

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aryl hydrocarbon receptor (Rattus norvegicus) | BDBM50240990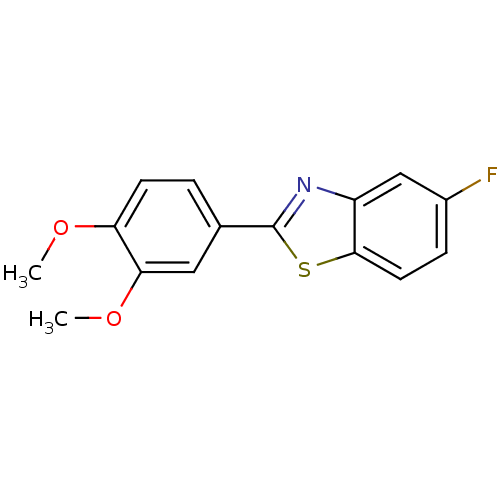 (2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole | 5-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Displacement of [3H]tetrachlorodibenzo-p-dioxin from aryl hydrocarbon receptor in CRL:WI rat liver cytosol | J Med Chem 51: 5135-9 (2008) Article DOI: 10.1021/jm800418z BindingDB Entry DOI: 10.7270/Q23X86F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50211726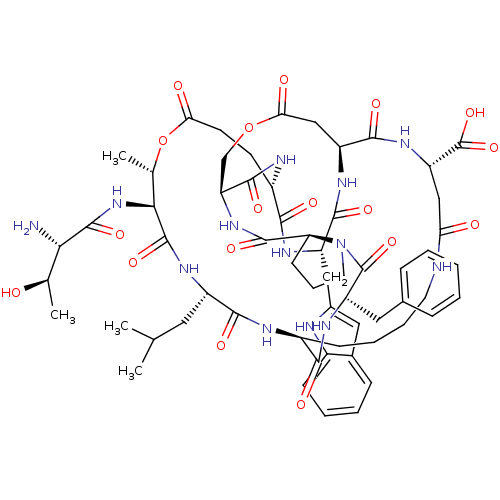 (CHEMBL403896 | FR-901451) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase | Bioorg Med Chem 15: 4618-28 (2007) Article DOI: 10.1016/j.bmc.2007.03.082 BindingDB Entry DOI: 10.7270/Q2D21ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50080264 (1N-[21-benzyl-29-[(Z)-ethylidene]-13,14,23-trihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase | Bioorg Med Chem 15: 4618-28 (2007) Article DOI: 10.1016/j.bmc.2007.03.082 BindingDB Entry DOI: 10.7270/Q2D21ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50211725 ((2S)-2-{[(3S,9S,12S,15S,18S,21R,26R,29S,32S)-21-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase | Bioorg Med Chem 15: 4618-28 (2007) Article DOI: 10.1016/j.bmc.2007.03.082 BindingDB Entry DOI: 10.7270/Q2D21ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50211723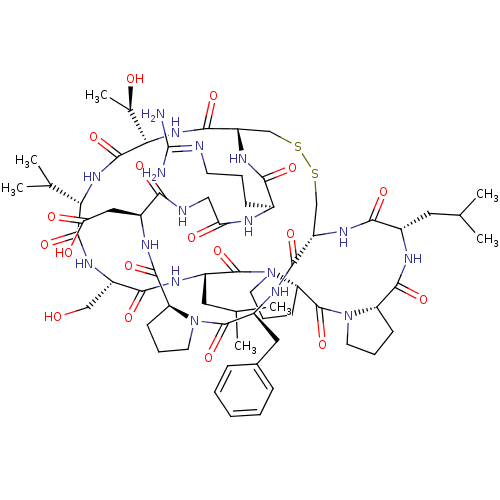 (2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase | Bioorg Med Chem 15: 4618-28 (2007) Article DOI: 10.1016/j.bmc.2007.03.082 BindingDB Entry DOI: 10.7270/Q2D21ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50211721 ((2S)-2-{[(3S,9S,12S,15S,18S,21R,26R,29S,32S)-21-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase | Bioorg Med Chem 15: 4618-28 (2007) Article DOI: 10.1016/j.bmc.2007.03.082 BindingDB Entry DOI: 10.7270/Q2D21ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50211722 ((2S)-2-{[(3S,9S,12S,15S,18S,21R,26R,29S,32S)-21-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase | Bioorg Med Chem 15: 4618-28 (2007) Article DOI: 10.1016/j.bmc.2007.03.082 BindingDB Entry DOI: 10.7270/Q2D21ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063777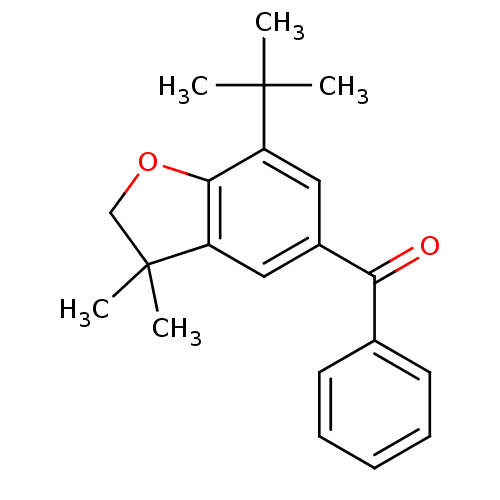 ((7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofuran-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063780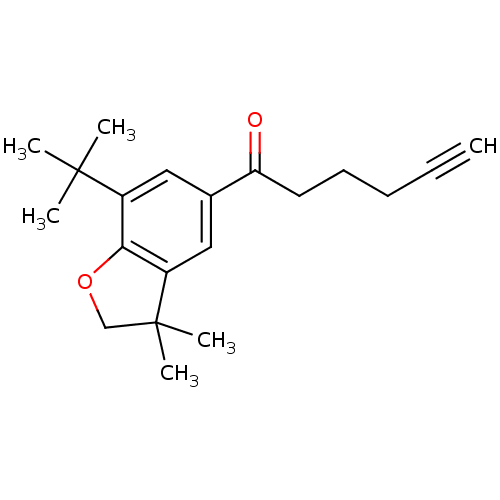 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Rattus norvegicus) | BDBM50240990 (2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole | 5-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Displacement of [3H]tetrachlorodibenzo-p-dioxin from aryl hydrocarbon receptor in CRL:WI rat liver cytosol | J Med Chem 51: 5135-9 (2008) Article DOI: 10.1021/jm800418z BindingDB Entry DOI: 10.7270/Q23X86F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063779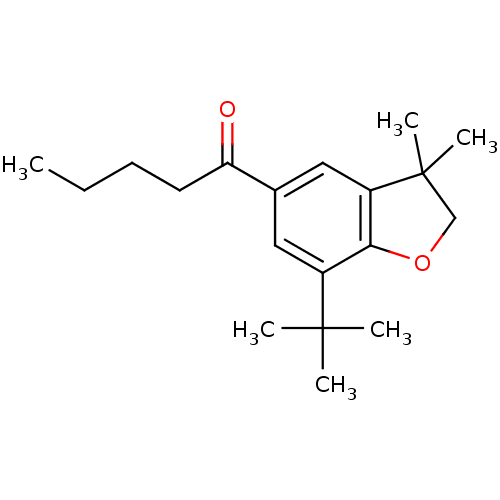 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50063780 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 1 (COX-1) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor p65 (Homo sapiens (Human)) | BDBM50196926 (CHEMBL438139 | Rocaglamide | US10085988, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Greensboro Curated by ChEMBL | Assay Description Inhibition of NFkappa p65 isolated from nuclear extract of human HeLa cells by ELISA | J Nat Prod 74: 1126-31 (2011) Article DOI: 10.1021/np200062x BindingDB Entry DOI: 10.7270/Q25M6621 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor p65 (Homo sapiens (Human)) | BDBM50196926 (CHEMBL438139 | Rocaglamide | US10085988, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of NFkappa p65 in nuclear extract of human HeLa cells assessed as blockade of NFkappa p65 binding to biotinylated-consesus sequence by ELI... | J Nat Prod 74: 1117-25 (2011) Article DOI: 10.1021/np200051j BindingDB Entry DOI: 10.7270/Q20C4W31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063778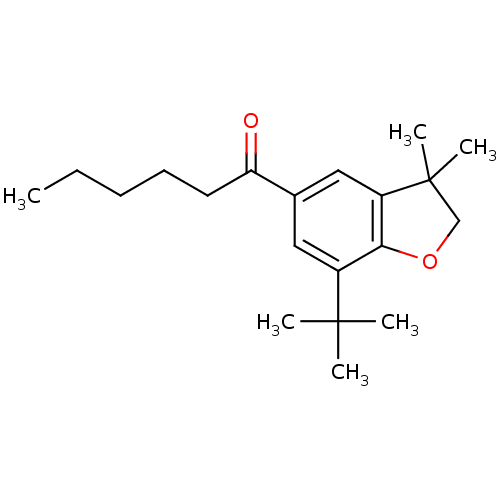 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50063781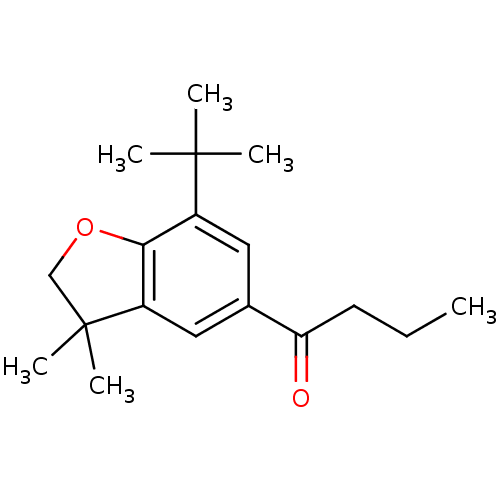 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 1 (COX-1) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063781 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063783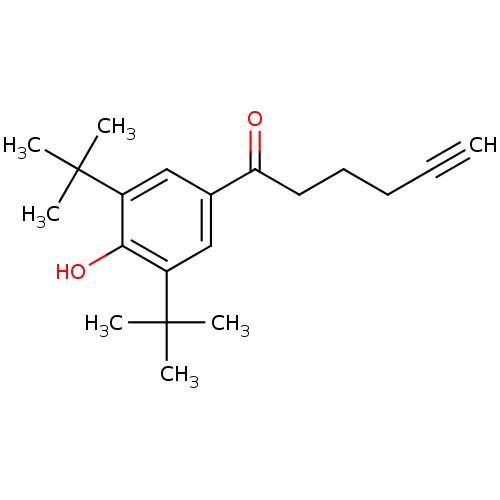 (1-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-hex-5-yn-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063789 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50063777 ((7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofuran-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 1 (COX-1) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063787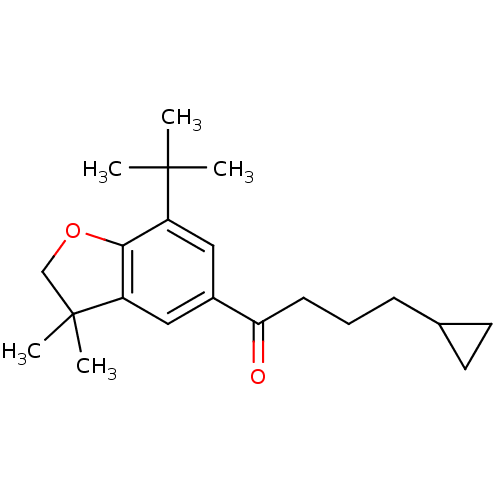 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50063783 (1-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-hex-5-yn-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 1 (COX-1) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063782 ((E)-1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor p65 (Homo sapiens (Human)) | BDBM50336457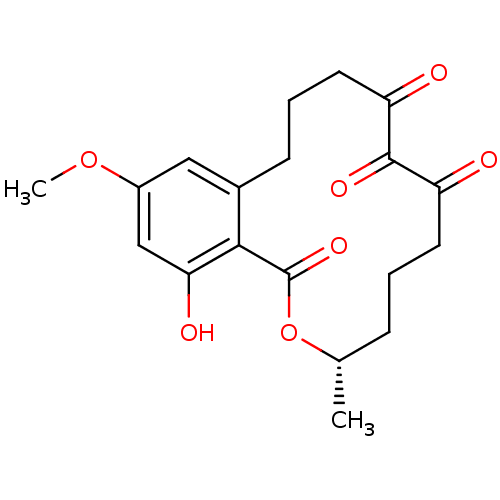 ((3S,5Z,8S,9R,11E)-8,9,16-trihydroxy-14-methoxy-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Greensboro Curated by ChEMBL | Assay Description Inhibition of NFkappa p65 isolated from nuclear extract of human HeLa cells by ELISA | J Nat Prod 74: 1126-31 (2011) Article DOI: 10.1021/np200062x BindingDB Entry DOI: 10.7270/Q25M6621 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063788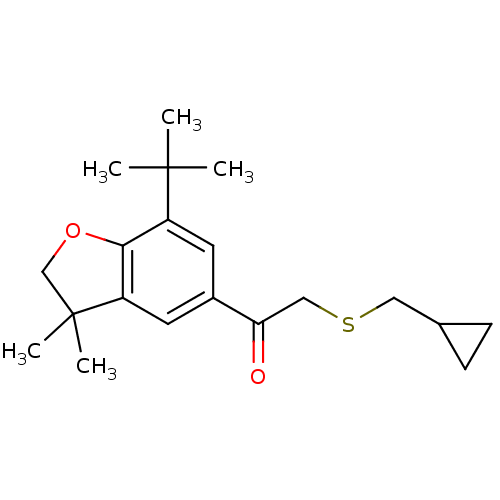 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50063788 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 1 (COX-1) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor p65 (Homo sapiens (Human)) | BDBM50347543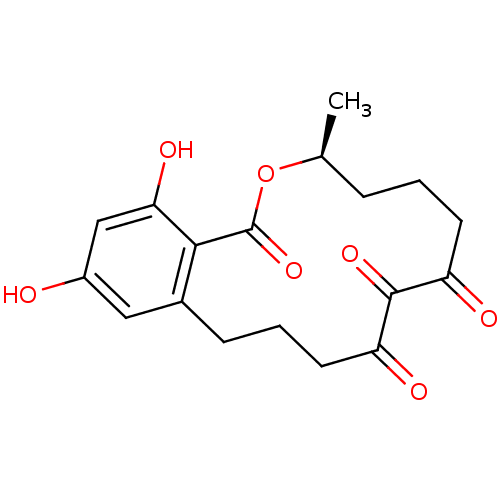 (CHEMBL1801948) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Greensboro Curated by ChEMBL | Assay Description Inhibition of NFkappa p65 isolated from nuclear extract of human HeLa cells by ELISA | J Nat Prod 74: 1126-31 (2011) Article DOI: 10.1021/np200062x BindingDB Entry DOI: 10.7270/Q25M6621 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50063782 ((E)-1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 1 (COX-1) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50063789 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 1 (COX-1) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50063779 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 1 (COX-1) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063785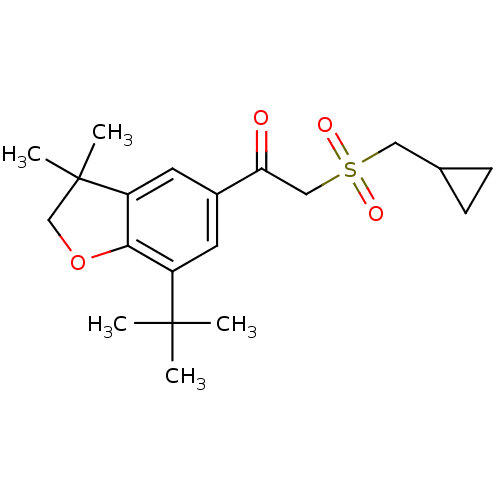 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063790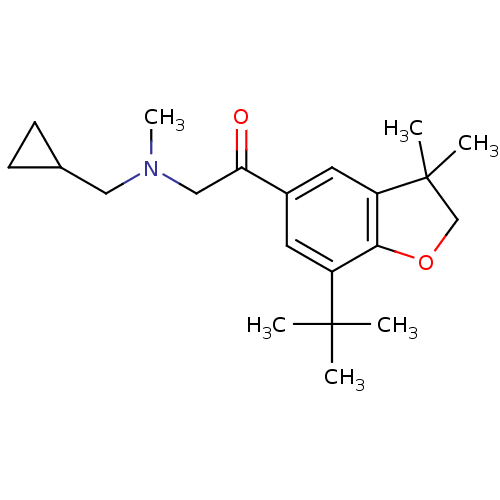 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor p65 (Homo sapiens (Human)) | BDBM50346334 (1,3,7-trihydroxy-2,4-diisoprenylxanthone | CHEMBL1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of NFkappa p65 in nuclear extract of human HeLa cells assessed as blockade of NFkappa p65 binding to biotinylated-consesus sequence by ELI... | J Nat Prod 74: 1117-25 (2011) Article DOI: 10.1021/np200051j BindingDB Entry DOI: 10.7270/Q20C4W31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50063783 (1-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-hex-5-yn-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description The compound was tested for the concentration (in microM) to inhibit 50% of 5-Lipoxygenase (5-LOX) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50009859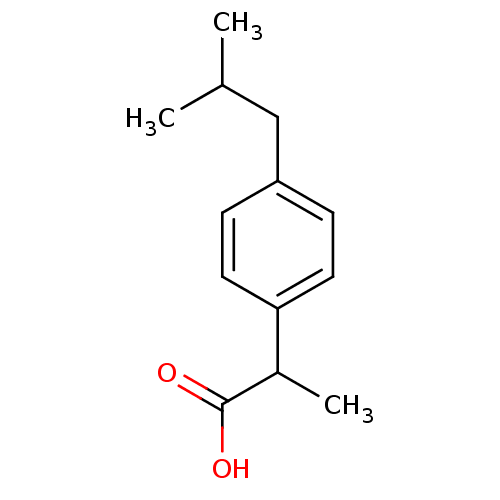 ((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 1 (COX-1) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063784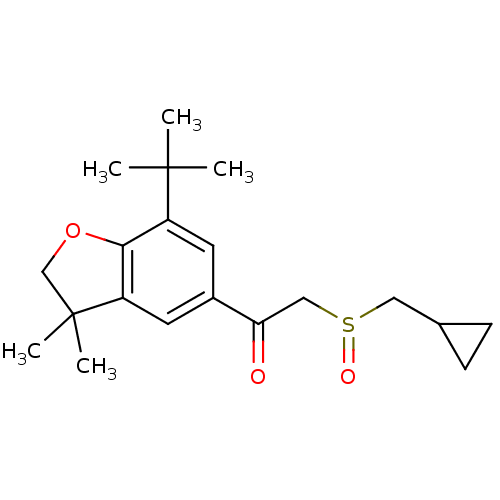 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50063786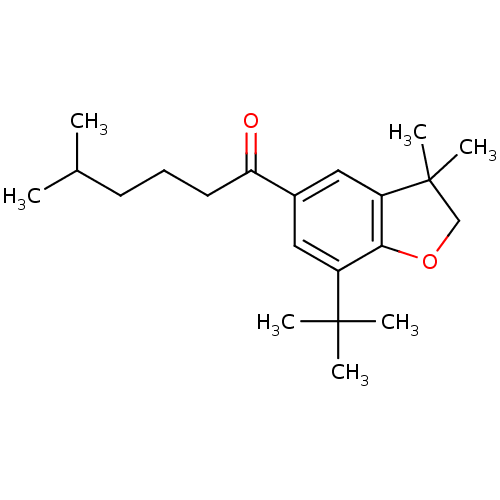 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50063777 ((7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofuran-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description The compound was tested for the concentration (in microM) to inhibit 50% of 5-Lipoxygenase (5-LOX) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50063785 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 1 (COX-1) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50063780 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description The compound was tested for the concentration (in microM) to inhibit 50% of 5-Lipoxygenase (5-LOX) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate lyase (Candida albicans) | BDBM50274515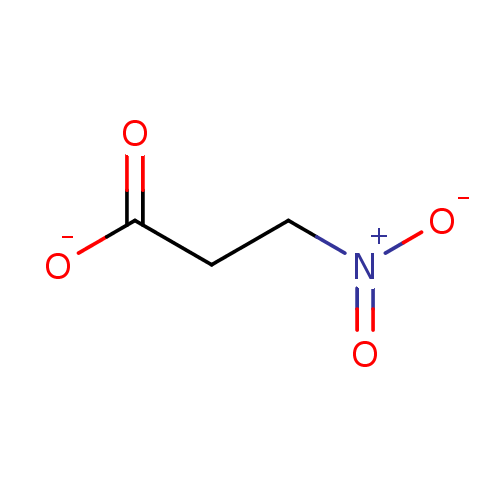 (3-nitropropanoic acid | 3-nitropropionic acid | Bo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of East Anglia Curated by ChEMBL | Assay Description Inhibition of Candida albicans isocitrate lyase after 30 mins | Bioorg Med Chem Lett 21: 7142-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.072 BindingDB Entry DOI: 10.7270/Q28G8M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50063787 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 1 (COX-1) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50063789 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description The compound was tested for the concentration (in microM) to inhibit 50% of 5-Lipoxygenase (5-LOX) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50063787 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description The compound was tested for the concentration (in microM) to inhibit 50% of 5-Lipoxygenase (5-LOX) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50063781 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description The compound was tested for the concentration (in microM) to inhibit 50% of 5-Lipoxygenase (5-LOX) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor p65 (Homo sapiens (Human)) | BDBM50346341 (6-O-benzoyl-alpha-mangostin | CHEMBL1782245) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of NFkappa p65 in nuclear extract of human HeLa cells assessed as blockade of NFkappa p65 binding to biotinylated-consesus sequence by ELI... | J Nat Prod 74: 1117-25 (2011) Article DOI: 10.1021/np200051j BindingDB Entry DOI: 10.7270/Q20C4W31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor p65 (Homo sapiens (Human)) | BDBM50346344 (7-O-Methylcochinchinone A | CHEMBL1782582) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of NFkappa p65 in nuclear extract of human HeLa cells assessed as blockade of NFkappa p65 binding to biotinylated-consesus sequence by ELI... | J Nat Prod 74: 1117-25 (2011) Article DOI: 10.1021/np200051j BindingDB Entry DOI: 10.7270/Q20C4W31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor p65 (Homo sapiens (Human)) | BDBM50346345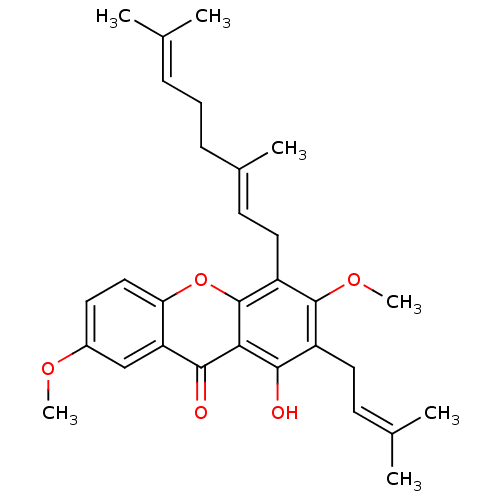 (3,7-Di-O-methylcochinchinone A | CHEMBL1782583) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of NFkappa p65 in nuclear extract of human HeLa cells assessed as blockade of NFkappa p65 binding to biotinylated-consesus sequence by ELI... | J Nat Prod 74: 1117-25 (2011) Article DOI: 10.1021/np200051j BindingDB Entry DOI: 10.7270/Q20C4W31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor p65 (Homo sapiens (Human)) | BDBM50214969 (1,3,6-Trihydroxy-7-methoxy-2,8-bis-(3-methyl-but-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of NFkappa p65 in nuclear extract of human HeLa cells assessed as blockade of NFkappa p65 binding to biotinylated-consesus sequence by ELI... | J Nat Prod 74: 1117-25 (2011) Article DOI: 10.1021/np200051j BindingDB Entry DOI: 10.7270/Q20C4W31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50063778 (1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 1 (COX-1) and is expressed in IC50. | J Med Chem 41: 1112-23 (1998) Article DOI: 10.1021/jm970679q BindingDB Entry DOI: 10.7270/Q2K936N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 91 total ) | Next | Last >> |