

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50232153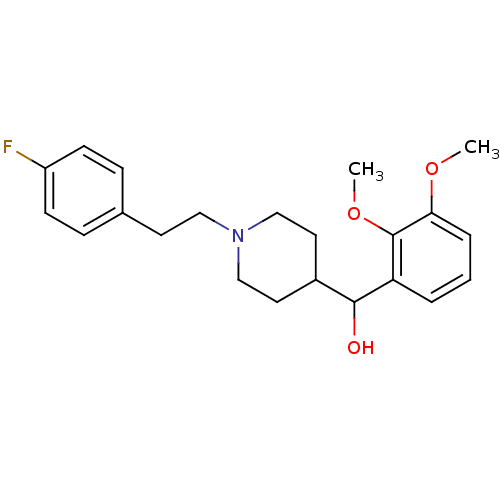 ((1-(4-fluorophenethyl)piperidin-4-yl)(2,3-dimethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | -55.8 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM139371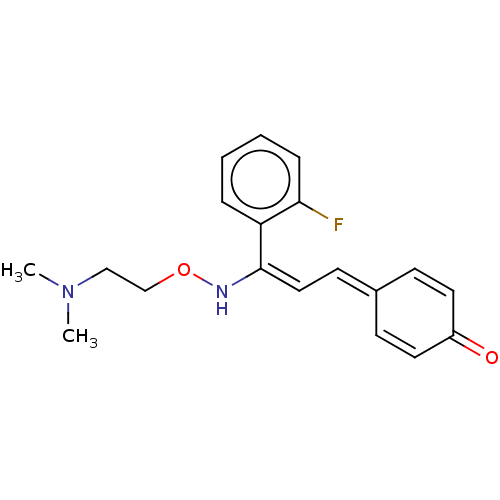 (eplivanserin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.0460 | -54.8 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0830 | -53.5 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.440 | -49.6 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM139370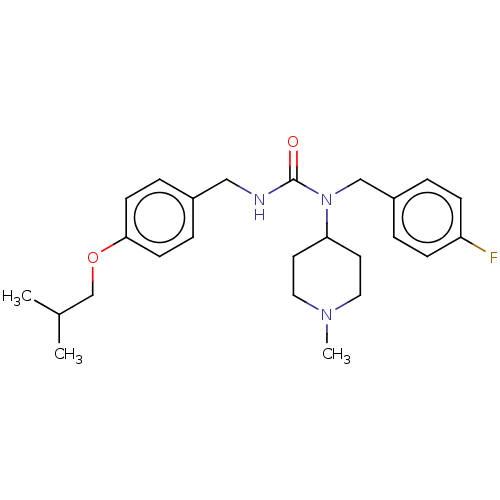 (ACP-103 | Nuplazid | Pimavanserin | Pimavanserin h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 0.5 | -49.3 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM22869 (6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.550 | -49.1 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM139371 (eplivanserin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.30 | -47.1 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM22869 (6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | -47.1 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM139370 (ACP-103 | Nuplazid | Pimavanserin | Pimavanserin h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 1.60 | -46.7 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50232153 ((1-(4-fluorophenethyl)piperidin-4-yl)(2,3-dimethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80 | -44.7 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals Inc. | Assay Description R-SAT assays were performed as described previously (Weiner et al., 2001), with the following modifications. In brief, NIH-3T3 cells were grown to 80... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50465693 (CHEMBL4283608) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as inhibition of 5-oxo-ETE-induced calcium mobilization incubated for 2 mins follow... | J Med Chem 61: 5934-5948 (2018) Article DOI: 10.1021/acs.jmedchem.8b00154 BindingDB Entry DOI: 10.7270/Q22F7R48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50465682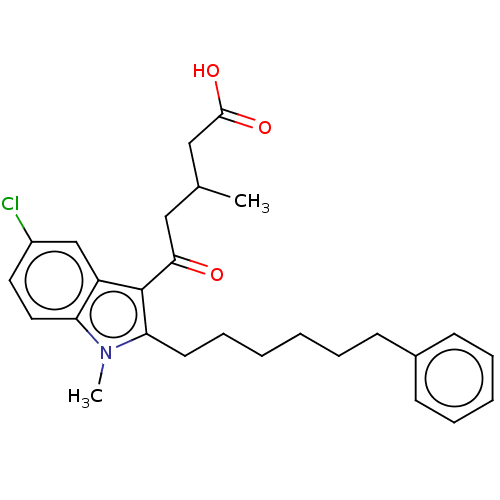 (CHEMBL4287941) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as inhibition of 5-oxo-ETE-induced calcium mobilization incubated for 2 mins follow... | J Med Chem 61: 5934-5948 (2018) Article DOI: 10.1021/acs.jmedchem.8b00154 BindingDB Entry DOI: 10.7270/Q22F7R48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50465685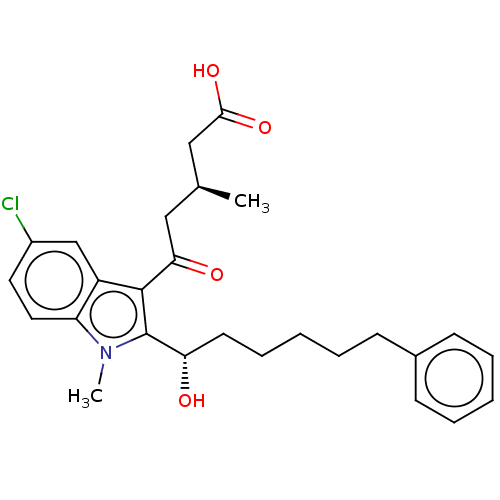 (CHEMBL4281149) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as inhibition of 5-oxo-ETE-induced calcium mobilization incubated for 2 mins follow... | J Med Chem 61: 5934-5948 (2018) Article DOI: 10.1021/acs.jmedchem.8b00154 BindingDB Entry DOI: 10.7270/Q22F7R48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM139371 (eplivanserin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals Inc. | Assay Description R-SAT assays were performed as described previously (Weiner et al., 2001), with the following modifications. In brief, NIH-3T3 cells were grown to 80... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50232153 ((1-(4-fluorophenethyl)piperidin-4-yl)(2,3-dimethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals Inc. | Assay Description R-SAT assays were performed as described previously (Weiner et al., 2001), with the following modifications. In brief, NIH-3T3 cells were grown to 80... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM139370 (ACP-103 | Nuplazid | Pimavanserin | Pimavanserin h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals Inc. | Assay Description R-SAT assays were performed as described previously (Weiner et al., 2001), with the following modifications. In brief, NIH-3T3 cells were grown to 80... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50465690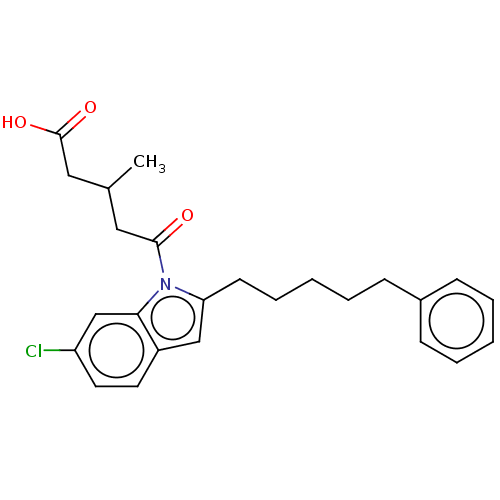 (CHEMBL4282356) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as inhibition of 5-oxo-ETE-induced calcium mobilization incubated for 2 mins follow... | J Med Chem 61: 5934-5948 (2018) Article DOI: 10.1021/acs.jmedchem.8b00154 BindingDB Entry DOI: 10.7270/Q22F7R48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50054792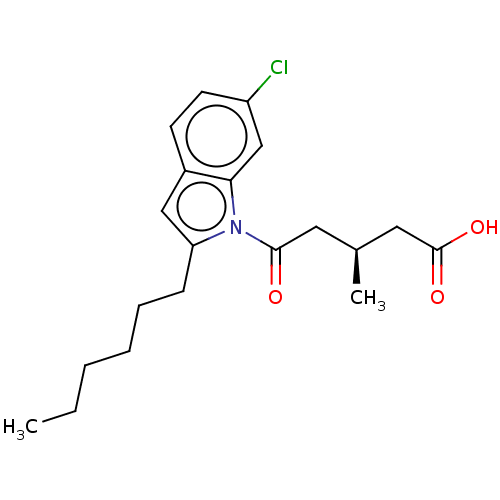 (CHEMBL3341973) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as inhibition of calcium mobilization by fluorescence assay | ACS Med Chem Lett 5: 815-9 (2014) Article DOI: 10.1021/ml500161v BindingDB Entry DOI: 10.7270/Q2H70HHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50054794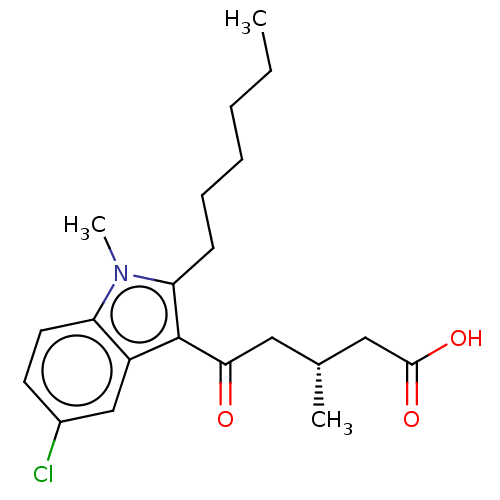 (CHEMBL3341971) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as inhibition of calcium mobilization by fluorescence assay | ACS Med Chem Lett 5: 815-9 (2014) Article DOI: 10.1021/ml500161v BindingDB Entry DOI: 10.7270/Q2H70HHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals Inc. | Assay Description R-SAT assays were performed as described previously (Weiner et al., 2001), with the following modifications. In brief, NIH-3T3 cells were grown to 80... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50024458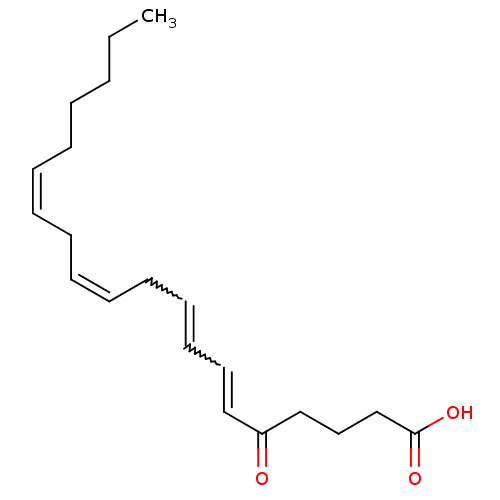 (5-oxo-ETE | CHEMBL18028 | ETE-5-Oxo) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Desensitization of 5-oxo-ETE receptor in indo-1-labeled human neutrophils assessed as inhibition of 5-oxo-ETE-induced calcium mobilization | Bioorg Med Chem Lett 21: 1987-90 (2011) Article DOI: 10.1016/j.bmcl.2011.02.021 BindingDB Entry DOI: 10.7270/Q2XW4K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM35948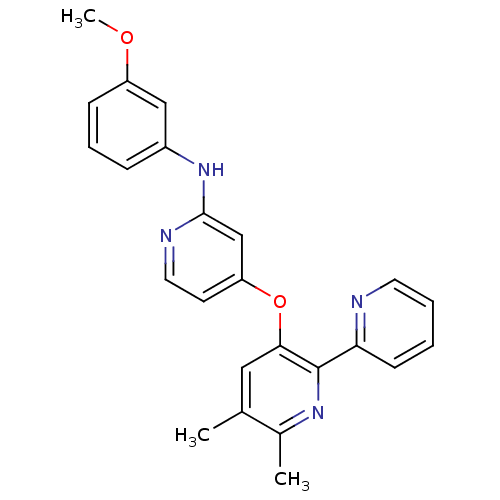 (4-pyridinoxy-2-anilinopyridine-based compound, 12) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | 32 | n/a | n/a | 7.4 | 23 |
AstraZeneca | Assay Description A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... | J Med Chem 52: 7901-5 (2009) Article DOI: 10.1021/jm900807w BindingDB Entry DOI: 10.7270/Q2KS6PXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals Inc. | Assay Description R-SAT assays were performed as described previously (Weiner et al., 2001), with the following modifications. In brief, NIH-3T3 cells were grown to 80... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50054793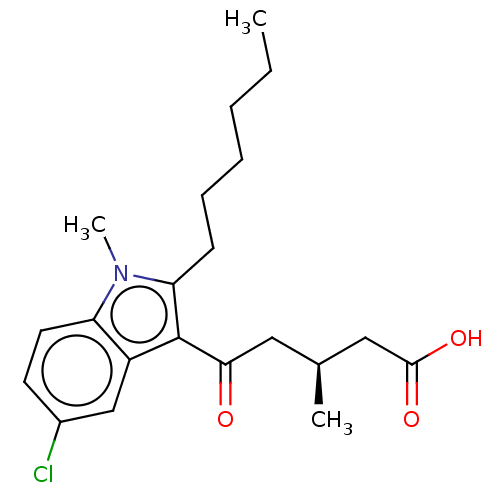 (CHEMBL3341972) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as inhibition of calcium mobilization by fluorescence assay | ACS Med Chem Lett 5: 815-9 (2014) Article DOI: 10.1021/ml500161v BindingDB Entry DOI: 10.7270/Q2H70HHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50054793 (CHEMBL3341972) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as inhibition of 5-oxo-ETE-induced calcium mobilization incubated for 2 mins follow... | J Med Chem 61: 5934-5948 (2018) Article DOI: 10.1021/acs.jmedchem.8b00154 BindingDB Entry DOI: 10.7270/Q22F7R48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50446952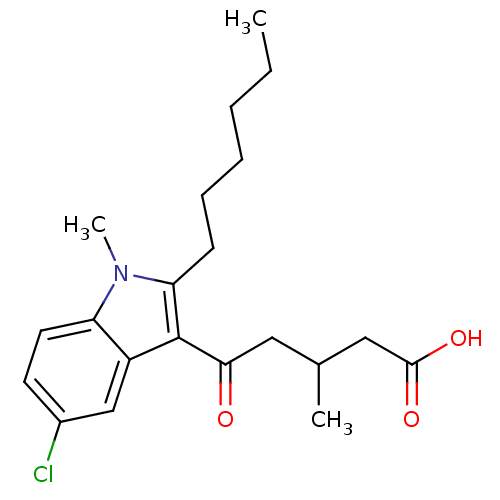 (CHEMBL3115775) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor (unknown origin) expressed in indo-1 loaded neutrophils assessed as inhibition of 5-oxo-ETE-induced calcium mobil... | J Med Chem 57: 364-77 (2014) Article DOI: 10.1021/jm401292m BindingDB Entry DOI: 10.7270/Q2N87C82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50054793 (CHEMBL3341972) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Claude Pepper Institute and Department of Chemistry, Florida Institute of Technology, 150 West University Boulevard, Melbourne, FL 32901, USA. Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as inhibition of 5-oxo-ETE-induced calcium mobilization incubated for 2 mins follow... | Bioorg Med Chem Lett 27: 4770-4776 (2017) Article DOI: 10.1016/j.bmcl.2017.08.034 BindingDB Entry DOI: 10.7270/Q2W098DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM35949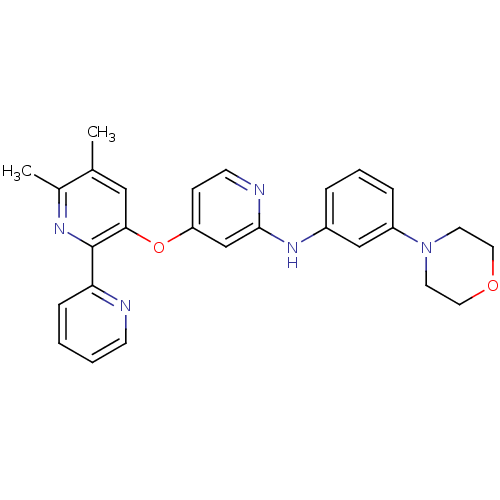 (4-pyridinoxy-2-anilinopyridine-based compound, 13) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | 62 | n/a | n/a | 7.4 | 23 |
AstraZeneca | Assay Description A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... | J Med Chem 52: 7901-5 (2009) Article DOI: 10.1021/jm900807w BindingDB Entry DOI: 10.7270/Q2KS6PXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50446953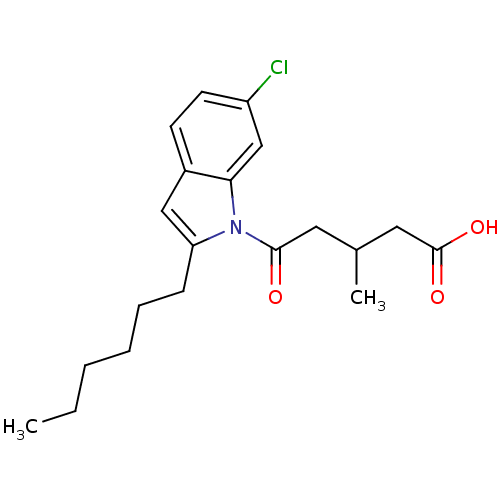 (CHEMBL3115774) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as inhibition of calcium mobilization by fluorescence assay | ACS Med Chem Lett 5: 815-9 (2014) Article DOI: 10.1021/ml500161v BindingDB Entry DOI: 10.7270/Q2H70HHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM35946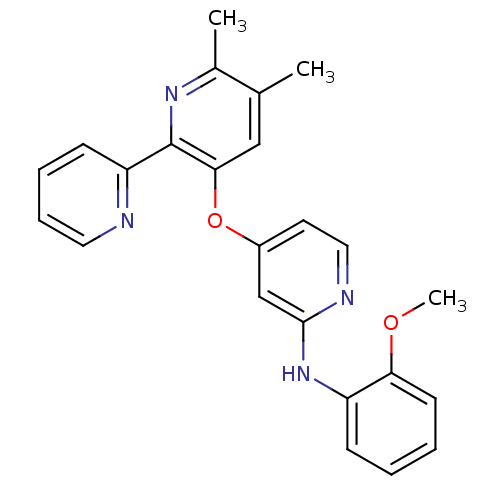 (4-pyridinoxy-2-anilinopyridine-based compound, 10) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | 114 | n/a | n/a | 7.4 | 23 |
AstraZeneca | Assay Description A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... | J Med Chem 52: 7901-5 (2009) Article DOI: 10.1021/jm900807w BindingDB Entry DOI: 10.7270/Q2KS6PXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50514979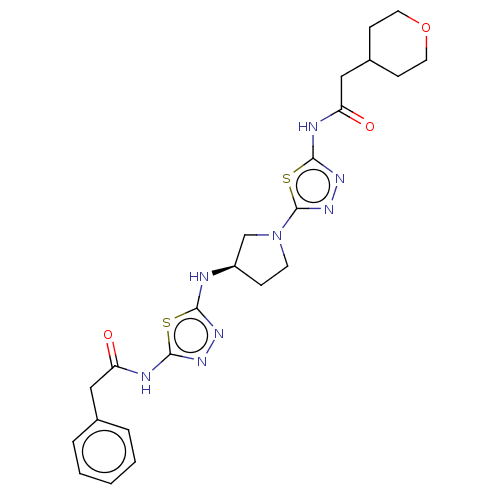 (CHEMBL4457936) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50446953 (CHEMBL3115774) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor (unknown origin) expressed in indo-1 loaded neutrophils assessed as inhibition of 5-oxo-ETE-induced calcium mobil... | J Med Chem 57: 364-77 (2014) Article DOI: 10.1021/jm401292m BindingDB Entry DOI: 10.7270/Q2N87C82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM109086 (US10793535, Cmpd ID 727 | US8604016, 670 | US99382...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50341133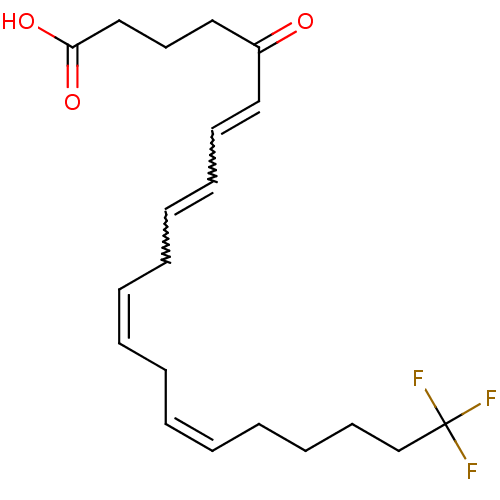 ((6E,8Z,11Z,14Z)-20,20,20-trifluoro-5-oxoicosa-6,8,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Desensitization of 5-oxo-ETE receptor in indo-1-labeled human neutrophils assessed as inhibition of 5-oxo-ETE-induced calcium mobilization | Bioorg Med Chem Lett 21: 1987-90 (2011) Article DOI: 10.1016/j.bmcl.2011.02.021 BindingDB Entry DOI: 10.7270/Q2XW4K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50514986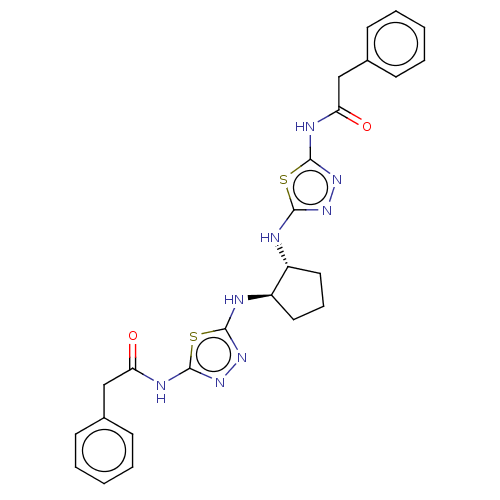 (CHEMBL4437956) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50514985 (CHEMBL4573635) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50465692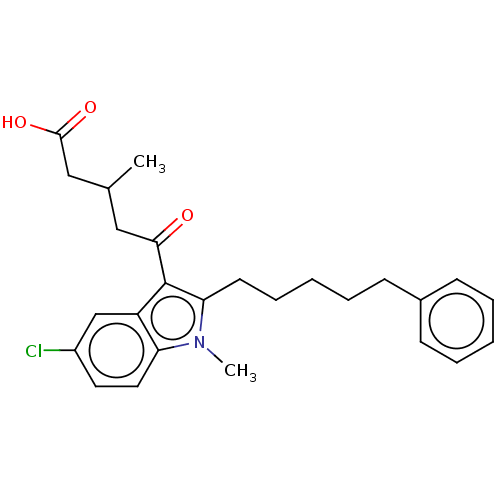 (CHEMBL4284672) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as inhibition of 5-oxo-ETE-induced calcium mobilization incubated for 2 mins follow... | J Med Chem 61: 5934-5948 (2018) Article DOI: 10.1021/acs.jmedchem.8b00154 BindingDB Entry DOI: 10.7270/Q22F7R48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50465681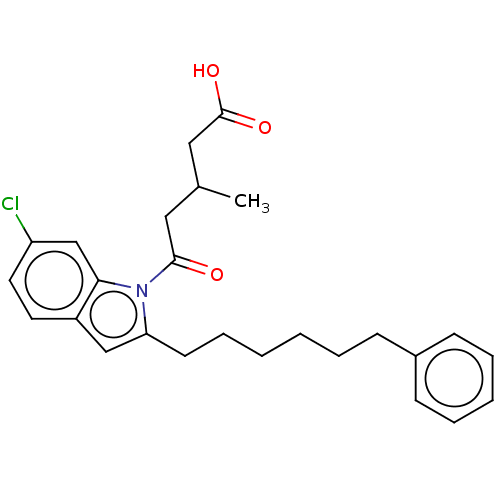 (CHEMBL4277299) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as inhibition of 5-oxo-ETE-induced calcium mobilization incubated for 2 mins follow... | J Med Chem 61: 5934-5948 (2018) Article DOI: 10.1021/acs.jmedchem.8b00154 BindingDB Entry DOI: 10.7270/Q22F7R48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM35953 (4-pyridinoxy-2-anilinopyridine-based compound, 17) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | 31 | n/a | n/a | 7.4 | 23 |
AstraZeneca | Assay Description A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... | J Med Chem 52: 7901-5 (2009) Article DOI: 10.1021/jm900807w BindingDB Entry DOI: 10.7270/Q2KS6PXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM35941 (4-pyridinoxy-2-anilinopyridine-based compound, 5) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | 20 | n/a | n/a | 7.4 | 23 |
AstraZeneca | Assay Description A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... | J Med Chem 52: 7901-5 (2009) Article DOI: 10.1021/jm900807w BindingDB Entry DOI: 10.7270/Q2KS6PXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM22869 (6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals Inc. | Assay Description R-SAT assays were performed as described previously (Weiner et al., 2001), with the following modifications. In brief, NIH-3T3 cells were grown to 80... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50514975 (CHEMBL4473143) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50150109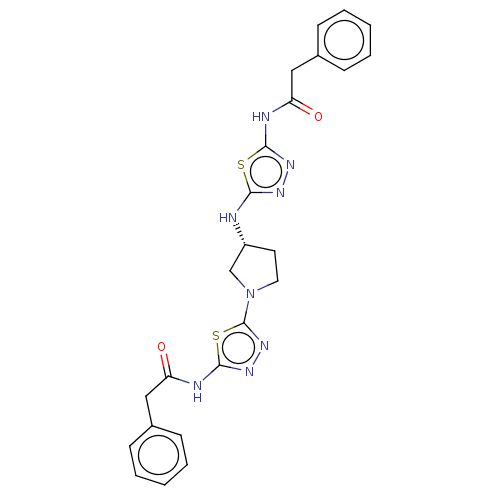 (CHEMBL3770355) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50446952 (CHEMBL3115775) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Claude Pepper Institute and Department of Chemistry, Florida Institute of Technology, 150 West University Boulevard, Melbourne, FL 32901, USA. Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as inhibition of 5-oxo-ETE-induced calcium mobilization incubated for 2 mins follow... | Bioorg Med Chem Lett 27: 4770-4776 (2017) Article DOI: 10.1016/j.bmcl.2017.08.034 BindingDB Entry DOI: 10.7270/Q2W098DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50514977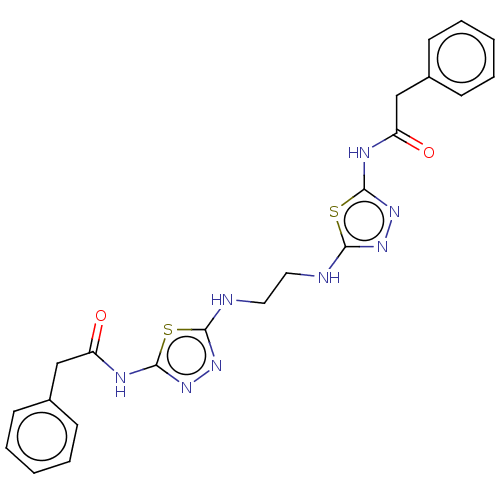 (CHEMBL4461749) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50446953 (CHEMBL3115774) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as inhibition of 5-oxo-ETE-induced calcium mobilization incubated for 2 mins follow... | J Med Chem 61: 5934-5948 (2018) Article DOI: 10.1021/acs.jmedchem.8b00154 BindingDB Entry DOI: 10.7270/Q22F7R48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50446952 (CHEMBL3115775) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as inhibition of 5-oxo-ETE-induced calcium mobilization incubated for 2 mins follow... | J Med Chem 61: 5934-5948 (2018) Article DOI: 10.1021/acs.jmedchem.8b00154 BindingDB Entry DOI: 10.7270/Q22F7R48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50514982 (CHEMBL4469711) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxoeicosanoid receptor 1 (Homo sapiens (Human)) | BDBM50465695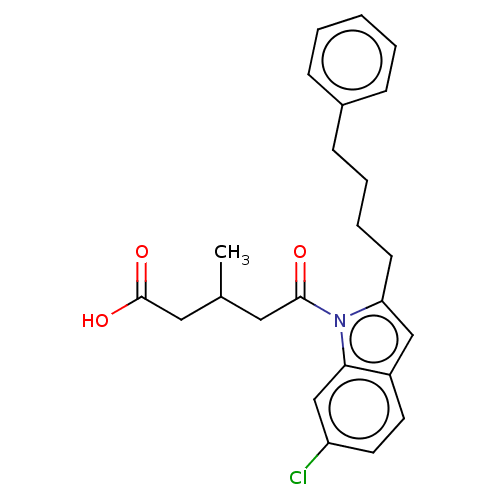 (CHEMBL4291346) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Institute of Technology Curated by ChEMBL | Assay Description Antagonist activity at OXE receptor in human neutrophils assessed as inhibition of 5-oxo-ETE-induced calcium mobilization incubated for 2 mins follow... | J Med Chem 61: 5934-5948 (2018) Article DOI: 10.1021/acs.jmedchem.8b00154 BindingDB Entry DOI: 10.7270/Q22F7R48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 188 total ) | Next | Last >> |