 Found 11659 hits with Last Name = 'hegde' and Initial = 's'
Found 11659 hits with Last Name = 'hegde' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM82505
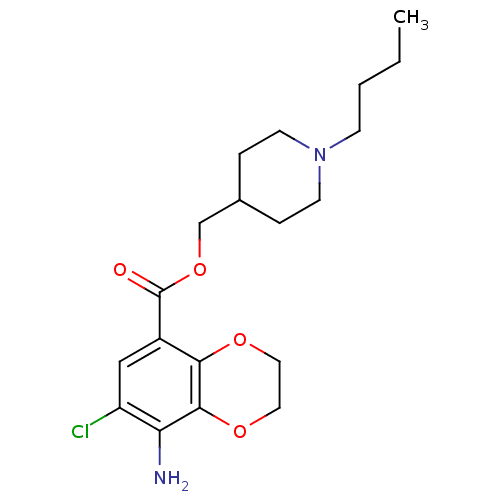
(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
FASEB J 10: 1398-407 (1996)
Article DOI: 10.1096/fasebj.10.12.8903510
BindingDB Entry DOI: 10.7270/Q2TQ602G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM85020
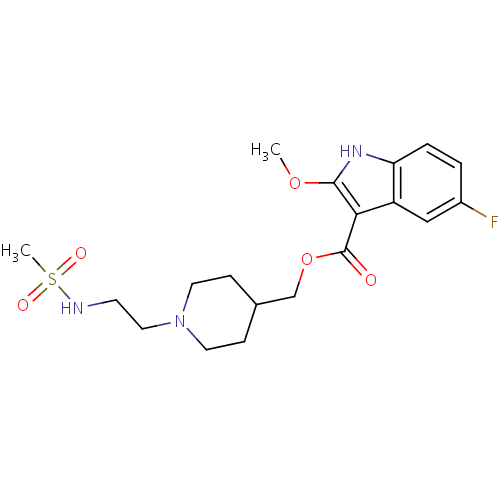
(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
FASEB J 10: 1398-407 (1996)
Article DOI: 10.1096/fasebj.10.12.8903510
BindingDB Entry DOI: 10.7270/Q2TQ602G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084436
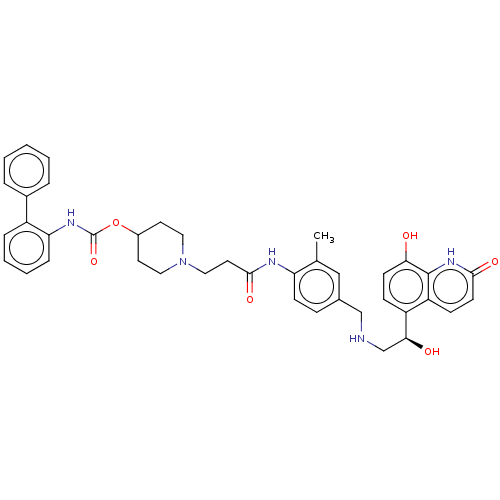
(CHEMBL3426693)Show SMILES Cc1cc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)ccc1NC(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C40H43N5O6/c1-26-23-27(24-41-25-36(47)31-12-15-35(46)39-32(31)13-16-37(48)44-39)11-14-33(26)42-38(49)19-22-45-20-17-29(18-21-45)51-40(50)43-34-10-6-5-9-30(34)28-7-3-2-4-8-28/h2-16,23,29,36,41,46-47H,17-22,24-25H2,1H3,(H,42,49)(H,43,50)(H,44,48)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
FASEB J 10: 1398-407 (1996)
Article DOI: 10.1096/fasebj.10.12.8903510
BindingDB Entry DOI: 10.7270/Q2TQ602G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084443
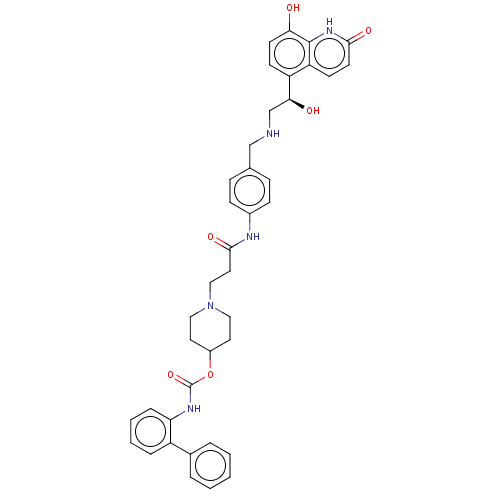
(CHEMBL3426687)Show SMILES O[C@@H](CNCc1ccc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C39H41N5O6/c45-34-16-14-31(32-15-17-36(47)43-38(32)34)35(46)25-40-24-26-10-12-28(13-11-26)41-37(48)20-23-44-21-18-29(19-22-44)50-39(49)42-33-9-5-4-8-30(33)27-6-2-1-3-7-27/h1-17,29,35,40,45-46H,18-25H2,(H,41,48)(H,42,49)(H,43,47)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
FASEB J 10: 1398-407 (1996)
Article DOI: 10.1096/fasebj.10.12.8903510
BindingDB Entry DOI: 10.7270/Q2TQ602G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084432
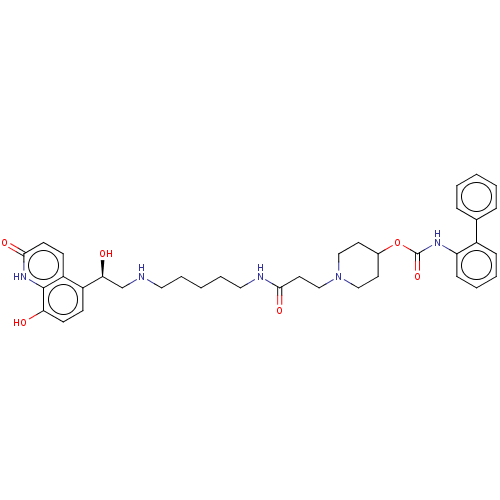
(CHEMBL3426697)Show SMILES O[C@@H](CNCCCCCNC(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C37H45N5O6/c43-32-15-13-29(30-14-16-35(46)41-36(30)32)33(44)25-38-20-7-2-8-21-39-34(45)19-24-42-22-17-27(18-23-42)48-37(47)40-31-12-6-5-11-28(31)26-9-3-1-4-10-26/h1,3-6,9-16,27,33,38,43-44H,2,7-8,17-25H2,(H,39,45)(H,40,47)(H,41,46)/t33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 by dansylamide (DNSA) competition assay |
Bioorg Med Chem Lett 23: 939-43 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.058
BindingDB Entry DOI: 10.7270/Q2VM4G54 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50337878
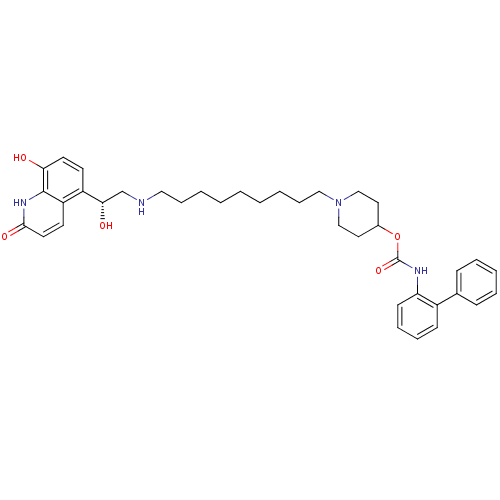
((R)-1-(9-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro...)Show SMILES O[C@@H](CNCCCCCCCCCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C38H48N4O5/c43-34-19-17-31(32-18-20-36(45)41-37(32)34)35(44)27-39-23-11-4-2-1-3-5-12-24-42-25-21-29(22-26-42)47-38(46)40-33-16-10-9-15-30(33)28-13-7-6-8-14-28/h6-10,13-20,29,35,39,43-44H,1-5,11-12,21-27H2,(H,40,46)(H,41,45)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084439
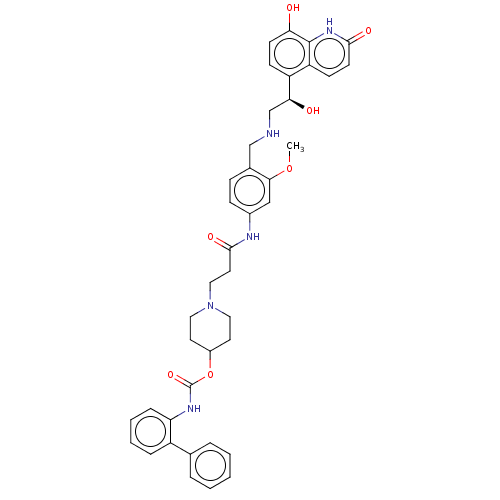
(CHEMBL3426691)Show SMILES COc1cc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)ccc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C40H43N5O7/c1-51-36-23-28(12-11-27(36)24-41-25-35(47)31-13-15-34(46)39-32(31)14-16-37(48)44-39)42-38(49)19-22-45-20-17-29(18-21-45)52-40(50)43-33-10-6-5-9-30(33)26-7-3-2-4-8-26/h2-16,23,29,35,41,46-47H,17-22,24-25H2,1H3,(H,42,49)(H,43,50)(H,44,48)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084442
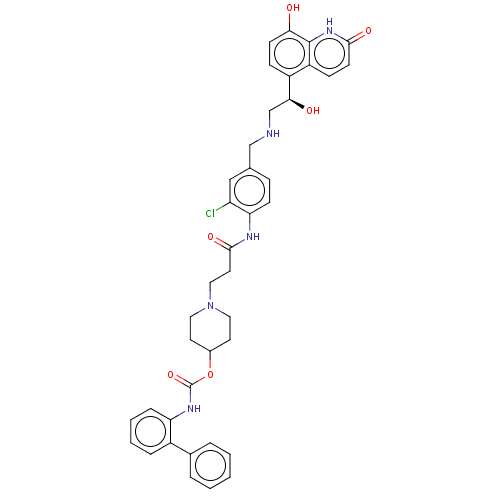
(CHEMBL3426688)Show SMILES O[C@@H](CNCc1ccc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c(Cl)c1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C39H40ClN5O6/c40-31-22-25(23-41-24-35(47)29-11-14-34(46)38-30(29)12-15-36(48)44-38)10-13-33(31)42-37(49)18-21-45-19-16-27(17-20-45)51-39(50)43-32-9-5-4-8-28(32)26-6-2-1-3-7-26/h1-15,22,27,35,41,46-47H,16-21,23-24H2,(H,42,49)(H,43,50)(H,44,48)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084440
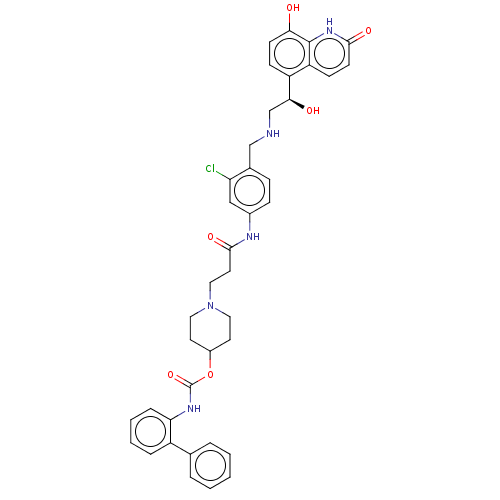
(CHEMBL3426690)Show SMILES O[C@@H](CNCc1ccc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)cc1Cl)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C39H40ClN5O6/c40-32-22-27(11-10-26(32)23-41-24-35(47)30-12-14-34(46)38-31(30)13-15-36(48)44-38)42-37(49)18-21-45-19-16-28(17-20-45)51-39(50)43-33-9-5-4-8-29(33)25-6-2-1-3-7-25/h1-15,22,28,35,41,46-47H,16-21,23-24H2,(H,42,49)(H,43,50)(H,44,48)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084424
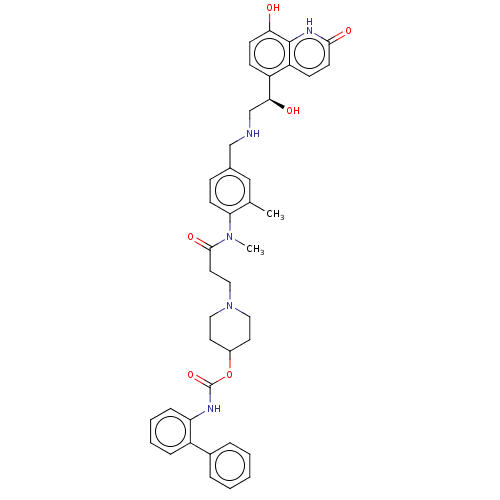
(CHEMBL3426705)Show SMILES CN(C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1ccc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1C |r| Show InChI InChI=1S/C41H45N5O6/c1-27-24-28(25-42-26-37(48)32-13-16-36(47)40-33(32)14-17-38(49)44-40)12-15-35(27)45(2)39(50)20-23-46-21-18-30(19-22-46)52-41(51)43-34-11-7-6-10-31(34)29-8-4-3-5-9-29/h3-17,24,30,37,42,47-48H,18-23,25-26H2,1-2H3,(H,43,51)(H,44,49)/t37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 by dansylamide (DNSA) competition assay |
Bioorg Med Chem Lett 23: 939-43 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.058
BindingDB Entry DOI: 10.7270/Q2VM4G54 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084433
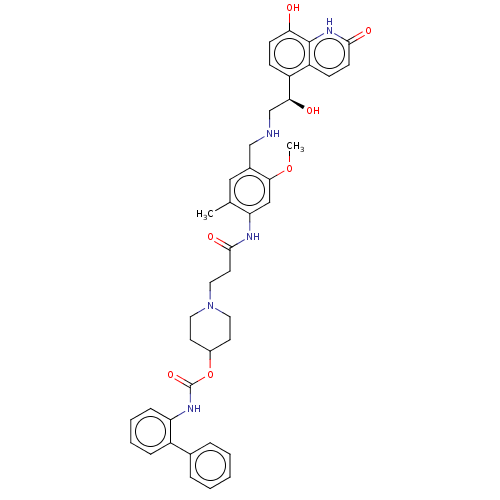
(CHEMBL3426696)Show SMILES COc1cc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c(C)cc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C41H45N5O7/c1-26-22-28(24-42-25-36(48)31-12-14-35(47)40-32(31)13-15-38(49)45-40)37(52-2)23-34(26)43-39(50)18-21-46-19-16-29(17-20-46)53-41(51)44-33-11-7-6-10-30(33)27-8-4-3-5-9-27/h3-15,22-23,29,36,42,47-48H,16-21,24-25H2,1-2H3,(H,43,50)(H,44,51)(H,45,49)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084426
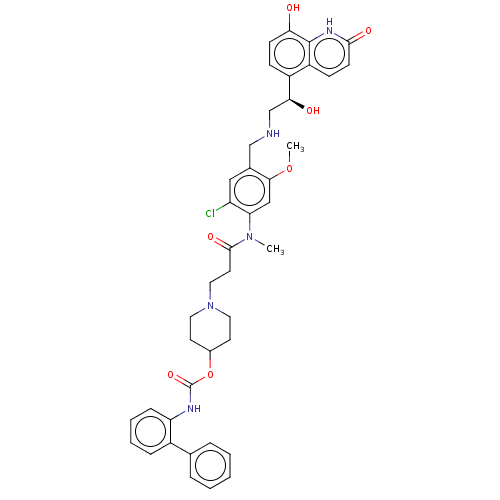
(CHEMBL3426703)Show SMILES COc1cc(N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c(Cl)cc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C41H44ClN5O7/c1-46(34-23-37(53-2)27(22-32(34)42)24-43-25-36(49)30-12-14-35(48)40-31(30)13-15-38(50)45-40)39(51)18-21-47-19-16-28(17-20-47)54-41(52)44-33-11-7-6-10-29(33)26-8-4-3-5-9-26/h3-15,22-23,28,36,43,48-49H,16-21,24-25H2,1-2H3,(H,44,52)(H,45,50)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084431
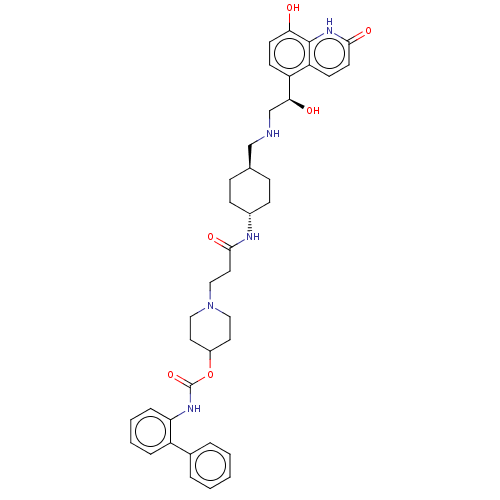
(CHEMBL3426698)Show SMILES O[C@@H](CNC[C@H]1CC[C@@H](CC1)NC(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:5.4,wD:1.0,8.11,(.27,-3.7,;1.33,-3.08,;2.67,-3.85,;2.67,-5.39,;4.01,-6.16,;4.01,-7.7,;5.35,-8.47,;5.35,-10.01,;4.01,-10.78,;2.68,-10.01,;2.68,-8.47,;4.01,-12.32,;5.35,-13.09,;6.42,-12.47,;5.35,-14.63,;6.69,-15.4,;6.69,-16.94,;8.03,-17.71,;8.03,-19.25,;6.69,-20.02,;5.36,-19.25,;5.36,-17.71,;6.69,-21.56,;5.35,-22.33,;4.29,-21.71,;5.35,-23.87,;4.02,-24.64,;2.68,-23.86,;1.35,-24.63,;1.34,-26.17,;2.67,-26.94,;4.01,-26.18,;5.34,-26.95,;6.68,-26.19,;8.01,-26.96,;8,-28.5,;6.66,-29.27,;5.33,-28.49,;1.33,-1.54,;2.66,-.77,;2.66,.77,;1.33,1.54,;1.33,2.77,;,.77,;-1.33,1.54,;-2.68,.77,;-3.75,1.39,;-2.68,-.77,;-1.33,-1.54,;,-.77,)| Show InChI InChI=1S/C39H47N5O6/c45-34-16-14-31(32-15-17-36(47)43-38(32)34)35(46)25-40-24-26-10-12-28(13-11-26)41-37(48)20-23-44-21-18-29(19-22-44)50-39(49)42-33-9-5-4-8-30(33)27-6-2-1-3-7-27/h1-9,14-17,26,28-29,35,40,45-46H,10-13,18-25H2,(H,41,48)(H,42,49)(H,43,47)/t26-,28-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM85026
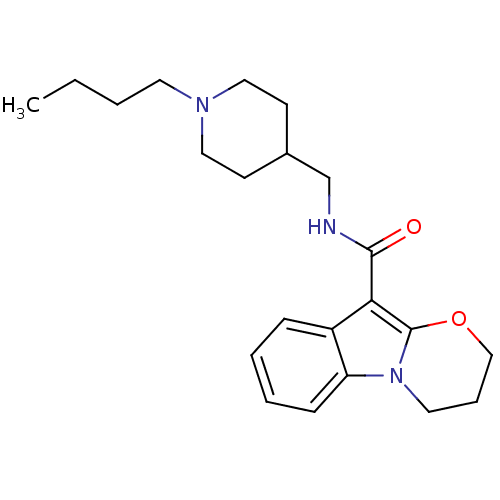
(N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...)Show InChI InChI=1S/C22H31N3O2/c1-2-3-11-24-13-9-17(10-14-24)16-23-21(26)20-18-7-4-5-8-19(18)25-12-6-15-27-22(20)25/h4-5,7-8,17H,2-3,6,9-16H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
FASEB J 10: 1398-407 (1996)
Article DOI: 10.1096/fasebj.10.12.8903510
BindingDB Entry DOI: 10.7270/Q2TQ602G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084434
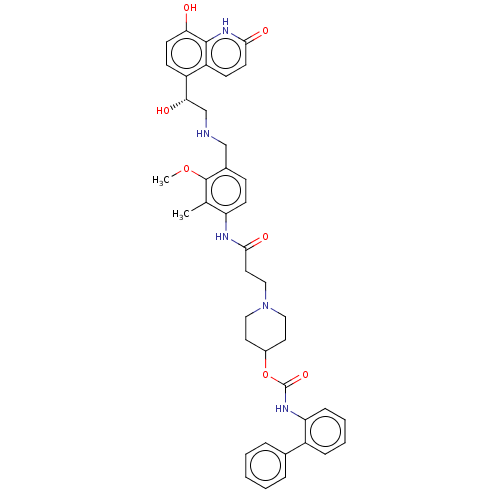
(CHEMBL3426695)Show SMILES COc1c(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)ccc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c1C |r| Show InChI InChI=1S/C41H45N5O7/c1-26-33(15-12-28(40(26)52-2)24-42-25-36(48)31-13-16-35(47)39-32(31)14-17-37(49)45-39)43-38(50)20-23-46-21-18-29(19-22-46)53-41(51)44-34-11-7-6-10-30(34)27-8-4-3-5-9-27/h3-17,29,36,42,47-48H,18-25H2,1-2H3,(H,43,50)(H,44,51)(H,45,49)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Imidazoleglycerol-phosphate dehydratase
(Cryptococcus neoformans) | BDBM50079743
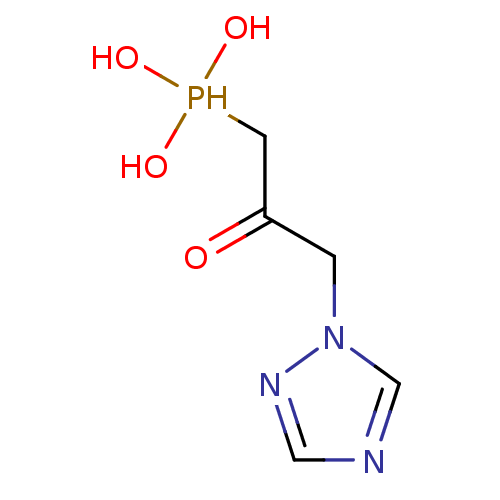
((2-Hydroxy-3-[1,2,4]triazol-1-yl-propyl)-phosphoni...)Show InChI InChI=1S/C5H10N3O4P/c9-5(2-13(10,11)12)1-8-4-6-3-7-8/h3-4,10-13H,1-2H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monsanto Company
Curated by ChEMBL
| Assay Description
Binding affinity imidazole glycerol phosphate dehydratase (IGPD) obtained from Cryptococcus neoformans |
Bioorg Med Chem Lett 9: 2053-8 (1999)
BindingDB Entry DOI: 10.7270/Q29P30T6 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084425
(CHEMBL3426704 | US9394275, I-25)Show SMILES COc1cc(ccc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12)N(C)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C41H45N5O7/c1-45(29-13-12-28(37(24-29)52-2)25-42-26-36(48)32-14-16-35(47)40-33(32)15-17-38(49)44-40)39(50)20-23-46-21-18-30(19-22-46)53-41(51)43-34-11-7-6-10-31(34)27-8-4-3-5-9-27/h3-17,24,30,36,42,47-48H,18-23,25-26H2,1-2H3,(H,43,51)(H,44,49)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084430

(CHEMBL3426699)Show SMILES O[C@@H](CNCc1ccc(cc1)C(=O)NCCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C39H41N5O6/c45-34-16-14-31(32-15-17-36(47)43-37(32)34)35(46)25-40-24-26-10-12-28(13-11-26)38(48)41-20-23-44-21-18-29(19-22-44)50-39(49)42-33-9-5-4-8-30(33)27-6-2-1-3-7-27/h1-17,29,35,40,45-46H,18-25H2,(H,41,48)(H,42,49)(H,43,47)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50490460

(CHEMBL2325977)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(=O)Nc1nnc(s1)S(N)(=O)=O |r| Show InChI InChI=1S/C22H36N4O6S2/c1-2-3-6-9-15(27)12-13-17-16(18(28)14-19(17)29)10-7-4-5-8-11-20(30)24-21-25-26-22(33-21)34(23,31)32/h4,7,12-13,15-19,27-29H,2-3,5-6,8-11,14H2,1H3,(H2,23,31,32)(H,24,25,30)/b7-4-,13-12+/t15-,16+,17+,18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 by dansylamide (DNSA) competition assay |
Bioorg Med Chem Lett 23: 939-43 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.058
BindingDB Entry DOI: 10.7270/Q2VM4G54 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084438
(CHEMBL3426692)Show SMILES COc1c(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)ccc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c1Cl |r| Show InChI InChI=1S/C40H42ClN5O7/c1-52-39-26(23-42-24-34(48)29-12-15-33(47)38-30(29)13-16-35(49)45-38)11-14-32(37(39)41)43-36(50)19-22-46-20-17-27(18-21-46)53-40(51)44-31-10-6-5-9-28(31)25-7-3-2-4-8-25/h2-16,27,34,42,47-48H,17-24H2,1H3,(H,43,50)(H,44,51)(H,45,49)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM82273

(CAS_167710-87-4 | RS 39604)Show SMILES COc1cc(COc2cc(N)c(Cl)cc2C(=O)CCC2CCN(CCNS(C)(=O)=O)CC2)cc(OC)c1 Show InChI InChI=1S/C26H36ClN3O6S/c1-34-20-12-19(13-21(14-20)35-2)17-36-26-16-24(28)23(27)15-22(26)25(31)5-4-18-6-9-30(10-7-18)11-8-29-37(3,32)33/h12-16,18,29H,4-11,17,28H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
FASEB J 10: 1398-407 (1996)
Article DOI: 10.1096/fasebj.10.12.8903510
BindingDB Entry DOI: 10.7270/Q2TQ602G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM82273

(CAS_167710-87-4 | RS 39604)Show SMILES COc1cc(COc2cc(N)c(Cl)cc2C(=O)CCC2CCN(CCNS(C)(=O)=O)CC2)cc(OC)c1 Show InChI InChI=1S/C26H36ClN3O6S/c1-34-20-12-19(13-21(14-20)35-2)17-36-26-16-24(28)23(27)15-22(26)25(31)5-4-18-6-9-30(10-7-18)11-8-29-37(3,32)33/h12-16,18,29H,4-11,17,28H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 1087-95 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb15922.x
BindingDB Entry DOI: 10.7270/Q2707ZX4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084427
(CHEMBL3426702)Show SMILES CN(CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)C(=O)c1ccc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1 |r| Show InChI InChI=1S/C40H43N5O6/c1-44(23-24-45-21-19-30(20-22-45)51-40(50)42-34-10-6-5-9-31(34)28-7-3-2-4-8-28)39(49)29-13-11-27(12-14-29)25-41-26-36(47)32-15-17-35(46)38-33(32)16-18-37(48)43-38/h2-18,30,36,41,46-47H,19-26H2,1H3,(H,42,50)(H,43,48)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50503902

(CHEMBL4588688)Show SMILES CCOc1nc(CC)c(CNC(=O)[C@@H](CS)CC(C)C)n1Cc1ccc(cc1F)-c1ccccc1C(O)=O |r| Show InChI InChI=1S/C29H36FN3O4S/c1-5-25-26(15-31-27(34)21(17-38)13-18(3)4)33(29(32-25)37-6-2)16-20-12-11-19(14-24(20)30)22-9-7-8-10-23(22)28(35)36/h7-12,14,18,21,38H,5-6,13,15-17H2,1-4H3,(H,31,34)(H,35,36)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US Inc.
Curated by ChEMBL
| Assay Description
Displacement of Europium-labeled angiotensin-2 from human AT1 receptor expressed in CHOK1 cell membranes after 120 mins by DELFIA |
ACS Med Chem Lett 10: 86-91 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00462
BindingDB Entry DOI: 10.7270/Q2D50R6V |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084423
(CHEMBL3426706)Show SMILES O[C@@H](CNCCc1cccc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C40H43N5O6/c46-35-15-13-32(33-14-16-37(48)44-39(33)35)36(47)26-41-21-17-27-7-6-10-29(25-27)42-38(49)20-24-45-22-18-30(19-23-45)51-40(50)43-34-12-5-4-11-31(34)28-8-2-1-3-9-28/h1-16,25,30,36,41,46-47H,17-24,26H2,(H,42,49)(H,43,50)(H,44,48)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50503896
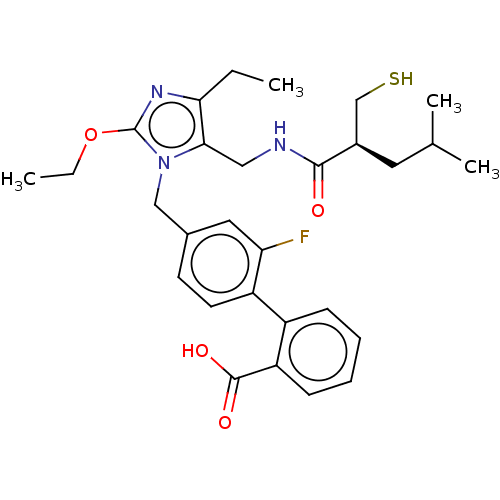
(CHEMBL4445778)Show SMILES CCOc1nc(CC)c(CNC(=O)[C@@H](CS)CC(C)C)n1Cc1ccc(c(F)c1)-c1ccccc1C(O)=O |r| Show InChI InChI=1S/C29H36FN3O4S/c1-5-25-26(15-31-27(34)20(17-38)13-18(3)4)33(29(32-25)37-6-2)16-19-11-12-22(24(30)14-19)21-9-7-8-10-23(21)28(35)36/h7-12,14,18,20,38H,5-6,13,15-17H2,1-4H3,(H,31,34)(H,35,36)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US Inc.
Curated by ChEMBL
| Assay Description
Displacement of Europium-labeled angiotensin-2 from human AT1 receptor expressed in CHOK1 cell membranes after 120 mins by DELFIA |
ACS Med Chem Lett 10: 86-91 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00462
BindingDB Entry DOI: 10.7270/Q2D50R6V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50503894

(CHEMBL4591218)Show SMILES CCOc1nc(CC)c(CNC(=O)[C@@H](CS)CC(C)C)n1Cc1ccc(cc1)-c1ccccc1C(O)=O |r| Show InChI InChI=1S/C29H37N3O4S/c1-5-25-26(16-30-27(33)22(18-37)15-19(3)4)32(29(31-25)36-6-2)17-20-11-13-21(14-12-20)23-9-7-8-10-24(23)28(34)35/h7-14,19,22,37H,5-6,15-18H2,1-4H3,(H,30,33)(H,34,35)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US Inc.
Curated by ChEMBL
| Assay Description
Displacement of Europium-labeled angiotensin-2 from human AT1 receptor expressed in CHOK1 cell membranes after 120 mins by DELFIA |
ACS Med Chem Lett 10: 86-91 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00462
BindingDB Entry DOI: 10.7270/Q2D50R6V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50503875
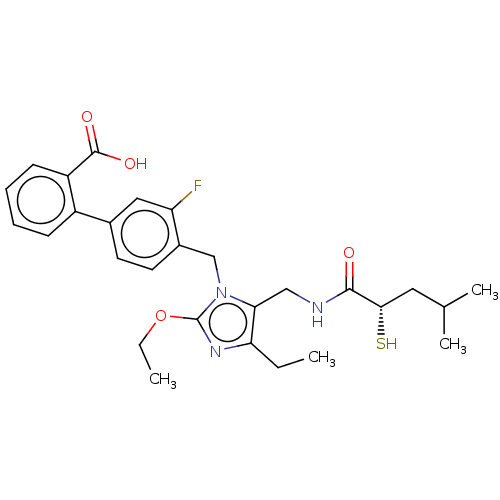
(CHEMBL4435800)Show SMILES CCOc1nc(CC)c(CNC(=O)[C@@H](S)CC(C)C)n1Cc1ccc(cc1F)-c1ccccc1C(O)=O |r| Show InChI InChI=1S/C28H34FN3O4S/c1-5-23-24(15-30-26(33)25(37)13-17(3)4)32(28(31-23)36-6-2)16-19-12-11-18(14-22(19)29)20-9-7-8-10-21(20)27(34)35/h7-12,14,17,25,37H,5-6,13,15-16H2,1-4H3,(H,30,33)(H,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US Inc.
Curated by ChEMBL
| Assay Description
Displacement of Europium-labeled angiotensin-2 from human AT1 receptor expressed in CHOK1 cell membranes after 120 mins by DELFIA |
ACS Med Chem Lett 10: 86-91 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00462
BindingDB Entry DOI: 10.7270/Q2D50R6V |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084435
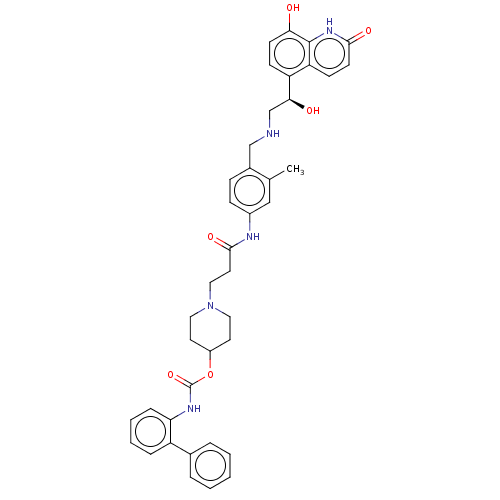
(CHEMBL3426694)Show SMILES Cc1cc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)ccc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C40H43N5O6/c1-26-23-29(12-11-28(26)24-41-25-36(47)32-13-15-35(46)39-33(32)14-16-37(48)44-39)42-38(49)19-22-45-20-17-30(18-21-45)51-40(50)43-34-10-6-5-9-31(34)27-7-3-2-4-8-27/h2-16,23,30,36,41,46-47H,17-22,24-25H2,1H3,(H,42,49)(H,43,50)(H,44,48)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50490454

(CHEMBL2325981)Show SMILES CC(C)OC(=O)CCC\C=C/C[C@H]1[C@@H](O)C[C@@H](O)[C@@H]1\C=C\C(=O)Nc1nnc(s1)S(N)(=O)=O |r| Show InChI InChI=1S/C20H30N4O7S2/c1-12(2)31-18(28)8-6-4-3-5-7-13-14(16(26)11-15(13)25)9-10-17(27)22-19-23-24-20(32-19)33(21,29)30/h3,5,9-10,12-16,25-26H,4,6-8,11H2,1-2H3,(H2,21,29,30)(H,22,23,27)/b5-3-,10-9+/t13-,14-,15+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 by dansylamide (DNSA) competition assay |
Bioorg Med Chem Lett 23: 939-43 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.058
BindingDB Entry DOI: 10.7270/Q2VM4G54 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084441
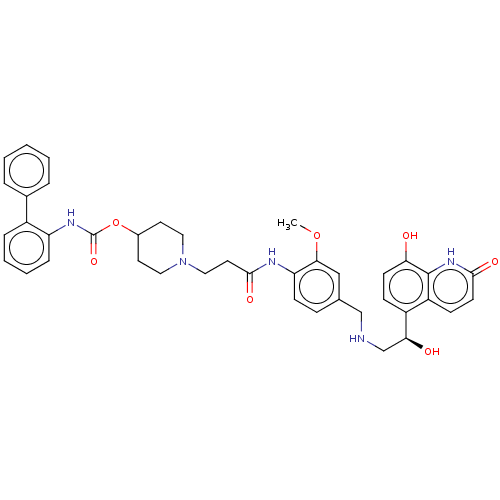
(CHEMBL3426689)Show SMILES COc1cc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)ccc1NC(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C40H43N5O7/c1-51-36-23-26(24-41-25-35(47)30-12-15-34(46)39-31(30)13-16-37(48)44-39)11-14-33(36)42-38(49)19-22-45-20-17-28(18-21-45)52-40(50)43-32-10-6-5-9-29(32)27-7-3-2-4-8-27/h2-16,23,28,35,41,46-47H,17-22,24-25H2,1H3,(H,42,49)(H,43,50)(H,44,48)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM85028

(1-[4-Amino-5-chloro-2-(3,5-dimethoxybenzyloxy)phen...)Show SMILES COc1cc(COc2cc(N)c(Cl)cc2C(=O)CCCCN2CCCCC2)cc(OC)c1 Show InChI InChI=1S/C25H33ClN2O4/c1-30-19-12-18(13-20(14-19)31-2)17-32-25-16-23(27)22(26)15-21(25)24(29)8-4-7-11-28-9-5-3-6-10-28/h12-16H,3-11,17,27H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
FASEB J 10: 1398-407 (1996)
Article DOI: 10.1096/fasebj.10.12.8903510
BindingDB Entry DOI: 10.7270/Q2TQ602G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084421

(CHEMBL3426708)Show SMILES COc1ccc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)cc1CCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C41H45N5O7/c1-52-37-15-11-29(25-28(37)17-21-42-26-36(48)32-12-14-35(47)40-33(32)13-16-38(49)45-40)43-39(50)20-24-46-22-18-30(19-23-46)53-41(51)44-34-10-6-5-9-31(34)27-7-3-2-4-8-27/h2-16,25,30,36,42,47-48H,17-24,26H2,1H3,(H,43,50)(H,44,51)(H,45,49)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084437
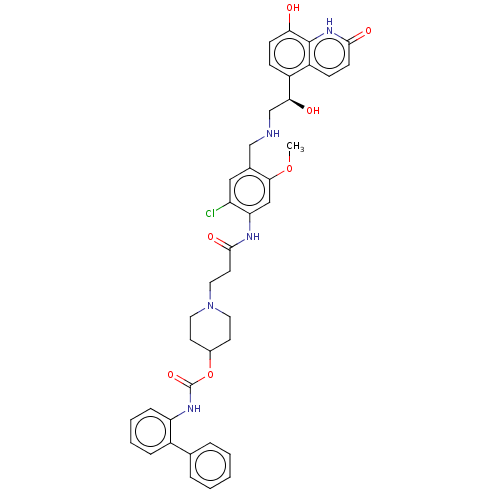
(Batefenterol | GSK961081 | GSK961081A | TD-5959)Show SMILES COc1cc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c(Cl)cc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C40H42ClN5O7/c1-52-36-22-33(31(41)21-26(36)23-42-24-35(48)29-11-13-34(47)39-30(29)12-14-37(49)45-39)43-38(50)17-20-46-18-15-27(16-19-46)53-40(51)44-32-10-6-5-9-28(32)25-7-3-2-4-8-25/h2-14,21-22,27,35,42,47-48H,15-20,23-24H2,1H3,(H,43,50)(H,44,51)(H,45,49)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50503885

(CHEMBL4438878)Show SMILES CCCOc1nc(CC)c(CNC(=O)[C@@H](CS)CC(C)C)n1Cc1ccc(cc1)-c1ccccc1C(O)=O |r| Show InChI InChI=1S/C30H39N3O4S/c1-5-15-37-30-32-26(6-2)27(17-31-28(34)23(19-38)16-20(3)4)33(30)18-21-11-13-22(14-12-21)24-9-7-8-10-25(24)29(35)36/h7-14,20,23,38H,5-6,15-19H2,1-4H3,(H,31,34)(H,35,36)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US Inc.
Curated by ChEMBL
| Assay Description
Displacement of Europium-labeled angiotensin-2 from human AT1 receptor expressed in CHOK1 cell membranes after 120 mins by DELFIA |
ACS Med Chem Lett 10: 86-91 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00462
BindingDB Entry DOI: 10.7270/Q2D50R6V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50056401
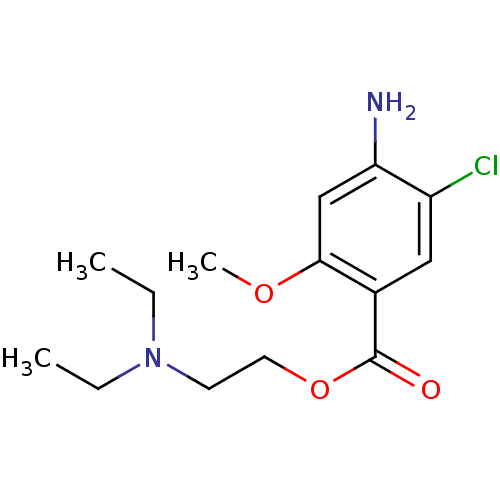
(2-diethylaminoethyl [2-methoxy-4-amino-5-chloro]be...)Show InChI InChI=1S/C14H21ClN2O3/c1-4-17(5-2)6-7-20-14(18)10-8-11(15)12(16)9-13(10)19-3/h8-9H,4-7,16H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
FASEB J 10: 1398-407 (1996)
Article DOI: 10.1096/fasebj.10.12.8903510
BindingDB Entry DOI: 10.7270/Q2TQ602G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084428
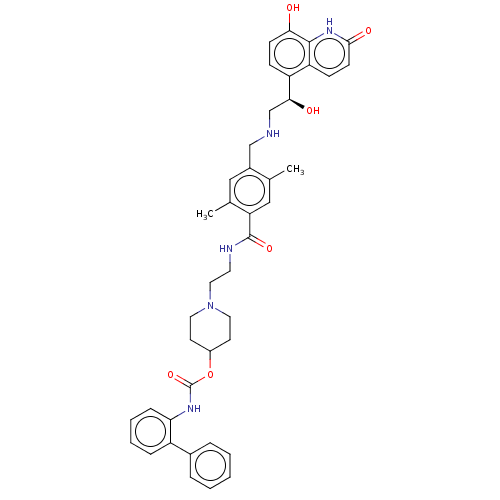
(CHEMBL3426701)Show SMILES Cc1cc(C(=O)NCCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c(C)cc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C41H45N5O6/c1-26-23-34(27(2)22-29(26)24-42-25-37(48)32-12-14-36(47)39-33(32)13-15-38(49)45-39)40(50)43-18-21-46-19-16-30(17-20-46)52-41(51)44-35-11-7-6-10-31(35)28-8-4-3-5-9-28/h3-15,22-23,30,37,42,47-48H,16-21,24-25H2,1-2H3,(H,43,50)(H,44,51)(H,45,49)/t37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50056415
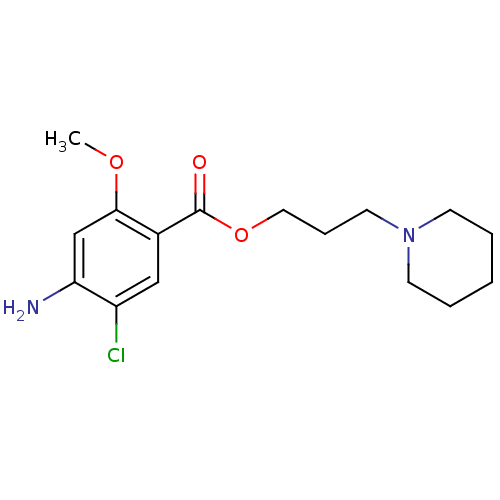
(2-piperidinopropyl 4-amino-5-chloro-2-methoxybenzo...)Show InChI InChI=1S/C16H23ClN2O3/c1-21-15-11-14(18)13(17)10-12(15)16(20)22-9-5-8-19-6-3-2-4-7-19/h10-11H,2-9,18H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
FASEB J 10: 1398-407 (1996)
Article DOI: 10.1096/fasebj.10.12.8903510
BindingDB Entry DOI: 10.7270/Q2TQ602G |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US Inc.
Curated by ChEMBL
| Assay Description
Displacement of Europium-labeled angiotensin-2 from human AT1 receptor expressed in CHOK1 cell membranes after 120 mins by DELFIA |
ACS Med Chem Lett 10: 86-91 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00462
BindingDB Entry DOI: 10.7270/Q2D50R6V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50503887
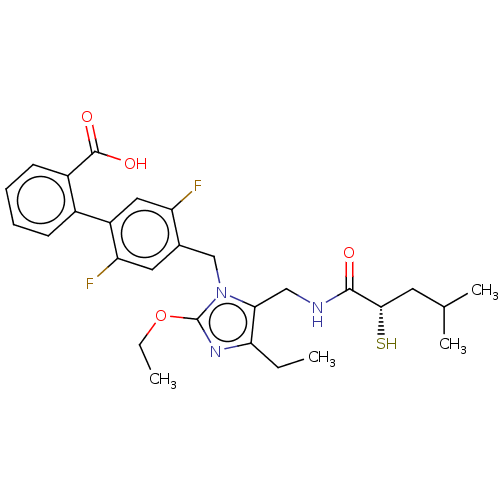
(CHEMBL4543532)Show SMILES CCOc1nc(CC)c(CNC(=O)[C@@H](S)CC(C)C)n1Cc1cc(F)c(cc1F)-c1ccccc1C(O)=O |r| Show InChI InChI=1S/C28H33F2N3O4S/c1-5-23-24(14-31-26(34)25(38)11-16(3)4)33(28(32-23)37-6-2)15-17-12-22(30)20(13-21(17)29)18-9-7-8-10-19(18)27(35)36/h7-10,12-13,16,25,38H,5-6,11,14-15H2,1-4H3,(H,31,34)(H,35,36)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US Inc.
Curated by ChEMBL
| Assay Description
Displacement of Europium-labeled angiotensin-2 from human AT1 receptor expressed in CHOK1 cell membranes after 120 mins by DELFIA |
ACS Med Chem Lett 10: 86-91 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00462
BindingDB Entry DOI: 10.7270/Q2D50R6V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50490462

(CHEMBL2325976)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(=O)N(CC)[C@H]1CN(CCCOC)S(=O)(=O)C2SC(=CC12)S(N)(=O)=O |r,c:41| Show InChI InChI=1S/C32H55N3O9S3/c1-4-6-9-13-23(36)16-17-25-24(28(37)21-29(25)38)14-10-7-8-11-15-30(39)35(5-2)27-22-34(18-12-19-44-3)47(42,43)32-26(27)20-31(45-32)46(33,40)41/h7,10,16-17,20,23-29,32,36-38H,4-6,8-9,11-15,18-19,21-22H2,1-3H3,(H2,33,40,41)/b10-7-,17-16+/t23-,24+,25+,26?,27-,28-,29+,32?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 by dansylamide (DNSA) competition assay |
Bioorg Med Chem Lett 23: 939-43 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.058
BindingDB Entry DOI: 10.7270/Q2VM4G54 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50503893
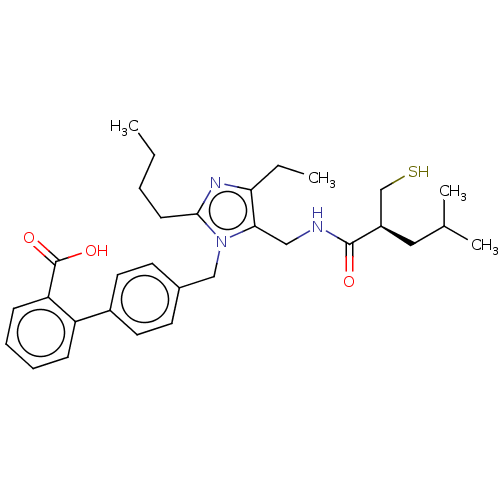
(CHEMBL4454778)Show SMILES CCCCc1nc(CC)c(CNC(=O)[C@@H](CS)CC(C)C)n1Cc1ccc(cc1)-c1ccccc1C(O)=O |r| Show InChI InChI=1S/C31H41N3O3S/c1-5-7-12-29-33-27(6-2)28(18-32-30(35)24(20-38)17-21(3)4)34(29)19-22-13-15-23(16-14-22)25-10-8-9-11-26(25)31(36)37/h8-11,13-16,21,24,38H,5-7,12,17-20H2,1-4H3,(H,32,35)(H,36,37)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US Inc.
Curated by ChEMBL
| Assay Description
Displacement of Europium-labeled angiotensin-2 from human AT1 receptor expressed in CHOK1 cell membranes after 120 mins by DELFIA |
ACS Med Chem Lett 10: 86-91 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00462
BindingDB Entry DOI: 10.7270/Q2D50R6V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50503897
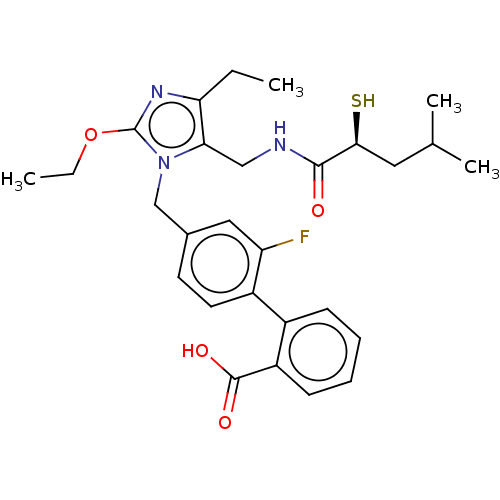
(CHEMBL4474870)Show SMILES CCOc1nc(CC)c(CNC(=O)[C@@H](S)CC(C)C)n1Cc1ccc(c(F)c1)-c1ccccc1C(O)=O |r| Show InChI InChI=1S/C28H34FN3O4S/c1-5-23-24(15-30-26(33)25(37)13-17(3)4)32(28(31-23)36-6-2)16-18-11-12-20(22(29)14-18)19-9-7-8-10-21(19)27(34)35/h7-12,14,17,25,37H,5-6,13,15-16H2,1-4H3,(H,30,33)(H,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US Inc.
Curated by ChEMBL
| Assay Description
Displacement of Europium-labeled angiotensin-2 from human AT1 receptor expressed in CHOK1 cell membranes after 120 mins by DELFIA |
ACS Med Chem Lett 10: 86-91 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00462
BindingDB Entry DOI: 10.7270/Q2D50R6V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50364378
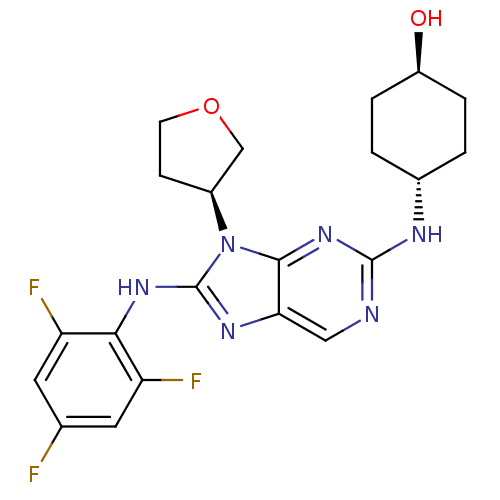
(CHEMBL1950289)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cc(F)cc3F)n([C@H]3CCOC3)c2n1 |r,wU:1.0,wD:4.7,25.26,(-6.26,-6.49,;-5.49,-5.16,;-6.25,-3.82,;-5.49,-2.5,;-3.95,-2.49,;-3.17,-3.82,;-3.94,-5.16,;-3.19,-1.16,;-1.65,-1.15,;-.89,.17,;.66,.18,;1.42,-1.15,;2.92,-1.46,;3.09,-2.98,;4.42,-3.75,;5.75,-2.97,;5.74,-1.45,;4.4,-.69,;7.06,-.67,;8.41,-1.43,;9.74,-.66,;8.41,-2.97,;7.08,-3.75,;7.08,-5.29,;1.69,-3.61,;1.23,-5.08,;-.23,-5.56,;-.23,-7.1,;1.24,-7.57,;2.14,-6.32,;.66,-2.48,;-.88,-2.48,)| Show InChI InChI=1S/C21H23F3N6O2/c22-11-7-15(23)18(16(24)8-11)28-21-27-17-9-25-20(26-12-1-3-14(31)4-2-12)29-19(17)30(21)13-5-6-32-10-13/h7-9,12-14,31H,1-6,10H2,(H,27,28)(H,25,26,29)/t12-,13-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of hexa-His-tagged JNK2 expressed in baculoviral system using GST-tagged cJun as substrate preincubated for 15 mins prior ATP addition mea... |
Bioorg Med Chem Lett 22: 1433-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.027
BindingDB Entry DOI: 10.7270/Q2C829SK |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50503900
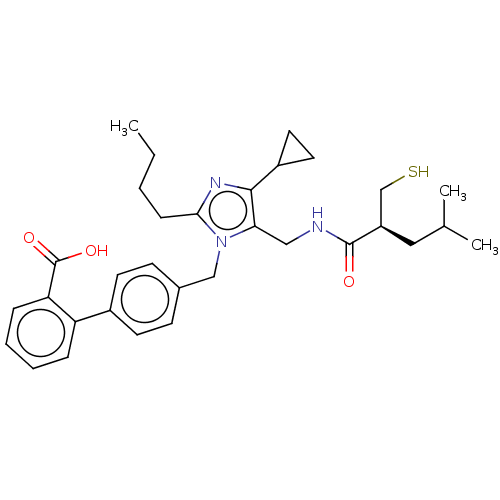
(CHEMBL4456544)Show SMILES CCCCc1nc(C2CC2)c(CNC(=O)[C@@H](CS)CC(C)C)n1Cc1ccc(cc1)-c1ccccc1C(O)=O |r| Show InChI InChI=1S/C32H41N3O3S/c1-4-5-10-29-34-30(24-15-16-24)28(18-33-31(36)25(20-39)17-21(2)3)35(29)19-22-11-13-23(14-12-22)26-8-6-7-9-27(26)32(37)38/h6-9,11-14,21,24-25,39H,4-5,10,15-20H2,1-3H3,(H,33,36)(H,37,38)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US Inc.
Curated by ChEMBL
| Assay Description
Displacement of Europium-labeled angiotensin-2 from human AT1 receptor expressed in CHOK1 cell membranes after 120 mins by DELFIA |
ACS Med Chem Lett 10: 86-91 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00462
BindingDB Entry DOI: 10.7270/Q2D50R6V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data