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using prephosphorylated polypeptide substrate after 30 mins by Glow-type luminescence assay in presence of 1... |
J Med Chem 54: 4042-56 (2011)
Article DOI: 10.1021/jm1016279
BindingDB Entry DOI: 10.7270/Q29887C1 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM50014884

(CHEMBL3262038)Show SMILES Cc1nc2c(nccn2c1-c1cnn(CC(F)(F)F)c1)N1CCOCC1 Show InChI InChI=1S/C16H17F3N6O/c1-11-13(12-8-21-24(9-12)10-16(17,18)19)25-3-2-20-14(15(25)22-11)23-4-6-26-7-5-23/h2-3,8-9H,4-7,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant PDE10A expressed in baculovirus infected Sf9 cells using [3H]cAMP as substrate after 60 mins by TopCount scintillation ... |
J Med Chem 57: 4196-212 (2014)
Article DOI: 10.1021/jm500073h
BindingDB Entry DOI: 10.7270/Q2X068K6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322772
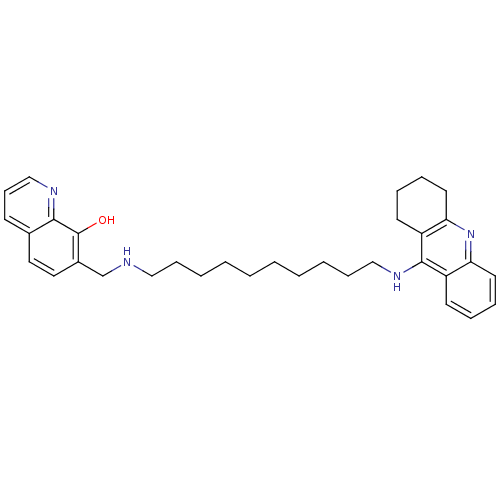
(7-{[10-(1,2,3,4-Tetrahydroacridin-9-ylamino)decyla...)Show SMILES Oc1c(CNCCCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C33H42N4O/c38-33-26(20-19-25-14-13-23-35-31(25)33)24-34-21-11-5-3-1-2-4-6-12-22-36-32-27-15-7-9-17-29(27)37-30-18-10-8-16-28(30)32/h7,9,13-15,17,19-20,23,34,38H,1-6,8,10-12,16,18,21-22,24H2,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50311996
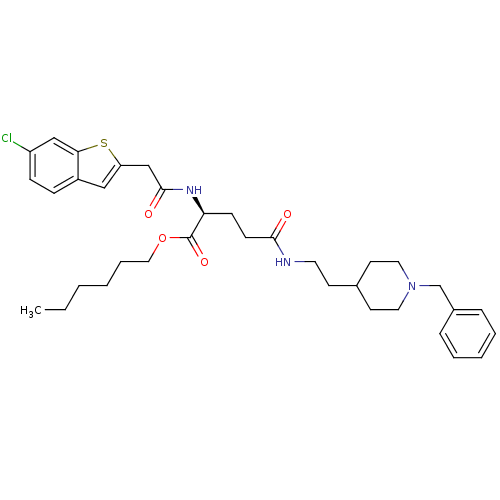
((S)-hexyl 5-(2-(1-benzylpiperidin-4-yl)ethylamino)...)Show SMILES CCCCCCOC(=O)[C@H](CCC(=O)NCCC1CCN(Cc2ccccc2)CC1)NC(=O)Cc1cc2ccc(Cl)cc2s1 |r| Show InChI InChI=1S/C35H46ClN3O4S/c1-2-3-4-8-21-43-35(42)31(38-34(41)24-30-22-28-11-12-29(36)23-32(28)44-30)13-14-33(40)37-18-15-26-16-19-39(20-17-26)25-27-9-6-5-7-10-27/h5-7,9-12,22-23,26,31H,2-4,8,13-21,24-25H2,1H3,(H,37,40)(H,38,41)/t31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in horse serum by Ellman method |
J Med Chem 52: 7249-57 (2009)
Article DOI: 10.1021/jm900628z
BindingDB Entry DOI: 10.7270/Q2668DB1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322774
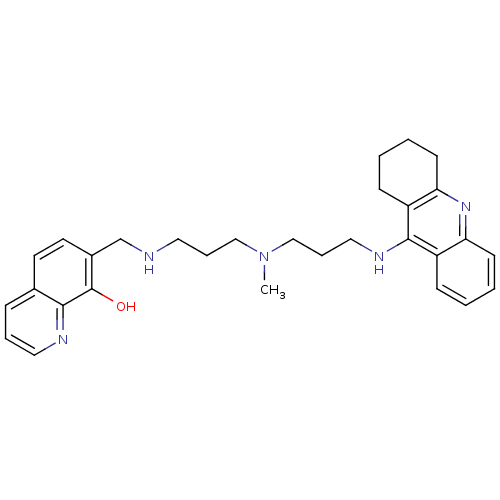
(7-{{3-{Methyl[3-(1,2,3,4-tetrahydroacridin-9-ylami...)Show SMILES CN(CCCNCc1ccc2cccnc2c1O)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H37N5O/c1-35(19-7-16-31-21-23-15-14-22-9-6-17-32-28(22)30(23)36)20-8-18-33-29-24-10-2-4-12-26(24)34-27-13-5-3-11-25(27)29/h2,4,6,9-10,12,14-15,17,31,36H,3,5,7-8,11,13,16,18-21H2,1H3,(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM50014825
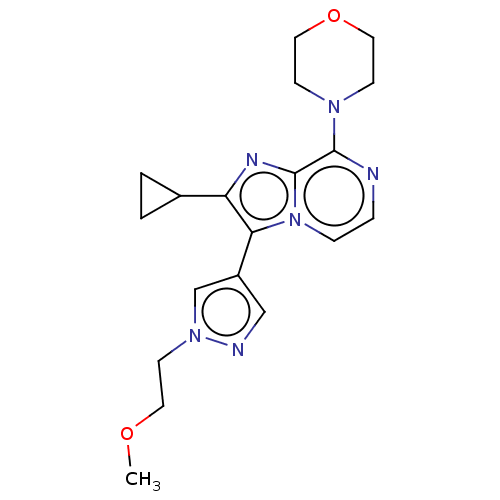
(CHEMBL3262031)Show SMILES COCCn1cc(cn1)-c1c(nc2c(nccn12)N1CCOCC1)C1CC1 Show InChI InChI=1S/C19H24N6O2/c1-26-9-8-24-13-15(12-21-24)17-16(14-2-3-14)22-19-18(20-4-5-25(17)19)23-6-10-27-11-7-23/h4-5,12-14H,2-3,6-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant PDE10A expressed in baculovirus infected Sf9 cells using [3H]cAMP as substrate after 60 mins by TopCount scintillation ... |
J Med Chem 57: 4196-212 (2014)
Article DOI: 10.1021/jm500073h
BindingDB Entry DOI: 10.7270/Q2X068K6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50322768

(5-Chloro-7-{[9-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(Cl)c2cccnc12 Show InChI InChI=1S/C32H39ClN4O/c33-27-21-23(32(38)31-24(27)15-12-20-36-31)22-34-18-10-4-2-1-3-5-11-19-35-30-25-13-6-8-16-28(25)37-29-17-9-7-14-26(29)30/h6,8,12-13,15-16,20-21,34,38H,1-5,7,9-11,14,17-19,22H2,(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM50014905
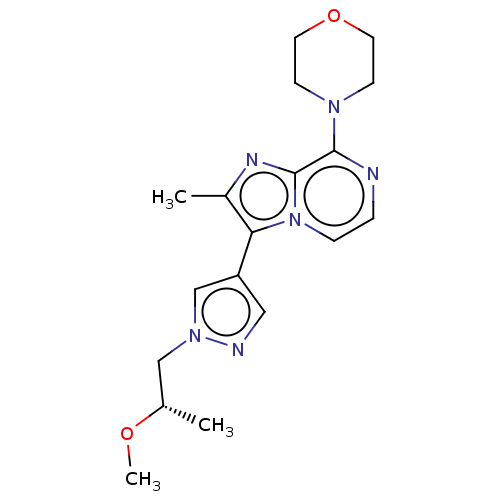
(CHEMBL3262043)Show SMILES CO[C@@H](C)Cn1cc(cn1)-c1c(C)nc2c(nccn12)N1CCOCC1 |r| Show InChI InChI=1S/C18H24N6O2/c1-13(25-3)11-23-12-15(10-20-23)16-14(2)21-18-17(19-4-5-24(16)18)22-6-8-26-9-7-22/h4-5,10,12-13H,6-9,11H2,1-3H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant PDE10A expressed in baculovirus infected Sf9 cells using [3H]cAMP as substrate after 60 mins by TopCount scintillation ... |
J Med Chem 57: 4196-212 (2014)
Article DOI: 10.1021/jm500073h
BindingDB Entry DOI: 10.7270/Q2X068K6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM50322772

(7-{[10-(1,2,3,4-Tetrahydroacridin-9-ylamino)decyla...)Show SMILES Oc1c(CNCCCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C33H42N4O/c38-33-26(20-19-25-14-13-23-35-31(25)33)24-34-21-11-5-3-1-2-4-6-12-22-36-32-27-15-7-9-17-29(27)37-30-18-10-8-16-28(30)32/h7,9,13-15,17,19-20,23,34,38H,1-6,8,10-12,16,18,21-22,24H2,(H,36,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM50014881

(CHEMBL3259842)Show InChI InChI=1S/C18H22N6O/c1-13-16(15-10-20-23(12-15)11-14-2-3-14)24-5-4-19-17(18(24)21-13)22-6-8-25-9-7-22/h4-5,10,12,14H,2-3,6-9,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant PDE10A expressed in baculovirus infected Sf9 cells using [3H]cAMP as substrate after 60 mins by TopCount scintillation ... |
J Med Chem 57: 4196-212 (2014)
Article DOI: 10.1021/jm500073h
BindingDB Entry DOI: 10.7270/Q2X068K6 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM50014906
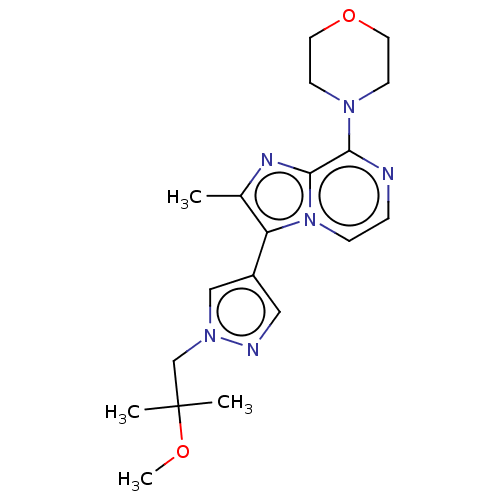
(CHEMBL3262044)Show SMILES COC(C)(C)Cn1cc(cn1)-c1c(C)nc2c(nccn12)N1CCOCC1 Show InChI InChI=1S/C19H26N6O2/c1-14-16(15-11-21-24(12-15)13-19(2,3)26-4)25-6-5-20-17(18(25)22-14)23-7-9-27-10-8-23/h5-6,11-12H,7-10,13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant PDE10A expressed in baculovirus infected Sf9 cells using [3H]cAMP as substrate after 60 mins by TopCount scintillation ... |
J Med Chem 57: 4196-212 (2014)
Article DOI: 10.1021/jm500073h
BindingDB Entry DOI: 10.7270/Q2X068K6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322777

(2-Methyl-7-{[10-(1,2,3,4-tetrahydroacridin-9-ylami...)Show SMILES Cc1ccc2ccc(CNCCCCCCCCCCNc3c4CCCCc4nc4ccccc34)c(O)c2n1 Show InChI InChI=1S/C34H44N4O/c1-25-18-19-26-20-21-27(34(39)32(26)37-25)24-35-22-12-6-4-2-3-5-7-13-23-36-33-28-14-8-10-16-30(28)38-31-17-11-9-15-29(31)33/h8,10,14,16,18-21,35,39H,2-7,9,11-13,15,17,22-24H2,1H3,(H,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50311996

((S)-hexyl 5-(2-(1-benzylpiperidin-4-yl)ethylamino)...)Show SMILES CCCCCCOC(=O)[C@H](CCC(=O)NCCC1CCN(Cc2ccccc2)CC1)NC(=O)Cc1cc2ccc(Cl)cc2s1 |r| Show InChI InChI=1S/C35H46ClN3O4S/c1-2-3-4-8-21-43-35(42)31(38-34(41)24-30-22-28-11-12-29(36)23-32(28)44-30)13-14-33(40)37-18-15-26-16-19-39(20-17-26)25-27-9-6-5-7-10-27/h5-7,9-12,22-23,26,31H,2-4,8,13-21,24-25H2,1H3,(H,37,40)(H,38,41)/t31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE in serum |
J Med Chem 52: 7249-57 (2009)
Article DOI: 10.1021/jm900628z
BindingDB Entry DOI: 10.7270/Q2668DB1 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322767

(2-Methyl-7-{[8-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Cc1ccc2ccc(CNCCCCCCCCNc3c4CCCCc4nc4ccccc34)c(O)c2n1 Show InChI InChI=1S/C32H40N4O/c1-23-16-17-24-18-19-25(32(37)30(24)35-23)22-33-20-10-4-2-3-5-11-21-34-31-26-12-6-8-14-28(26)36-29-15-9-7-13-27(29)31/h6,8,12,14,16-19,33,37H,2-5,7,9-11,13,15,20-22H2,1H3,(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322774

(7-{{3-{Methyl[3-(1,2,3,4-tetrahydroacridin-9-ylami...)Show SMILES CN(CCCNCc1ccc2cccnc2c1O)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H37N5O/c1-35(19-7-16-31-21-23-15-14-22-9-6-17-32-28(22)30(23)36)20-8-18-33-29-24-10-2-4-12-26(24)34-27-13-5-3-11-25(27)29/h2,4,6,9-10,12,14-15,17,31,36H,3,5,7-8,11,13,16,18-21H2,1H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaciones Biol�gicas (CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4D3 assessed after 60 mins by IMAP fluorescence polarization assay |
J Med Chem 57: 8590-607 (2014)
Article DOI: 10.1021/jm501090m
BindingDB Entry DOI: 10.7270/Q2S75HXC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322768

(5-Chloro-7-{[9-(1,2,3,4-tetrahydroacridin-9-ylamin...)Show SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(Cl)c2cccnc12 Show InChI InChI=1S/C32H39ClN4O/c33-27-21-23(32(38)31-24(27)15-12-20-36-31)22-34-18-10-4-2-1-3-5-11-19-35-30-25-13-6-8-16-28(25)37-29-17-9-7-14-26(29)30/h6,8,12-13,15-16,20-21,34,38H,1-5,7,9-11,14,17-19,22H2,(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM50014842

(CHEMBL3262032)Show InChI InChI=1S/C17H19N7O2/c1-25-7-6-23-12-13(11-20-23)15-14(10-18)21-17-16(19-2-3-24(15)17)22-4-8-26-9-5-22/h2-3,11-12H,4-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant PDE10A expressed in baculovirus infected Sf9 cells using [3H]cAMP as substrate after 60 mins by TopCount scintillation ... |
J Med Chem 57: 4196-212 (2014)
Article DOI: 10.1021/jm500073h
BindingDB Entry DOI: 10.7270/Q2X068K6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322771

(7-{[8-(1,2,3,4-Tetrahydroacridin-9-ylamino)octylam...)Show SMILES Oc1c(CNCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc2cccnc12 Show InChI InChI=1S/C31H38N4O/c36-31-24(18-17-23-12-11-21-33-29(23)31)22-32-19-9-3-1-2-4-10-20-34-30-25-13-5-7-15-27(25)35-28-16-8-6-14-26(28)30/h5,7,11-13,15,17-18,21,32,36H,1-4,6,8-10,14,16,19-20,22H2,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50277784

(CHEMBL484928 | N,N,2-trimethyl-5-nitrobenzenesulfo...)Show InChI InChI=1S/C9H12N2O4S/c1-7-4-5-8(11(12)13)6-9(7)16(14,15)10(2)3/h4-6H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaciones Biol�gicas (CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE7A1 assessed as inhibition of hydrolysis of [3H]cAMP after 20 mins by scintillation proximity assay |
J Med Chem 57: 8590-607 (2014)
Article DOI: 10.1021/jm501090m
BindingDB Entry DOI: 10.7270/Q2S75HXC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50322782

(5-Chloro-7-{{3-{methyl[3-(1,2,3,4-tetrahydroacridi...)Show SMILES CN(CCCNCc1cc(Cl)c2cccnc2c1O)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H36ClN5O/c1-36(17-7-14-32-20-21-19-25(31)22-11-6-15-34-29(22)30(21)37)18-8-16-33-28-23-9-2-4-12-26(23)35-27-13-5-3-10-24(27)28/h2,4,6,9,11-12,15,19,32,37H,3,5,7-8,10,13-14,16-18,20H2,1H3,(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocyte AChE by Ellman's reaction |
J Med Chem 53: 4927-37 (2010)
Article DOI: 10.1021/jm100329q
BindingDB Entry DOI: 10.7270/Q22Z15QR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM50059991
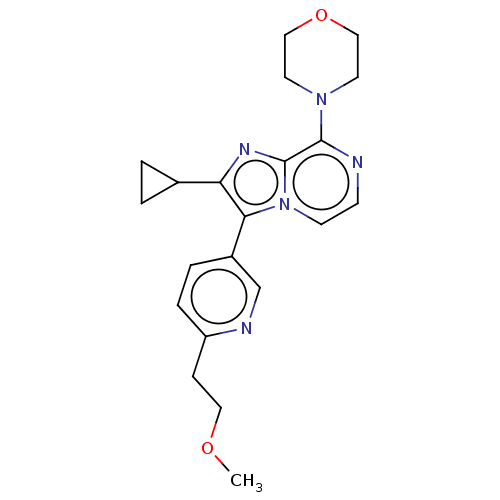
(CHEMBL3394362)Show SMILES COCCc1ccc(cn1)-c1c(nc2c(nccn12)N1CCOCC1)C1CC1 Show InChI InChI=1S/C21H25N5O2/c1-27-11-6-17-5-4-16(14-23-17)19-18(15-2-3-15)24-21-20(22-7-8-26(19)21)25-9-12-28-13-10-25/h4-5,7-8,14-15H,2-3,6,9-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant PDE10A expressed in Sf9 cells using [3H]cAMP substrate incubated for 60 mins by topcount scintillation counting method |
J Med Chem 58: 978-93 (2015)
Article DOI: 10.1021/jm501651a
BindingDB Entry DOI: 10.7270/Q2CV4KDK |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaciones Biol�gicas (CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B2 assessed after 60 mins by IMAP fluorescence polarization assay |
J Med Chem 57: 8590-607 (2014)
Article DOI: 10.1021/jm501090m
BindingDB Entry DOI: 10.7270/Q2S75HXC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data