

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052442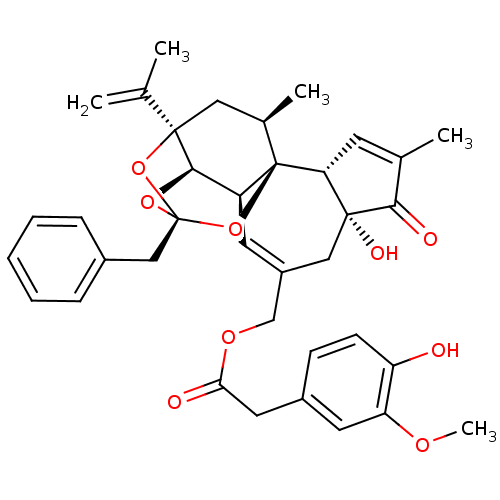 ((4-Hydroxy-3-methoxy-phenyl)-acetic acid (2R,3S,3a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding to Rat Vanilloid receptor 1 (VR1) expressing CHO cells compared to capsacin | J Med Chem 46: 3116-26 (2003) Article DOI: 10.1021/jm030089u BindingDB Entry DOI: 10.7270/Q2SB4551 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571836 ((Z)-3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbon...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50385670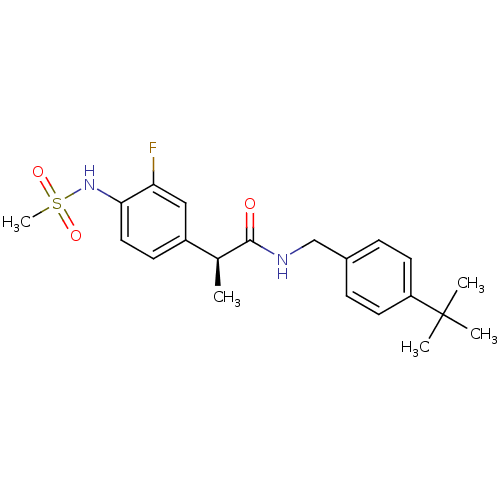 (CHEMBL2042399) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 receptor expressed in CHO cells assessed as decrease in capsaicin-induced intracellular 45Ca2+ uptake after 1 hr b... | Bioorg Med Chem 20: 215-24 (2011) Article DOI: 10.1016/j.bmc.2011.11.008 BindingDB Entry DOI: 10.7270/Q2MG7QJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571793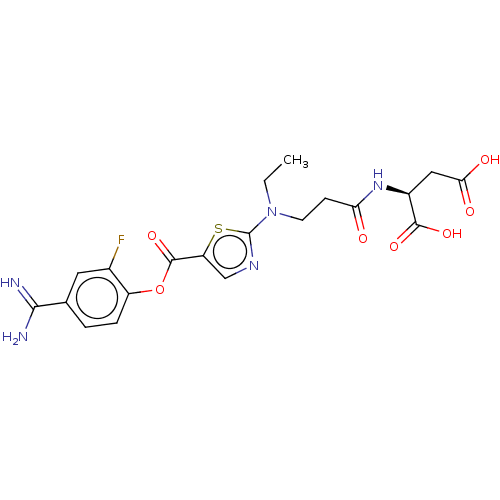 ((3-((5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571830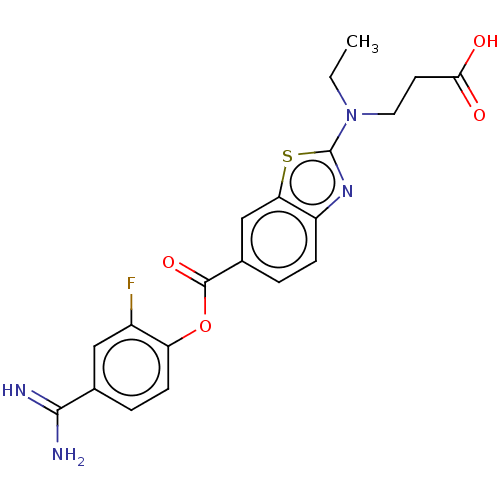 (3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571817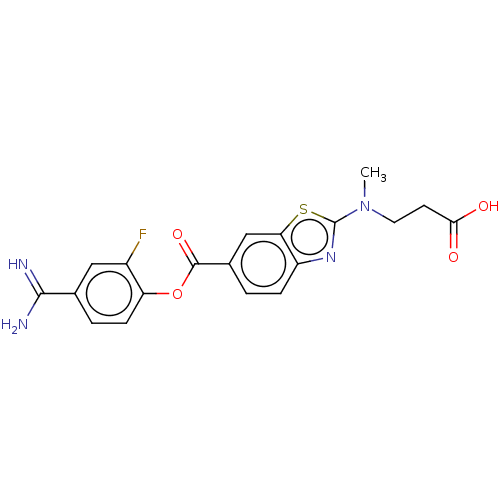 (3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571797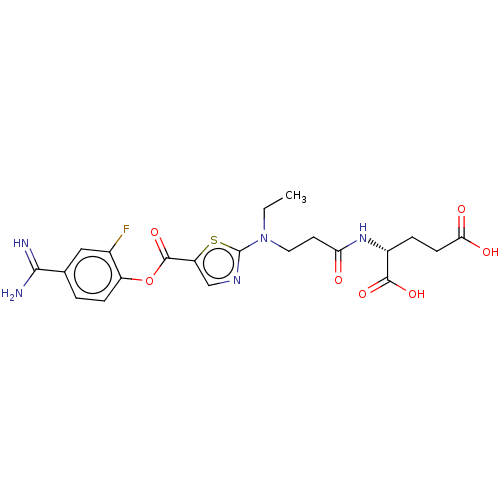 ((3-((5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571802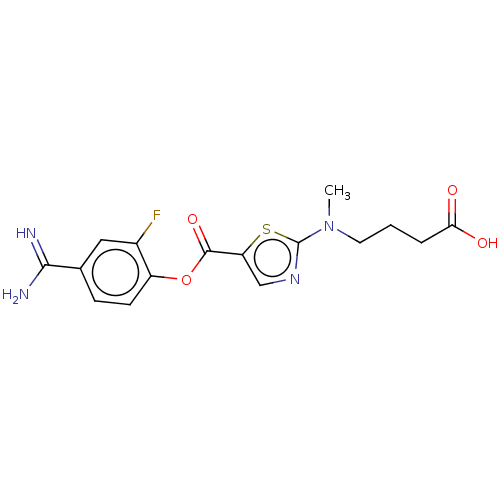 (4-((5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571815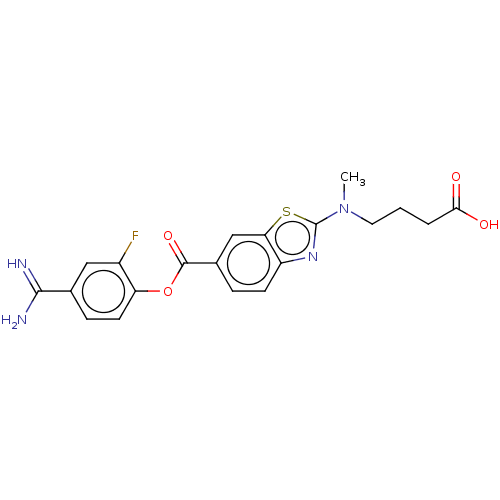 (4-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571814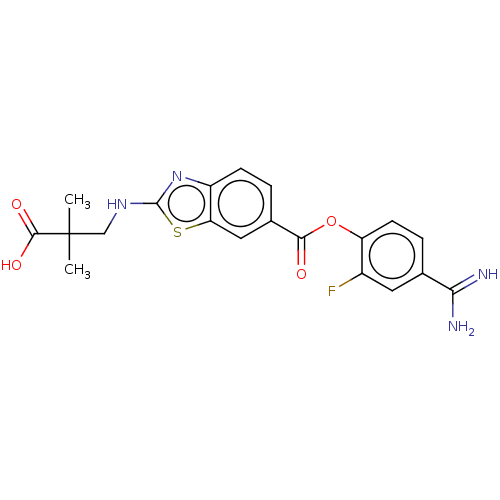 (3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571772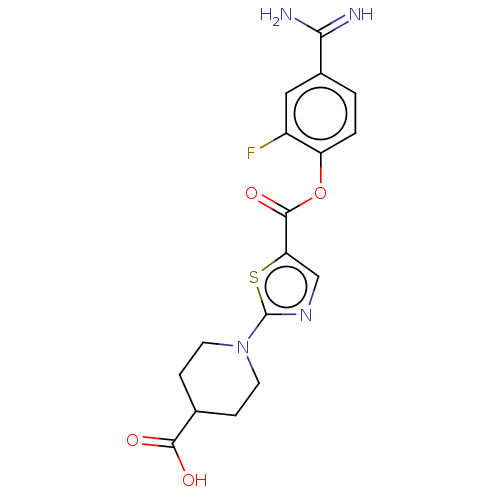 (1-(5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)th...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50300114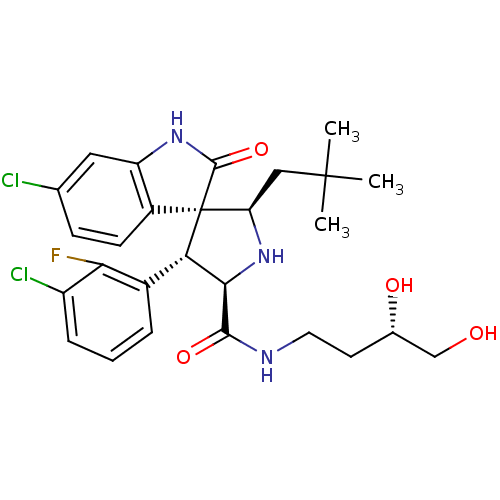 ((2'R,3S,4'S,5'R)-6-chloro-4'-(3-chloro-2-fluorophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of his-tagged human recombinant MDM2 binding to p53-based peptide PMDM6-F by fluorescence polarization assay | J Med Chem 52: 7970-3 (2009) Article DOI: 10.1021/jm901400z BindingDB Entry DOI: 10.7270/Q2N29X17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20286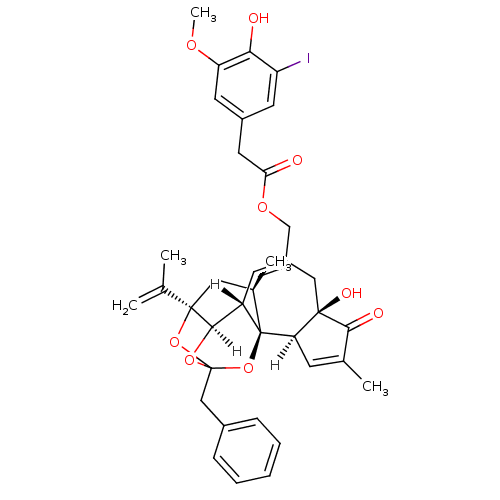 (5-I-RTX | 5-iodoresiniferatoxin | [(1R,2R,6R,10S,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.610 | -54.7 | n/a | n/a | 12.2 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571760 (3-((5-((4-carbamimidoyl-2-fluorophenoxy)carbonyl)t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571843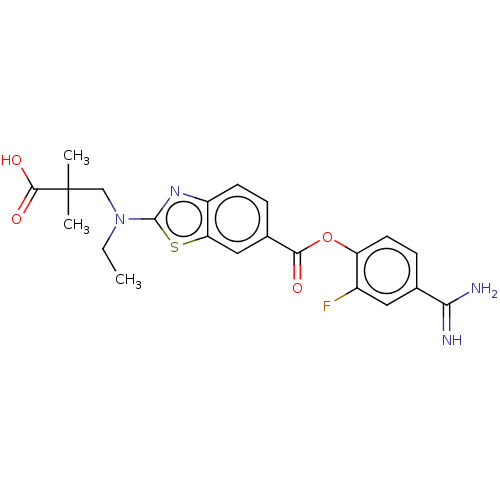 (3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50432760 (CHEMBL2348620) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to XIAP linker BIR2-BIR3 domain (unknown origin) after 3 hrs by competitive fluorescence polarization assay in presence of Smac-1F | J Med Chem 56: 3969-79 (2013) Article DOI: 10.1021/jm400216d BindingDB Entry DOI: 10.7270/Q2M61MNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to 5-HT2A | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50300115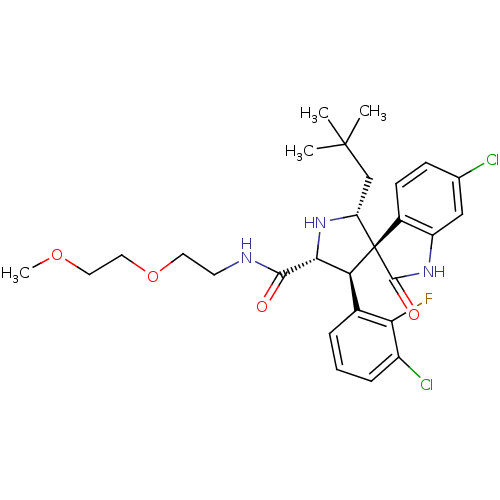 ((2'R,3S,4'S,5'R)-6-chloro-4'-(3-chloro-2-fluorophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of his-tagged human recombinant MDM2 binding to p53-based peptide PMDM6-F by fluorescence polarization assay | J Med Chem 52: 7970-3 (2009) Article DOI: 10.1021/jm901400z BindingDB Entry DOI: 10.7270/Q2N29X17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313283 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of 5-HT2A receptor | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313283 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to 5-HT2A | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571841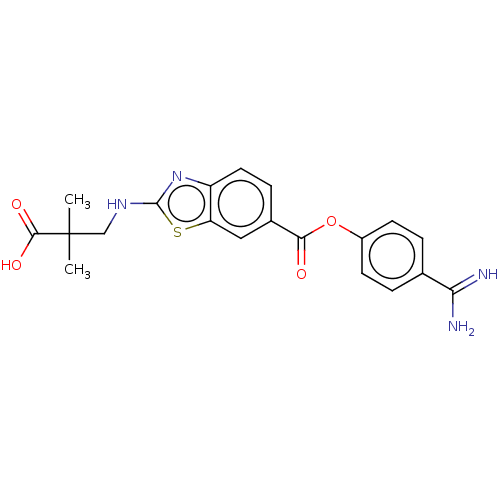 (3-((6-((4-Carbamimidoylphenoxy)carbonyl)benzo[d]th...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571766 (4-Carbamimidoyl-2-fluorophenyl 2-(4-(methoxycarbon...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571783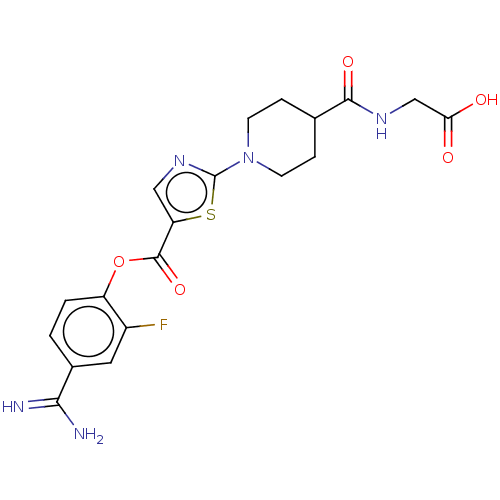 ((1-(5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50393507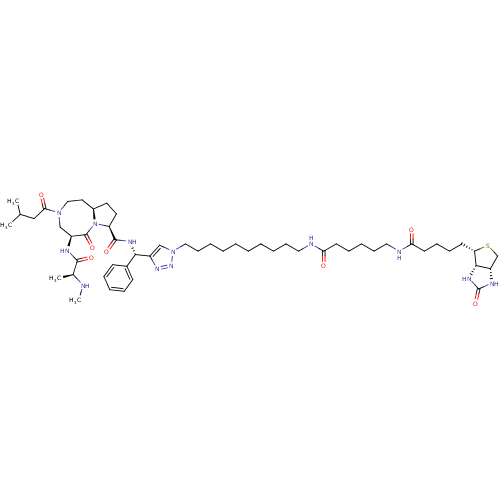 (CHEMBL2158053) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Competitive inhibition of human cIAP1 BIR3 domain expressed in Escherichia coli BL21(DE3) after 2 to 3 hrs by fluorescence polarization assay | J Med Chem 54: 2714-26 (2011) Article DOI: 10.1021/jm101505d BindingDB Entry DOI: 10.7270/Q2H70GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM26218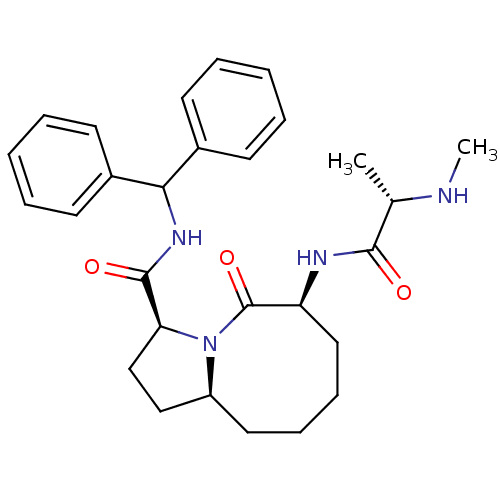 ((3S,6S,10aS)-N-(diphenylmethyl)-6-[(2S)-2-(methyla...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of fluorescently tagged SM5F from human cIAP-1 BIR3 expressed in Escherichia coli BL21 cells by fluorescence polarization assay | J Med Chem 51: 8158-62 (2008) Article DOI: 10.1021/jm801254r BindingDB Entry DOI: 10.7270/Q2BR8S2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571839 (4-Carbamimidoyl-2-fluorophenyl 2-((4-methoxy-4-oxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50273673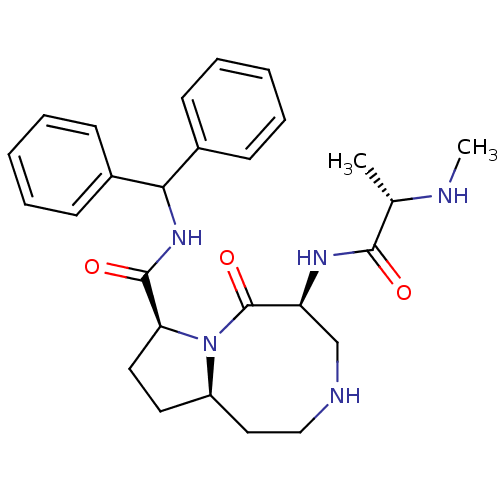 ((5S,8S,10aR)-N-Benzhydryl-5-((S)-2-(methylamino)pr...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of fluorescently tagged SM5F from human cIAP-1 BIR3 expressed in Escherichia coli BL21 cells by fluorescence polarization assay | J Med Chem 51: 8158-62 (2008) Article DOI: 10.1021/jm801254r BindingDB Entry DOI: 10.7270/Q2BR8S2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50273698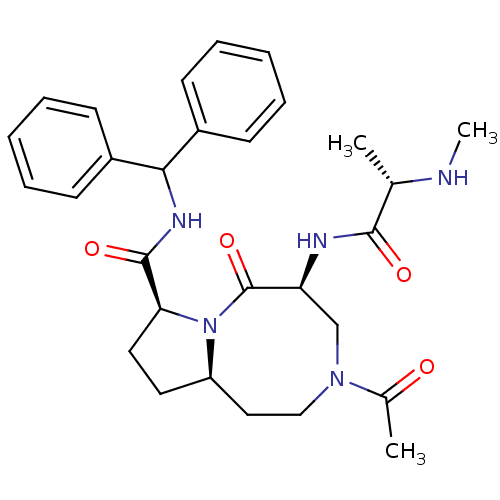 ((5S,8S,10aR)-3-Acetyl-N-benzhydryl-5-((S)-2-(methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of fluorescently tagged SM5F from human cIAP-1 BIR3 expressed in Escherichia coli BL21 cells by fluorescence polarization assay | J Med Chem 51: 8158-62 (2008) Article DOI: 10.1021/jm801254r BindingDB Entry DOI: 10.7270/Q2BR8S2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571840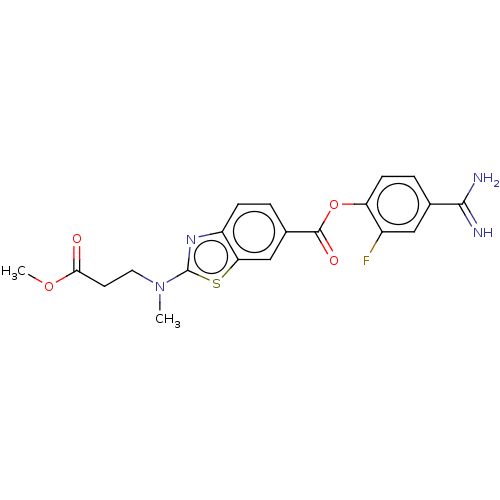 (4-Carbamimidoyl-2-fluorophenyl 2-((3-methoxy-3-oxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50432754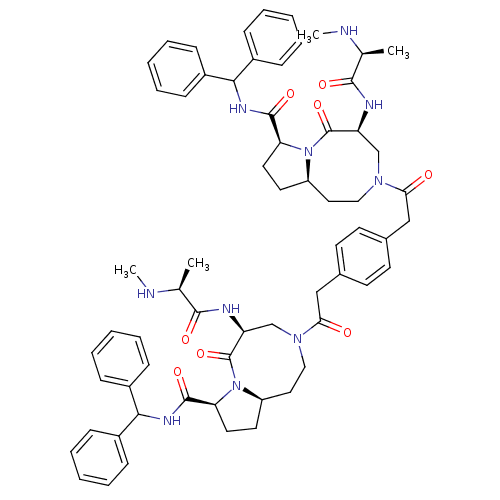 (CHEMBL2348614) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to XIAP linker BIR2-BIR3 domain (unknown origin) after 3 hrs by competitive fluorescence polarization assay in presence of Smac-1F | J Med Chem 56: 3969-79 (2013) Article DOI: 10.1021/jm400216d BindingDB Entry DOI: 10.7270/Q2M61MNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50432753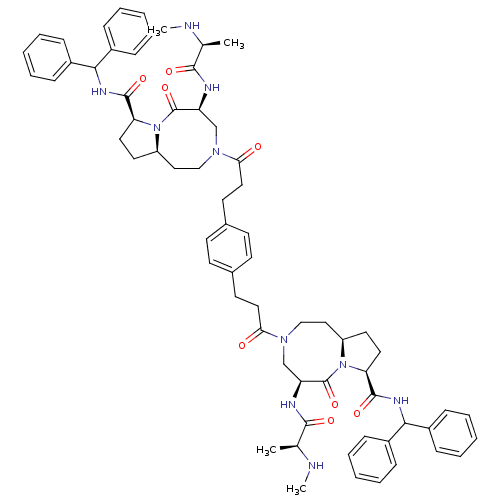 (CHEMBL2348613) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to XIAP linker BIR2-BIR3 domain (unknown origin) after 3 hrs by competitive fluorescence polarization assay in presence of Smac-1F | J Med Chem 56: 3969-79 (2013) Article DOI: 10.1021/jm400216d BindingDB Entry DOI: 10.7270/Q2M61MNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571789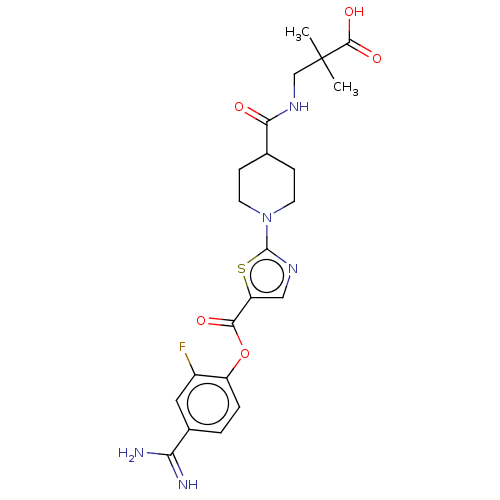 (3-(1-(5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50273699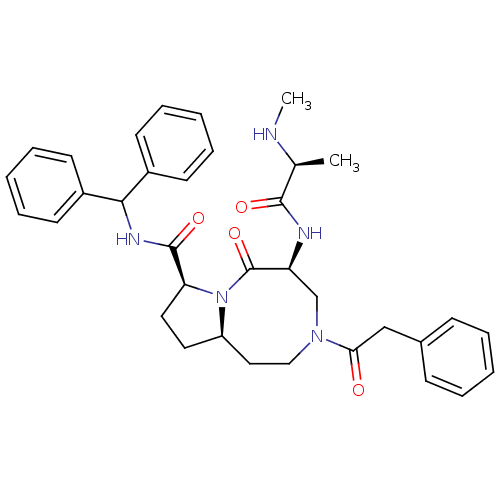 ((5S,8S,10aR)-N-Benzhydryl-5-((S)-2-(methyl-amino)p...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of fluorescently tagged SM5F from human cIAP-1 BIR3 expressed in Escherichia coli BL21 cells by fluorescence polarization assay | J Med Chem 51: 8158-62 (2008) Article DOI: 10.1021/jm801254r BindingDB Entry DOI: 10.7270/Q2BR8S2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50432759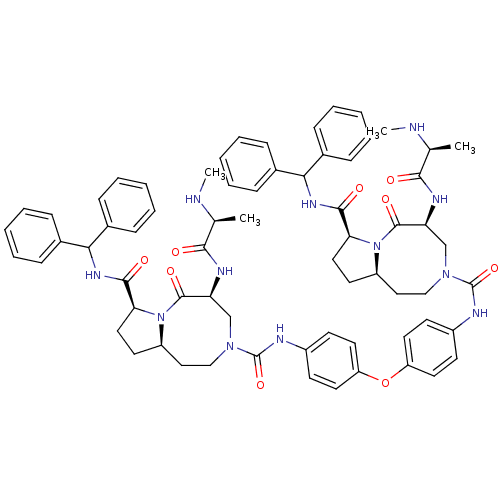 (CHEMBL2348619) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to cIAP1 BIR3 domain (unknown origin) after 3 hrs by competitive fluorescence polarization assay in presence of Smac-2F | J Med Chem 56: 3969-79 (2013) Article DOI: 10.1021/jm400216d BindingDB Entry DOI: 10.7270/Q2M61MNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50300116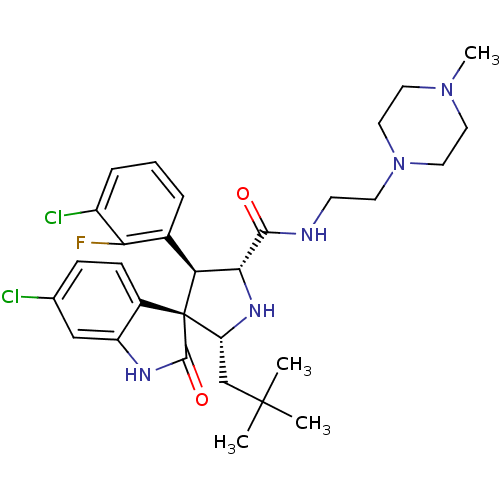 ((2'R,3S,4'S,5'R)-6-chloro-4'-(3-chloro-2-fluorophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of his-tagged human recombinant MDM2 binding to p53-based peptide PMDM6-F by fluorescence polarization assay | J Med Chem 52: 7970-3 (2009) Article DOI: 10.1021/jm901400z BindingDB Entry DOI: 10.7270/Q2N29X17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571787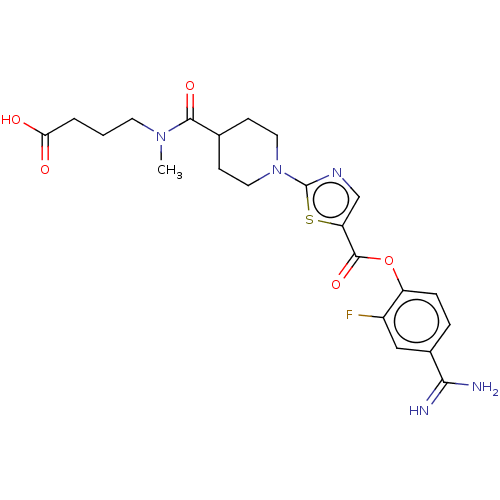 (4-(1-(5-((4-carbamimidoyl-2-fluorophenoxy)carbonyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50393507 (CHEMBL2158053) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Competitive inhibition of human cIAP2 BIR3 domain expressed in Escherichia coli BL21(DE3) after 2 to 3 hrs by fluorescence polarization assay | J Med Chem 54: 2714-26 (2011) Article DOI: 10.1021/jm101505d BindingDB Entry DOI: 10.7270/Q2H70GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31202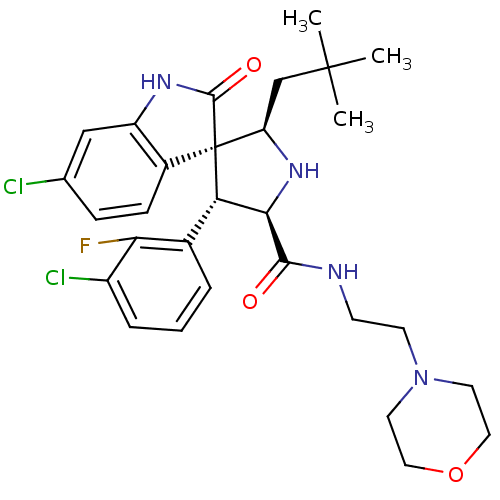 (JMC493432 Compound 8 | MI-63) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of his-tagged human recombinant MDM2 binding to p53-based peptide PMDM6-F by fluorescence polarization assay | J Med Chem 52: 7970-3 (2009) Article DOI: 10.1021/jm901400z BindingDB Entry DOI: 10.7270/Q2N29X17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571785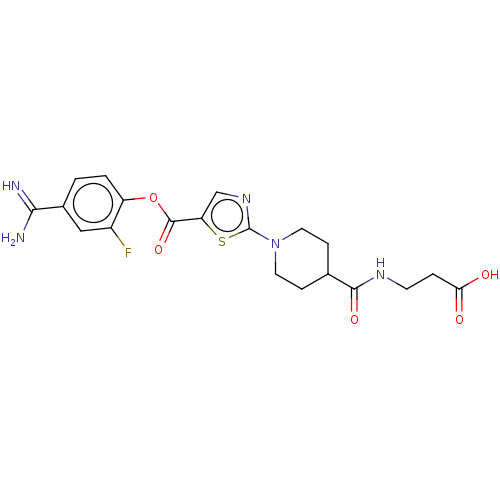 (3-(1-(5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50432762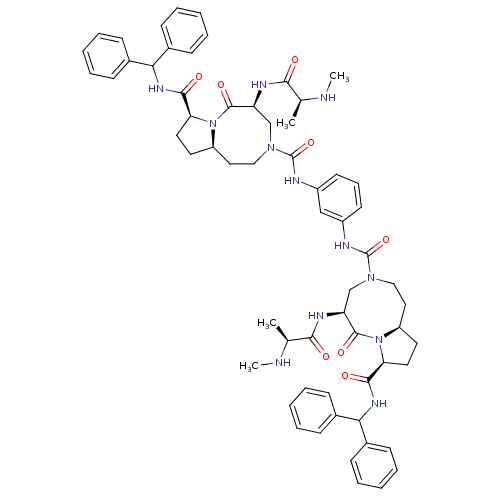 (CHEMBL2348622) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to XIAP linker BIR2-BIR3 domain (unknown origin) after 3 hrs by competitive fluorescence polarization assay in presence of Smac-1F | J Med Chem 56: 3969-79 (2013) Article DOI: 10.1021/jm400216d BindingDB Entry DOI: 10.7270/Q2M61MNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50432758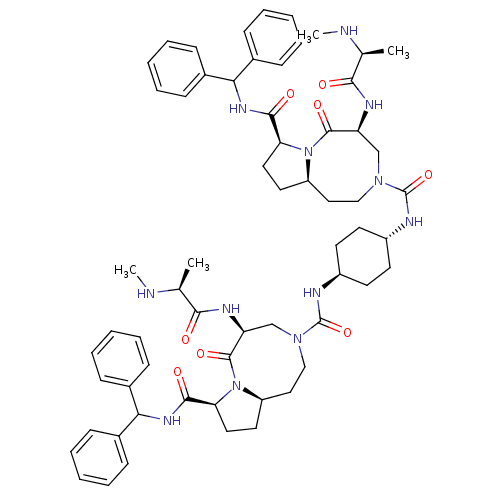 (CHEMBL2348618) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to XIAP linker BIR2-BIR3 domain (unknown origin) after 3 hrs by competitive fluorescence polarization assay in presence of Smac-1F | J Med Chem 56: 3969-79 (2013) Article DOI: 10.1021/jm400216d BindingDB Entry DOI: 10.7270/Q2M61MNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571781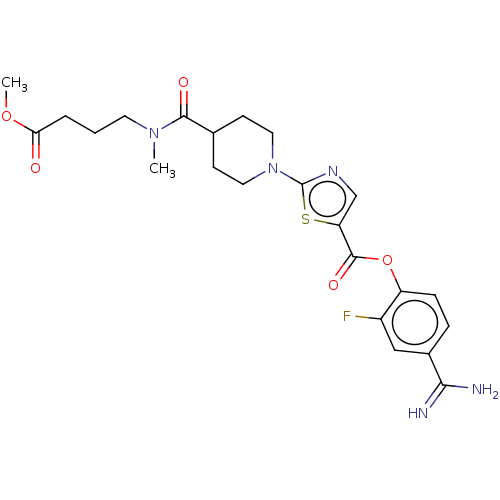 (4-Carbamimidoyl-2-fluorophenyl 2-(4-((4-methoxy-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571773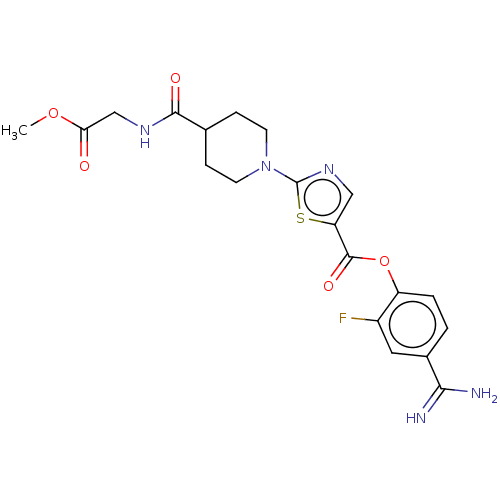 (4-Carbamimidoyl-2-fluorophenyl 2-(4-((2-methoxy-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 8 (Homo sapiens (Human)) | BDBM26218 ((3S,6S,10aS)-N-(diphenylmethyl)-6-[(2S)-2-(methyla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of fluorescently tagged SM5F from human cIAP-2 BIR3 expressed in Escherichia coli BL21 cells by fluorescence polarization assay | J Med Chem 51: 8158-62 (2008) Article DOI: 10.1021/jm801254r BindingDB Entry DOI: 10.7270/Q2BR8S2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20314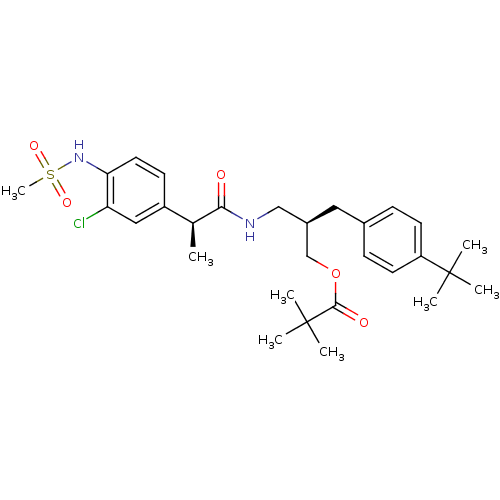 ((2R)-2-[(4-tert-butylphenyl)methyl]-3-[(2S)-2-(3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.83 | -51.9 | n/a | n/a | 5.20 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571824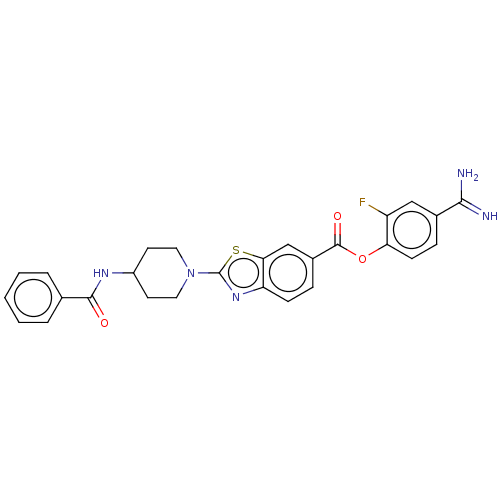 (4-Carbamimidoyl-2-fluorophenyl 2-(4-benzoamidopipe...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 8 (Homo sapiens (Human)) | BDBM50273698 ((5S,8S,10aR)-3-Acetyl-N-benzhydryl-5-((S)-2-(methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of fluorescently tagged SM5F from human cIAP-2 BIR3 expressed in Escherichia coli BL21 cells by fluorescence polarization assay | J Med Chem 51: 8158-62 (2008) Article DOI: 10.1021/jm801254r BindingDB Entry DOI: 10.7270/Q2BR8S2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50393505 (CHEMBL2158051) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Competitive inhibition of human cIAP1 BIR3 domain expressed in Escherichia coli BL21(DE3) after 2 to 3 hrs by fluorescence polarization assay | J Med Chem 54: 2714-26 (2011) Article DOI: 10.1021/jm101505d BindingDB Entry DOI: 10.7270/Q2H70GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50300118 ((2'R,3S,4'S,5'R)-6-chloro-4'-(3-chloro-2-fluorophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of his-tagged human recombinant MDM2 binding to p53-based peptide PMDM6-F by fluorescence polarization assay | J Med Chem 52: 7970-3 (2009) Article DOI: 10.1021/jm901400z BindingDB Entry DOI: 10.7270/Q2N29X17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50017594 (CHEMBL3288626) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from rat TRPV1 after 60 mins by competitive binding assay | Bioorg Med Chem Lett 24: 2685-8 (2014) Article DOI: 10.1016/j.bmcl.2014.04.054 BindingDB Entry DOI: 10.7270/Q26111VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 6454 total ) | Next | Last >> |