 Found 9453 hits with Last Name = 'subramani' and Initial = 's'
Found 9453 hits with Last Name = 'subramani' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50448583
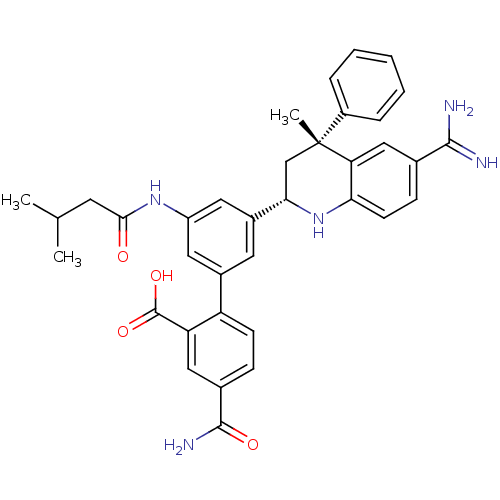
(CHEMBL3127491)Show SMILES CC(C)CC(=O)Nc1cc(cc(c1)-c1ccc(cc1C(O)=O)C(N)=O)[C@@H]1C[C@](C)(c2ccccc2)c2cc(ccc2N1)C(N)=N |r| Show InChI InChI=1S/C36H37N5O4/c1-20(2)13-32(42)40-26-15-23(27-11-9-22(34(39)43)17-28(27)35(44)45)14-24(16-26)31-19-36(3,25-7-5-4-6-8-25)29-18-21(33(37)38)10-12-30(29)41-31/h4-12,14-18,20,31,41H,13,19H2,1-3H3,(H3,37,38)(H2,39,43)(H,40,42)(H,44,45)/t31-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032873
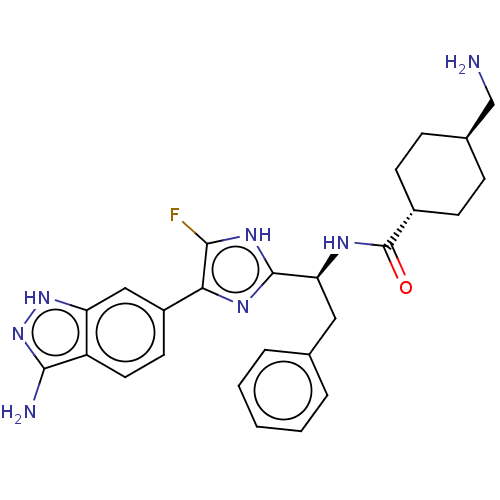
(CHEMBL3355684)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(F)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(38,-31.47,;39.32,-32.23,;39.32,-33.76,;40.64,-31.46,;41.96,-32.23,;40.64,-29.94,;41.95,-30.68,;15.71,-45.09,;16.47,-43.75,;18.01,-43.75,;18.79,-45.09,;20.33,-45.09,;21.09,-43.75,;20.33,-42.42,;18.79,-42.42,;22.63,-43.75,;23.41,-45.09,;23.41,-42.42,;24.95,-42.42,;25.71,-43.75,;27.25,-43.75,;28.03,-42.42,;29.57,-42.42,;30.33,-43.75,;29.57,-45.09,;28.03,-45.09,;25.71,-41.09,;25.09,-39.68,;26.23,-38.65,;27.56,-39.42,;28.97,-38.79,;27.24,-40.93,;26.07,-37.12,;27.32,-36.22,;27.16,-34.69,;25.75,-34.05,;25.27,-32.59,;26.18,-31.34,;23.73,-32.59,;23.25,-34.05,;24.5,-34.96,;24.66,-36.49,)| Show InChI InChI=1S/C26H30FN7O.C2HF3O2/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17;3-2(4,5)1(6)7/h1-5,10-11,13,16-17,21H,6-9,12,14,28H2,(H,30,35)(H,31,32)(H3,29,33,34);(H,6,7)/t16-,17-,21-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032874
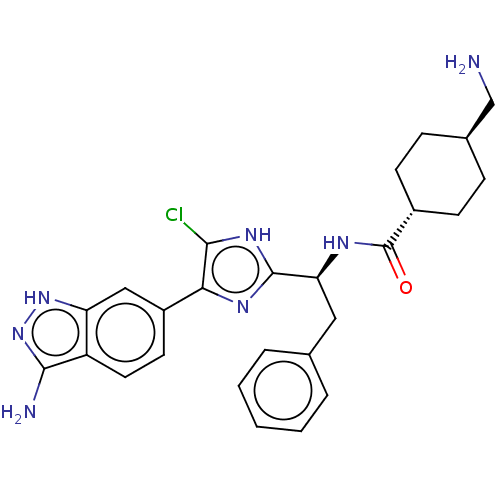
(CHEMBL3355683)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(34.53,-30.97,;35.85,-31.73,;35.85,-33.26,;37.17,-30.97,;38.49,-31.73,;37.17,-29.44,;38.49,-30.19,;15.69,-45.08,;16.46,-43.74,;18,-43.74,;18.77,-45.08,;20.31,-45.08,;21.08,-43.74,;20.31,-42.42,;18.77,-42.42,;22.62,-43.74,;23.39,-45.08,;23.39,-42.42,;24.93,-42.42,;25.7,-43.74,;27.24,-43.74,;28.01,-42.42,;29.55,-42.42,;30.32,-43.74,;29.55,-45.08,;28.01,-45.08,;25.7,-41.08,;25.07,-39.67,;26.21,-38.64,;27.55,-39.42,;28.96,-38.78,;27.23,-40.92,;26.05,-37.11,;27.3,-36.21,;27.14,-34.68,;25.73,-34.05,;25.26,-32.58,;26.16,-31.33,;23.72,-32.58,;23.24,-34.05,;24.49,-34.96,;24.65,-36.49,)| Show InChI InChI=1S/C26H30ClN7O.C2HF3O2/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17;3-2(4,5)1(6)7/h1-5,10-11,13,16-17,21H,6-9,12,14,28H2,(H,30,35)(H,31,32)(H3,29,33,34);(H,6,7)/t16-,17-,21-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032874

(CHEMBL3355683)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(34.53,-30.97,;35.85,-31.73,;35.85,-33.26,;37.17,-30.97,;38.49,-31.73,;37.17,-29.44,;38.49,-30.19,;15.69,-45.08,;16.46,-43.74,;18,-43.74,;18.77,-45.08,;20.31,-45.08,;21.08,-43.74,;20.31,-42.42,;18.77,-42.42,;22.62,-43.74,;23.39,-45.08,;23.39,-42.42,;24.93,-42.42,;25.7,-43.74,;27.24,-43.74,;28.01,-42.42,;29.55,-42.42,;30.32,-43.74,;29.55,-45.08,;28.01,-45.08,;25.7,-41.08,;25.07,-39.67,;26.21,-38.64,;27.55,-39.42,;28.96,-38.78,;27.23,-40.92,;26.05,-37.11,;27.3,-36.21,;27.14,-34.68,;25.73,-34.05,;25.26,-32.58,;26.16,-31.33,;23.72,-32.58,;23.24,-34.05,;24.49,-34.96,;24.65,-36.49,)| Show InChI InChI=1S/C26H30ClN7O.C2HF3O2/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17;3-2(4,5)1(6)7/h1-5,10-11,13,16-17,21H,6-9,12,14,28H2,(H,30,35)(H,31,32)(H3,29,33,34);(H,6,7)/t16-,17-,21-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis at 37 degC by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50448583

(CHEMBL3127491)Show SMILES CC(C)CC(=O)Nc1cc(cc(c1)-c1ccc(cc1C(O)=O)C(N)=O)[C@@H]1C[C@](C)(c2ccccc2)c2cc(ccc2N1)C(N)=N |r| Show InChI InChI=1S/C36H37N5O4/c1-20(2)13-32(42)40-26-15-23(27-11-9-22(34(39)43)17-28(27)35(44)45)14-24(16-26)31-19-36(3,25-7-5-4-6-8-25)29-18-21(33(37)38)10-12-30(29)41-31/h4-12,14-18,20,31,41H,13,19H2,1-3H3,(H3,37,38)(H2,39,43)(H,40,42)(H,44,45)/t31-,36+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50032873

(CHEMBL3355684)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(F)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(38,-31.47,;39.32,-32.23,;39.32,-33.76,;40.64,-31.46,;41.96,-32.23,;40.64,-29.94,;41.95,-30.68,;15.71,-45.09,;16.47,-43.75,;18.01,-43.75,;18.79,-45.09,;20.33,-45.09,;21.09,-43.75,;20.33,-42.42,;18.79,-42.42,;22.63,-43.75,;23.41,-45.09,;23.41,-42.42,;24.95,-42.42,;25.71,-43.75,;27.25,-43.75,;28.03,-42.42,;29.57,-42.42,;30.33,-43.75,;29.57,-45.09,;28.03,-45.09,;25.71,-41.09,;25.09,-39.68,;26.23,-38.65,;27.56,-39.42,;28.97,-38.79,;27.24,-40.93,;26.07,-37.12,;27.32,-36.22,;27.16,-34.69,;25.75,-34.05,;25.27,-32.59,;26.18,-31.34,;23.73,-32.59,;23.25,-34.05,;24.5,-34.96,;24.66,-36.49,)| Show InChI InChI=1S/C26H30FN7O.C2HF3O2/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17;3-2(4,5)1(6)7/h1-5,10-11,13,16-17,21H,6-9,12,14,28H2,(H,30,35)(H,31,32)(H3,29,33,34);(H,6,7)/t16-,17-,21-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032876

(CHEMBL3355681)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(F)[nH]1)-c1ccc(cc1)C(N)=O |r,wU:18.18,12.14,wD:9.7,(29.83,-22.98,;31.17,-23.75,;31.17,-25.29,;32.5,-22.98,;33.84,-23.75,;33.83,-22.19,;32.5,-21.43,;13.75,-29.96,;15.08,-30.74,;16.42,-29.97,;17.76,-30.75,;19.1,-29.98,;19.09,-28.44,;17.76,-27.67,;16.42,-28.43,;20.42,-27.67,;20.42,-26.13,;21.76,-28.44,;23.09,-27.67,;23.09,-26.13,;24.43,-25.36,;25.76,-26.13,;27.09,-25.36,;27.1,-23.82,;25.75,-23.05,;24.42,-23.82,;24.43,-28.44,;25.83,-27.81,;26.86,-28.95,;26.09,-30.29,;26.72,-31.7,;24.58,-29.97,;28.39,-28.8,;29.01,-27.39,;30.53,-27.23,;31.45,-28.47,;30.82,-29.88,;29.29,-30.04,;32.98,-28.31,;33.89,-29.55,;33.61,-26.9,)| Show InChI InChI=1S/C26H30FN5O2.C2HF3O2/c27-23-22(18-10-12-19(13-11-18)24(29)33)31-25(32-23)21(14-16-4-2-1-3-5-16)30-26(34)20-8-6-17(15-28)7-9-20;3-2(4,5)1(6)7/h1-5,10-13,17,20-21H,6-9,14-15,28H2,(H2,29,33)(H,30,34)(H,31,32);(H,6,7)/t17-,20-,21-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032875

(CHEMBL3355682)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(39.15,-34.6,;40.47,-35.36,;40.47,-36.89,;41.79,-34.6,;43.11,-35.36,;41.79,-33.07,;43.11,-33.82,;26.66,-34.02,;27.43,-35.36,;26.66,-36.68,;25.12,-36.68,;24.35,-38.02,;25.12,-39.35,;26.66,-39.35,;27.43,-38.02,;24.35,-40.69,;22.81,-40.69,;25.12,-42.02,;24.35,-43.36,;22.81,-43.36,;22.04,-44.69,;22.81,-46.02,;22.04,-47.35,;20.5,-47.35,;19.73,-46.02,;20.5,-44.69,;25.12,-44.69,;26.65,-44.85,;26.97,-46.36,;25.64,-47.13,;24.49,-46.1,;28.38,-46.98,;28.54,-48.51,;29.95,-49.15,;31.19,-48.24,;32.7,-48.56,;33.32,-49.96,;33.47,-47.22,;32.44,-46.08,;31.03,-46.71,;29.63,-46.08,)| Show InChI InChI=1S/C26H31N7O.C2HF3O2/c27-14-17-6-8-18(9-7-17)26(34)31-22(12-16-4-2-1-3-5-16)25-29-15-23(30-25)19-10-11-20-21(13-19)32-33-24(20)28;3-2(4,5)1(6)7/h1-5,10-11,13,15,17-18,22H,6-9,12,14,27H2,(H,29,30)(H,31,34)(H3,28,32,33);(H,6,7)/t17-,18-,22-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM24621
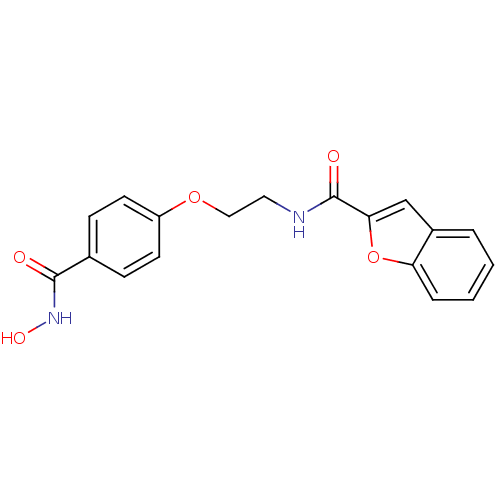
(CG-003 | N-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}-...)Show InChI InChI=1S/C18H16N2O5/c21-17(20-23)12-5-7-14(8-6-12)24-10-9-19-18(22)16-11-13-3-1-2-4-15(13)25-16/h1-8,11,23H,9-10H2,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032877
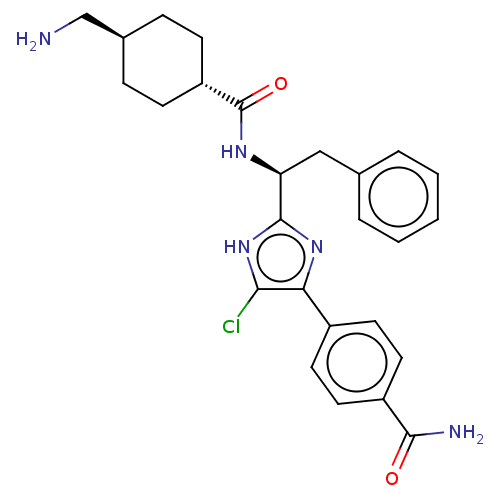
(CHEMBL3355680)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccc(cc1)C(N)=O |r,wU:18.18,12.14,wD:9.7,(29.83,-22.98,;31.17,-23.75,;31.17,-25.29,;32.5,-22.98,;33.84,-23.75,;33.83,-22.19,;32.5,-21.43,;13.75,-29.96,;15.08,-30.74,;16.42,-29.97,;17.76,-30.75,;19.1,-29.98,;19.09,-28.44,;17.76,-27.67,;16.42,-28.43,;20.42,-27.67,;20.42,-26.13,;21.76,-28.44,;23.09,-27.67,;23.09,-26.13,;24.43,-25.36,;25.76,-26.13,;27.09,-25.36,;27.1,-23.82,;25.75,-23.05,;24.42,-23.82,;24.43,-28.44,;25.83,-27.81,;26.86,-28.95,;26.09,-30.29,;26.72,-31.7,;24.58,-29.97,;28.39,-28.8,;29.01,-27.39,;30.53,-27.23,;31.45,-28.47,;30.82,-29.88,;29.29,-30.04,;32.98,-28.31,;33.89,-29.55,;33.61,-26.9,)| Show InChI InChI=1S/C26H30ClN5O2.C2HF3O2/c27-23-22(18-10-12-19(13-11-18)24(29)33)31-25(32-23)21(14-16-4-2-1-3-5-16)30-26(34)20-8-6-17(15-28)7-9-20;3-2(4,5)1(6)7/h1-5,10-13,17,20-21H,6-9,14-15,28H2,(H2,29,33)(H,30,34)(H,31,32);(H,6,7)/t17-,20-,21-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50032874

(CHEMBL3355683)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(34.53,-30.97,;35.85,-31.73,;35.85,-33.26,;37.17,-30.97,;38.49,-31.73,;37.17,-29.44,;38.49,-30.19,;15.69,-45.08,;16.46,-43.74,;18,-43.74,;18.77,-45.08,;20.31,-45.08,;21.08,-43.74,;20.31,-42.42,;18.77,-42.42,;22.62,-43.74,;23.39,-45.08,;23.39,-42.42,;24.93,-42.42,;25.7,-43.74,;27.24,-43.74,;28.01,-42.42,;29.55,-42.42,;30.32,-43.74,;29.55,-45.08,;28.01,-45.08,;25.7,-41.08,;25.07,-39.67,;26.21,-38.64,;27.55,-39.42,;28.96,-38.78,;27.23,-40.92,;26.05,-37.11,;27.3,-36.21,;27.14,-34.68,;25.73,-34.05,;25.26,-32.58,;26.16,-31.33,;23.72,-32.58,;23.24,-34.05,;24.49,-34.96,;24.65,-36.49,)| Show InChI InChI=1S/C26H30ClN7O.C2HF3O2/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17;3-2(4,5)1(6)7/h1-5,10-11,13,16-17,21H,6-9,12,14,28H2,(H,30,35)(H,31,32)(H3,29,33,34);(H,6,7)/t16-,17-,21-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM24620
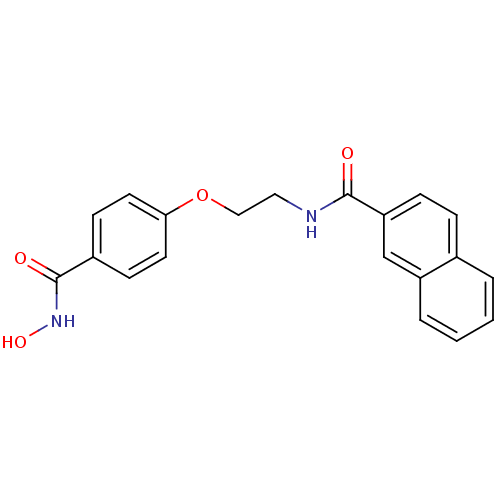
(CG-002 | N-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}n...)Show InChI InChI=1S/C20H18N2O4/c23-19(17-6-5-14-3-1-2-4-16(14)13-17)21-11-12-26-18-9-7-15(8-10-18)20(24)22-25/h1-10,13,25H,11-12H2,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM424912
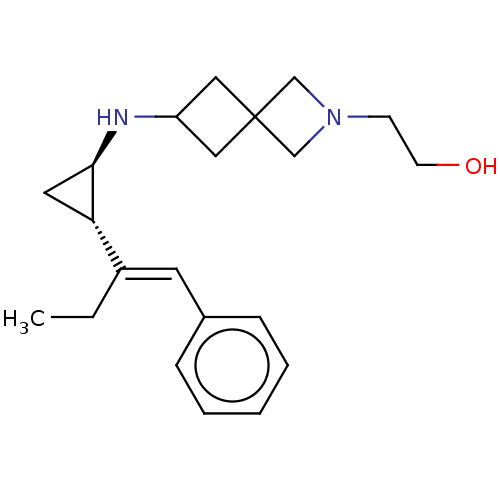
(2-(6-(((1R,2S)-2-((E)-1-phenylbut-1-en-2-yl)cyclop...)Show SMILES CC\C(=C/c1ccccc1)[C@@H]1C[C@H]1NC1CC2(C1)CN(CCO)C2 |r| Show InChI InChI=1S/C21H30N2O/c1-2-17(10-16-6-4-3-5-7-16)19-11-20(19)22-18-12-21(13-18)14-23(15-21)8-9-24/h3-7,10,18-20,22,24H,2,8-9,11-15H2,1H3/b17-10+/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Time dependant inhibition of recombinant N-terminal His-tagged LSD1 (unknown origin) expressed in Escherichia coli expression system assessed as inhi... |
ACS Med Chem Lett 11: 1213-1220 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00060
BindingDB Entry DOI: 10.7270/Q22N55VZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | -46.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50032876

(CHEMBL3355681)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(F)[nH]1)-c1ccc(cc1)C(N)=O |r,wU:18.18,12.14,wD:9.7,(29.83,-22.98,;31.17,-23.75,;31.17,-25.29,;32.5,-22.98,;33.84,-23.75,;33.83,-22.19,;32.5,-21.43,;13.75,-29.96,;15.08,-30.74,;16.42,-29.97,;17.76,-30.75,;19.1,-29.98,;19.09,-28.44,;17.76,-27.67,;16.42,-28.43,;20.42,-27.67,;20.42,-26.13,;21.76,-28.44,;23.09,-27.67,;23.09,-26.13,;24.43,-25.36,;25.76,-26.13,;27.09,-25.36,;27.1,-23.82,;25.75,-23.05,;24.42,-23.82,;24.43,-28.44,;25.83,-27.81,;26.86,-28.95,;26.09,-30.29,;26.72,-31.7,;24.58,-29.97,;28.39,-28.8,;29.01,-27.39,;30.53,-27.23,;31.45,-28.47,;30.82,-29.88,;29.29,-30.04,;32.98,-28.31,;33.89,-29.55,;33.61,-26.9,)| Show InChI InChI=1S/C26H30FN5O2.C2HF3O2/c27-23-22(18-10-12-19(13-11-18)24(29)33)31-25(32-23)21(14-16-4-2-1-3-5-16)30-26(34)20-8-6-17(15-28)7-9-20;3-2(4,5)1(6)7/h1-5,10-13,17,20-21H,6-9,14-15,28H2,(H2,29,33)(H,30,34)(H,31,32);(H,6,7)/t17-,20-,21-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032878
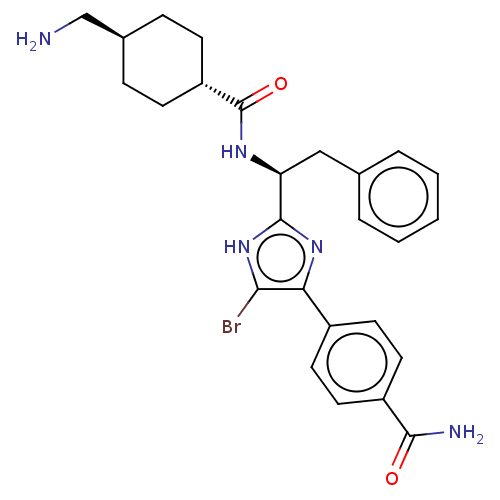
(CHEMBL3355679)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Br)[nH]1)-c1ccc(cc1)C(N)=O |r,wU:18.18,12.14,wD:9.7,(29.77,-17.68,;31.11,-18.45,;31.11,-19.99,;32.44,-17.68,;33.78,-18.45,;33.77,-16.89,;32.44,-16.14,;13.69,-24.66,;15.02,-25.44,;16.36,-24.67,;17.7,-25.45,;19.04,-24.68,;19.03,-23.14,;17.7,-22.37,;16.36,-23.13,;20.36,-22.37,;20.36,-20.83,;21.69,-23.14,;23.03,-22.37,;23.03,-20.83,;24.37,-20.06,;25.7,-20.83,;27.03,-20.07,;27.03,-18.52,;25.69,-17.75,;24.36,-18.53,;24.36,-23.14,;25.77,-22.51,;26.8,-23.65,;26.03,-24.99,;26.66,-26.41,;24.52,-24.67,;28.32,-23.5,;28.94,-22.09,;30.47,-21.93,;31.39,-23.18,;30.76,-24.58,;29.23,-24.74,;32.92,-23.01,;33.83,-24.25,;33.55,-21.6,)| Show InChI InChI=1S/C26H30BrN5O2.C2HF3O2/c27-23-22(18-10-12-19(13-11-18)24(29)33)31-25(32-23)21(14-16-4-2-1-3-5-16)30-26(34)20-8-6-17(15-28)7-9-20;3-2(4,5)1(6)7/h1-5,10-13,17,20-21H,6-9,14-15,28H2,(H2,29,33)(H,30,34)(H,31,32);(H,6,7)/t17-,20-,21-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50158869
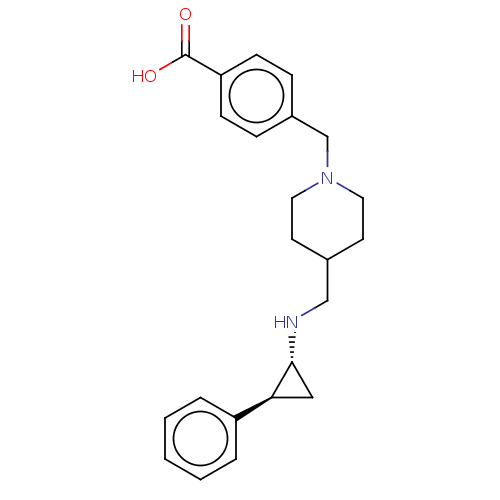
(CHEMBL3786182 | US10836743, Compound GSK-2879552 |...)Show SMILES OC(=O)c1ccc(CN2CCC(CN[C@@H]3C[C@H]3c3ccccc3)CC2)cc1 |r| Show InChI InChI=1S/C23H28N2O2/c26-23(27)20-8-6-18(7-9-20)16-25-12-10-17(11-13-25)15-24-22-14-21(22)19-4-2-1-3-5-19/h1-9,17,21-22,24H,10-16H2,(H,26,27)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Time dependant inhibition of recombinant N-terminal His-tagged LSD1 (unknown origin) expressed in Escherichia coli expression system assessed as inhi... |
ACS Med Chem Lett 11: 1213-1220 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00060
BindingDB Entry DOI: 10.7270/Q22N55VZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50032877

(CHEMBL3355680)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccc(cc1)C(N)=O |r,wU:18.18,12.14,wD:9.7,(29.83,-22.98,;31.17,-23.75,;31.17,-25.29,;32.5,-22.98,;33.84,-23.75,;33.83,-22.19,;32.5,-21.43,;13.75,-29.96,;15.08,-30.74,;16.42,-29.97,;17.76,-30.75,;19.1,-29.98,;19.09,-28.44,;17.76,-27.67,;16.42,-28.43,;20.42,-27.67,;20.42,-26.13,;21.76,-28.44,;23.09,-27.67,;23.09,-26.13,;24.43,-25.36,;25.76,-26.13,;27.09,-25.36,;27.1,-23.82,;25.75,-23.05,;24.42,-23.82,;24.43,-28.44,;25.83,-27.81,;26.86,-28.95,;26.09,-30.29,;26.72,-31.7,;24.58,-29.97,;28.39,-28.8,;29.01,-27.39,;30.53,-27.23,;31.45,-28.47,;30.82,-29.88,;29.29,-30.04,;32.98,-28.31,;33.89,-29.55,;33.61,-26.9,)| Show InChI InChI=1S/C26H30ClN5O2.C2HF3O2/c27-23-22(18-10-12-19(13-11-18)24(29)33)31-25(32-23)21(14-16-4-2-1-3-5-16)30-26(34)20-8-6-17(15-28)7-9-20;3-2(4,5)1(6)7/h1-5,10-13,17,20-21H,6-9,14-15,28H2,(H2,29,33)(H,30,34)(H,31,32);(H,6,7)/t17-,20-,21-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468103
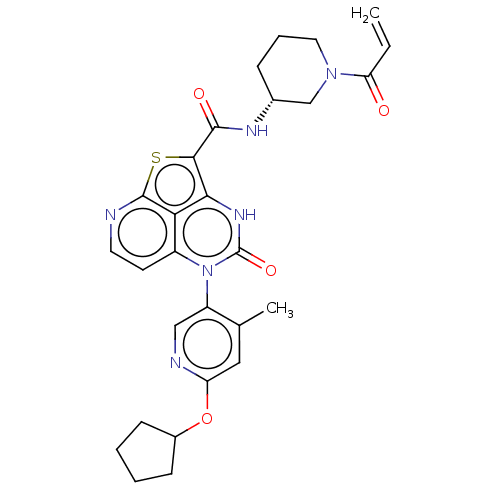
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(6-(cyclop...)Show SMILES Cc1cc(OC2CCCC2)ncc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:24.25,(-1.92,5.75,;-3.25,4.98,;-4.59,5.75,;-5.92,4.98,;-7.25,5.75,;-8.59,4.98,;-9.99,5.6,;-11.02,4.46,;-10.25,3.13,;-8.75,3.45,;-5.92,3.44,;-4.59,2.67,;-3.25,3.44,;-1.92,2.67,;-1.92,1.13,;-3.25,.36,;-3.25,-1.18,;-1.92,-1.95,;-.59,-1.18,;.88,-1.66,;1.78,-.41,;3.32,-.41,;4.09,.92,;4.09,-1.75,;5.63,-1.75,;6.4,-.41,;7.94,-.41,;8.71,-1.75,;7.94,-3.08,;6.4,-3.08,;8.71,-4.41,;7.94,-5.75,;10.25,-4.41,;11.02,-5.75,;.75,1.13,;.75,2.67,;-.59,3.44,;-.59,4.98,;-.59,.36,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Trypsin
(Homo sapiens (Human)) | BDBM50032874

(CHEMBL3355683)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(34.53,-30.97,;35.85,-31.73,;35.85,-33.26,;37.17,-30.97,;38.49,-31.73,;37.17,-29.44,;38.49,-30.19,;15.69,-45.08,;16.46,-43.74,;18,-43.74,;18.77,-45.08,;20.31,-45.08,;21.08,-43.74,;20.31,-42.42,;18.77,-42.42,;22.62,-43.74,;23.39,-45.08,;23.39,-42.42,;24.93,-42.42,;25.7,-43.74,;27.24,-43.74,;28.01,-42.42,;29.55,-42.42,;30.32,-43.74,;29.55,-45.08,;28.01,-45.08,;25.7,-41.08,;25.07,-39.67,;26.21,-38.64,;27.55,-39.42,;28.96,-38.78,;27.23,-40.92,;26.05,-37.11,;27.3,-36.21,;27.14,-34.68,;25.73,-34.05,;25.26,-32.58,;26.16,-31.33,;23.72,-32.58,;23.24,-34.05,;24.49,-34.96,;24.65,-36.49,)| Show InChI InChI=1S/C26H30ClN7O.C2HF3O2/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17;3-2(4,5)1(6)7/h1-5,10-11,13,16-17,21H,6-9,12,14,28H2,(H,30,35)(H,31,32)(H3,29,33,34);(H,6,7)/t16-,17-,21-;/m0./s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation trypsin using N-benzoyl-Ile-Glu-(OH, OMe)-Gly-Arg-pNA as substrate by spectrophotometric analysis |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468000
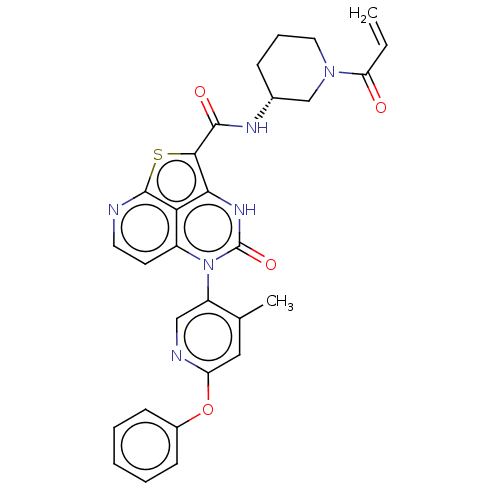
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(4-methyl-...)Show SMILES Cc1cc(Oc2ccccc2)ncc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wU:25.26,(-4.48,-.77,;-4.48,.77,;-5.82,1.54,;-5.82,3.08,;-7.15,3.85,;-8.48,3.08,;-9.82,3.85,;-11.15,3.08,;-11.15,1.54,;-9.82,.77,;-8.48,1.54,;-4.48,3.85,;-3.15,3.08,;-3.15,1.54,;-1.82,.77,;-1.82,-.77,;-3.15,-1.54,;-3.15,-3.08,;-1.82,-3.85,;-.48,-3.08,;1.01,-3.48,;1.94,-1.86,;3.48,-1.86,;4.25,-3.19,;4.25,-.53,;5.79,-.53,;6.56,-1.86,;8.1,-1.86,;8.87,-.53,;8.1,.81,;6.56,.81,;8.87,2.14,;8.1,3.48,;10.41,2.14,;11.15,3.43,;.85,-.77,;.85,.77,;-.48,1.54,;-.48,3.08,;-.48,-1.54,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467718
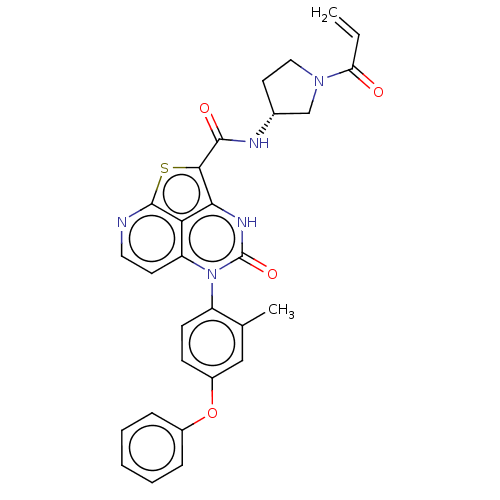
((R)-N-(1-Acryloylpyrrolidin-3-yl)-5-(2-methyl-4-(2...)Show SMILES Cc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:25.26,(-1,4.5,;-2.28,3.77,;-3.62,4.54,;-4.95,3.77,;-6.28,4.54,;-7.62,3.77,;-8.95,4.54,;-10.28,3.77,;-10.28,2.23,;-8.95,1.46,;-7.62,2.23,;-4.95,2.23,;-3.62,1.46,;-2.28,2.23,;-.95,1.46,;-.95,-.08,;-2.28,-.85,;-2.28,-2.39,;-.95,-3.16,;.39,-2.39,;1.85,-2.87,;2.76,-1.62,;4.29,-1.65,;5.09,-.33,;5.04,-3,;6.58,-3,;7.49,-1.75,;8.95,-2.23,;8.95,-3.77,;7.49,-4.24,;10.28,-4.54,;10.28,-6.08,;11.62,-3.77,;11.62,-2.23,;1.72,-.08,;1.72,1.46,;.39,2.23,;.39,3.77,;.39,-.85,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032858
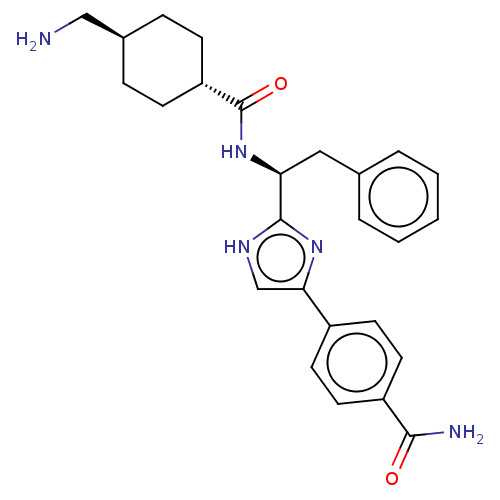
(CHEMBL3355670)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1ccc(cc1)C(N)=O |r,wU:18.18,12.14,wD:9.7,(24.17,-9.75,;25.51,-10.52,;25.51,-12.06,;26.84,-9.75,;28.17,-10.52,;28.17,-8.96,;26.84,-8.21,;8.49,-17.32,;9.81,-18.09,;11.15,-17.33,;12.49,-18.1,;13.83,-17.34,;13.83,-15.79,;12.5,-15.03,;11.16,-15.79,;15.16,-15.02,;15.16,-13.48,;16.49,-15.79,;17.83,-15.02,;17.83,-13.48,;19.16,-12.71,;20.49,-13.49,;21.83,-12.72,;21.83,-11.18,;20.49,-10.41,;19.16,-11.18,;19.16,-15.79,;20.56,-15.17,;21.59,-16.31,;20.82,-17.65,;19.32,-17.32,;23.12,-16.15,;23.74,-14.75,;25.27,-14.59,;26.18,-15.83,;25.55,-17.24,;24.02,-17.4,;27.71,-15.67,;28.62,-16.91,;28.34,-14.26,)| Show InChI InChI=1S/C26H31N5O2.C2HF3O2/c27-15-18-6-8-21(9-7-18)26(33)31-22(14-17-4-2-1-3-5-17)25-29-16-23(30-25)19-10-12-20(13-11-19)24(28)32;3-2(4,5)1(6)7/h1-5,10-13,16,18,21-22H,6-9,14-15,27H2,(H2,28,32)(H,29,30)(H,31,33);(H,6,7)/t18-,21-,22-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467367
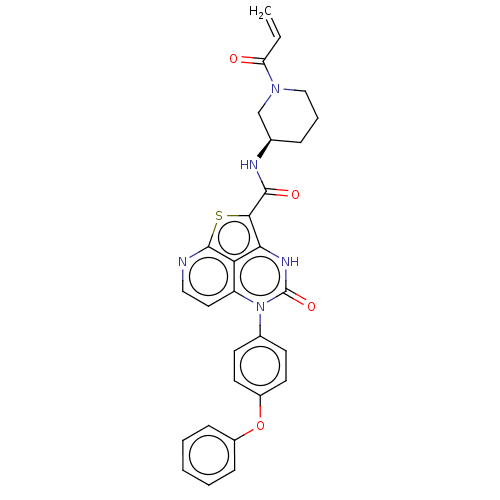
((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-(4-phenox...)Show SMILES C=CC(=O)N1CCC[C@H](C1)NC(=O)c1sc2nccc3n(-c4ccc(Oc5ccccc5)cc4)c(=O)[nH]c1c23 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM24618
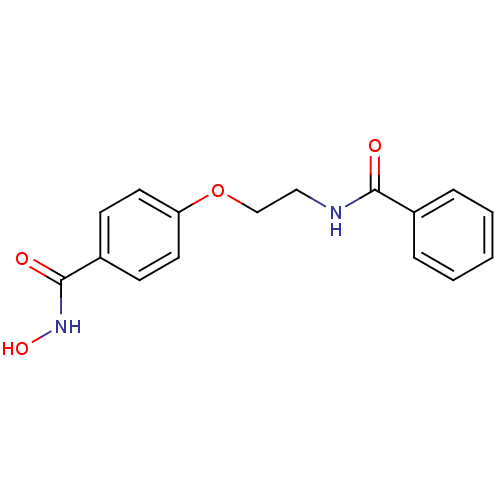
(CG-001 | N-hydroxy-4-[2-(phenylformamido)ethoxy]be...)Show InChI InChI=1S/C16H16N2O4/c19-15(12-4-2-1-3-5-12)17-10-11-22-14-8-6-13(7-9-14)16(20)18-21/h1-9,21H,10-11H2,(H,17,19)(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 43 | -41.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50032878

(CHEMBL3355679)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Br)[nH]1)-c1ccc(cc1)C(N)=O |r,wU:18.18,12.14,wD:9.7,(29.77,-17.68,;31.11,-18.45,;31.11,-19.99,;32.44,-17.68,;33.78,-18.45,;33.77,-16.89,;32.44,-16.14,;13.69,-24.66,;15.02,-25.44,;16.36,-24.67,;17.7,-25.45,;19.04,-24.68,;19.03,-23.14,;17.7,-22.37,;16.36,-23.13,;20.36,-22.37,;20.36,-20.83,;21.69,-23.14,;23.03,-22.37,;23.03,-20.83,;24.37,-20.06,;25.7,-20.83,;27.03,-20.07,;27.03,-18.52,;25.69,-17.75,;24.36,-18.53,;24.36,-23.14,;25.77,-22.51,;26.8,-23.65,;26.03,-24.99,;26.66,-26.41,;24.52,-24.67,;28.32,-23.5,;28.94,-22.09,;30.47,-21.93,;31.39,-23.18,;30.76,-24.58,;29.23,-24.74,;32.92,-23.01,;33.83,-24.25,;33.55,-21.6,)| Show InChI InChI=1S/C26H30BrN5O2.C2HF3O2/c27-23-22(18-10-12-19(13-11-18)24(29)33)31-25(32-23)21(14-16-4-2-1-3-5-16)30-26(34)20-8-6-17(15-28)7-9-20;3-2(4,5)1(6)7/h1-5,10-13,17,20-21H,6-9,14-15,28H2,(H2,29,33)(H,30,34)(H,31,32);(H,6,7)/t17-,20-,21-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50601991
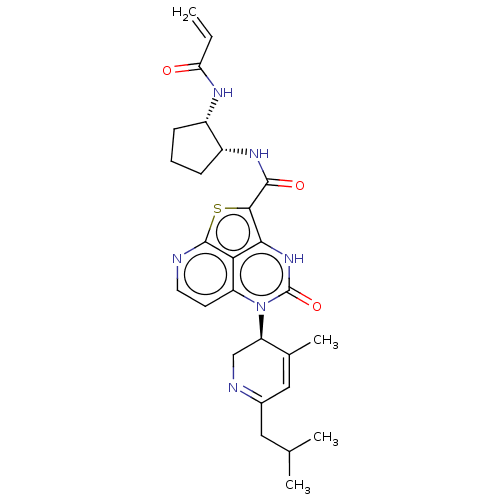
(CHEMBL5206366)Show SMILES CC(C)CC1=NC[C@H](C(C)=C1)n1c2ccnc3sc(C(=O)N[C@@H]4CCC[C@@H]4NC(=O)C=C)c([nH]c1=O)c23 |r,c:9,t:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50032875

(CHEMBL3355682)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(39.15,-34.6,;40.47,-35.36,;40.47,-36.89,;41.79,-34.6,;43.11,-35.36,;41.79,-33.07,;43.11,-33.82,;26.66,-34.02,;27.43,-35.36,;26.66,-36.68,;25.12,-36.68,;24.35,-38.02,;25.12,-39.35,;26.66,-39.35,;27.43,-38.02,;24.35,-40.69,;22.81,-40.69,;25.12,-42.02,;24.35,-43.36,;22.81,-43.36,;22.04,-44.69,;22.81,-46.02,;22.04,-47.35,;20.5,-47.35,;19.73,-46.02,;20.5,-44.69,;25.12,-44.69,;26.65,-44.85,;26.97,-46.36,;25.64,-47.13,;24.49,-46.1,;28.38,-46.98,;28.54,-48.51,;29.95,-49.15,;31.19,-48.24,;32.7,-48.56,;33.32,-49.96,;33.47,-47.22,;32.44,-46.08,;31.03,-46.71,;29.63,-46.08,)| Show InChI InChI=1S/C26H31N7O.C2HF3O2/c27-14-17-6-8-18(9-7-17)26(34)31-22(12-16-4-2-1-3-5-16)25-29-15-23(30-25)19-10-11-20-21(13-19)32-33-24(20)28;3-2(4,5)1(6)7/h1-5,10-11,13,15,17-18,22H,6-9,12,14,27H2,(H,29,30)(H,31,34)(H3,28,32,33);(H,6,7)/t17-,18-,22-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50032858

(CHEMBL3355670)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1ccc(cc1)C(N)=O |r,wU:18.18,12.14,wD:9.7,(24.17,-9.75,;25.51,-10.52,;25.51,-12.06,;26.84,-9.75,;28.17,-10.52,;28.17,-8.96,;26.84,-8.21,;8.49,-17.32,;9.81,-18.09,;11.15,-17.33,;12.49,-18.1,;13.83,-17.34,;13.83,-15.79,;12.5,-15.03,;11.16,-15.79,;15.16,-15.02,;15.16,-13.48,;16.49,-15.79,;17.83,-15.02,;17.83,-13.48,;19.16,-12.71,;20.49,-13.49,;21.83,-12.72,;21.83,-11.18,;20.49,-10.41,;19.16,-11.18,;19.16,-15.79,;20.56,-15.17,;21.59,-16.31,;20.82,-17.65,;19.32,-17.32,;23.12,-16.15,;23.74,-14.75,;25.27,-14.59,;26.18,-15.83,;25.55,-17.24,;24.02,-17.4,;27.71,-15.67,;28.62,-16.91,;28.34,-14.26,)| Show InChI InChI=1S/C26H31N5O2.C2HF3O2/c27-15-18-6-8-21(9-7-18)26(33)31-22(14-17-4-2-1-3-5-17)25-29-16-23(30-25)19-10-12-20(13-11-19)24(28)32;3-2(4,5)1(6)7/h1-5,10-13,16,18,21-22H,6-9,14-15,27H2,(H2,28,32)(H,29,30)(H,31,33);(H,6,7)/t18-,21-,22-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468010

((R)-N-(1-Acryloylpiperidin-3-yl)-5-(6-cyclobutoxy-...)Show SMILES Cc1cc(OC2CCC2)ncc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:23.24,(-1.8,6.31,;-3.13,5.54,;-4.47,6.31,;-5.8,5.54,;-7.14,6.31,;-8.47,5.54,;-9.96,5.93,;-10.36,4.45,;-8.87,4.05,;-5.8,4,;-4.47,3.23,;-3.13,4,;-1.8,3.23,;-1.8,1.69,;-3.13,.92,;-3.13,-.62,;-1.8,-1.39,;-.47,-.62,;1.14,-.89,;1.88,.36,;3.42,.36,;4.19,1.7,;4.19,-.97,;5.73,-.97,;6.5,.36,;8.04,.36,;8.82,-.97,;8.04,-2.3,;6.5,-2.3,;8.82,-3.64,;10.36,-3.64,;8.04,-4.97,;8.82,-6.31,;.87,1.69,;.87,3.23,;-.47,4,;-.47,5.54,;-.47,.92,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467836
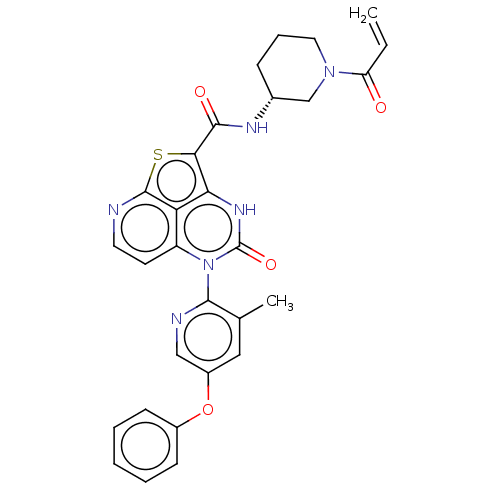
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(3-methyl-5-phe...)Show SMILES Cc1cc(Oc2ccccc2)cnc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:25.26,(-4.18,1.77,;-4.18,3.31,;-5.51,4.08,;-5.51,5.62,;-6.85,6.39,;-8.18,5.62,;-9.51,6.39,;-10.85,5.62,;-10.85,4.08,;-9.51,3.31,;-8.18,4.08,;-4.18,6.39,;-2.84,5.62,;-2.84,4.08,;-1.51,3.31,;-1.51,1.77,;-2.84,1,;-2.84,-.54,;-1.51,-1.31,;-.18,-.54,;1.16,-1.31,;2.37,.26,;3.91,.27,;4.66,1.61,;4.69,-1.06,;6.23,-1.06,;7,.28,;8.54,.28,;9.31,-1.06,;8.54,-2.39,;7,-2.39,;9.31,-3.72,;10.85,-3.72,;8.54,-5.06,;9.31,-6.39,;1.16,1.77,;1.16,3.31,;-.18,4.08,;-.18,5.62,;-.18,1,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032866
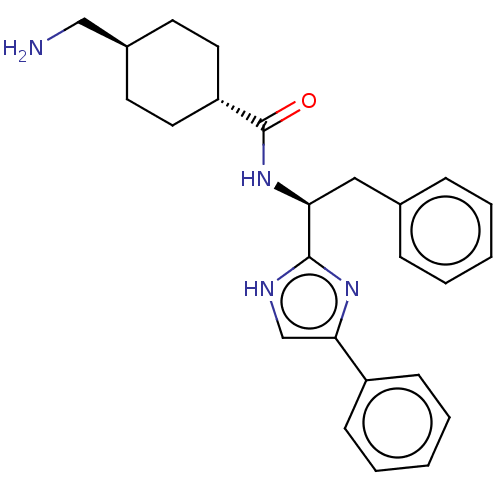
(CHEMBL3355664)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1ccccc1 |r,wU:18.18,12.14,wD:9.7,(25.97,-5.14,;27.3,-5.91,;27.3,-7.45,;28.64,-5.14,;29.97,-5.91,;29.96,-4.35,;28.64,-3.6,;10.28,-12.71,;11.61,-13.48,;12.94,-12.72,;14.28,-13.49,;15.62,-12.73,;15.62,-11.18,;14.29,-10.41,;12.95,-11.18,;16.95,-10.41,;16.95,-8.87,;18.28,-11.18,;19.62,-10.41,;19.62,-8.87,;20.96,-8.1,;22.29,-8.88,;23.62,-8.11,;23.62,-6.57,;22.28,-5.8,;20.95,-6.57,;20.95,-11.18,;22.36,-10.56,;23.39,-11.7,;22.62,-13.04,;21.11,-12.71,;24.92,-11.54,;25.54,-10.13,;27.06,-9.97,;27.98,-11.22,;27.35,-12.63,;25.82,-12.79,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468007
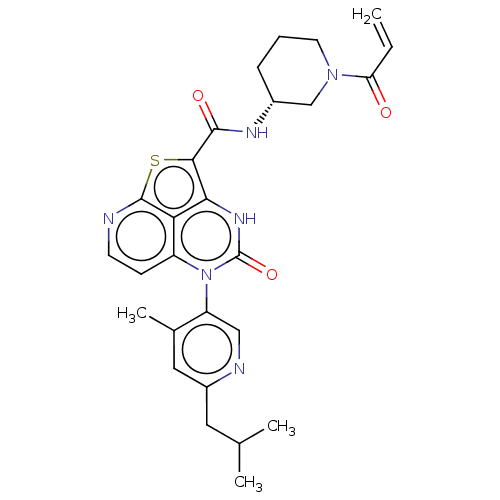
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(6-isobuty...)Show SMILES CC(C)Cc1cc(C)c(cn1)-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:22.22,(-9.16,1.88,;-9.16,3.42,;-10.5,4.19,;-7.83,4.19,;-6.5,3.42,;-6.5,1.88,;-5.16,1.11,;-5.16,-.49,;-3.83,1.88,;-3.83,3.42,;-5.16,4.19,;-2.5,1.11,;-2.5,-.43,;-3.83,-1.2,;-3.83,-2.74,;-2.5,-3.51,;-1.16,-2.74,;.32,-3.14,;1.26,-1.52,;2.8,-1.52,;3.57,-2.85,;3.57,-.19,;5.11,-.19,;5.88,1.15,;7.42,1.15,;8.19,-.19,;7.42,-1.52,;5.88,-1.52,;8.19,-2.85,;7.42,-4.19,;9.73,-2.85,;10.5,-1.52,;.17,-.43,;.17,1.11,;-1.16,1.88,;-1.16,3.42,;-1.16,-1.2,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032883
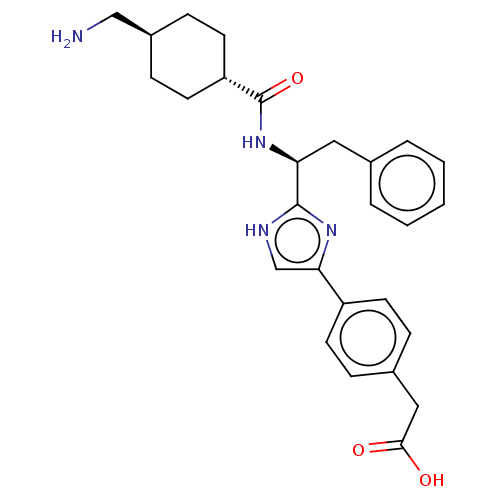
(CHEMBL3355674)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1ccc(CC(O)=O)cc1 |r,wU:18.18,12.14,wD:9.7,(29.77,-17.68,;31.11,-18.45,;31.11,-19.99,;32.44,-17.68,;33.77,-18.45,;33.77,-16.89,;32.44,-16.14,;13.69,-24.66,;15.02,-25.44,;16.35,-24.67,;17.7,-25.45,;19.03,-24.68,;19.03,-23.14,;17.7,-22.37,;16.36,-23.13,;20.36,-22.37,;20.36,-20.83,;21.69,-23.14,;23.03,-22.37,;23.03,-20.83,;24.37,-20.06,;25.7,-20.83,;27.03,-20.06,;27.03,-18.52,;25.69,-17.75,;24.36,-18.52,;24.36,-23.14,;25.76,-22.51,;26.8,-23.65,;26.03,-24.99,;24.52,-24.67,;28.32,-23.5,;28.94,-22.09,;30.47,-21.93,;31.38,-23.17,;32.92,-23.01,;33.82,-24.25,;33.2,-25.67,;35.36,-24.09,;30.76,-24.58,;29.23,-24.74,)| Show InChI InChI=1S/C27H32N4O3.C2HF3O2/c28-16-20-8-12-22(13-9-20)27(34)31-23(14-18-4-2-1-3-5-18)26-29-17-24(30-26)21-10-6-19(7-11-21)15-25(32)33;3-2(4,5)1(6)7/h1-7,10-11,17,20,22-23H,8-9,12-16,28H2,(H,29,30)(H,31,34)(H,32,33);(H,6,7)/t20-,22-,23-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468124
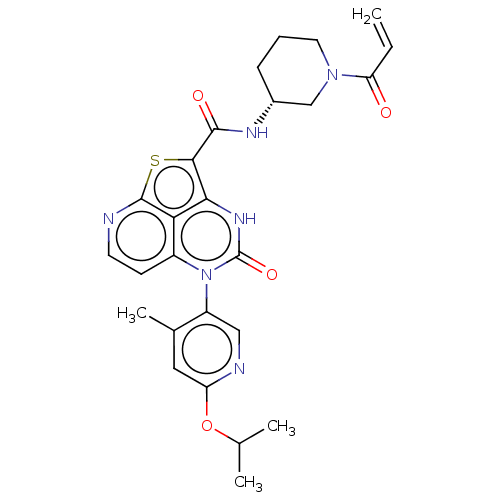
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(6-isoprop...)Show SMILES CC(C)Oc1cc(C)c(cn1)-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:22.22,(-10.22,5.69,;-8.88,4.92,;-8.88,3.38,;-7.55,5.69,;-6.22,4.92,;-4.88,5.69,;-3.55,4.92,;-2.22,5.69,;-3.55,3.38,;-4.88,2.61,;-6.22,3.38,;-2.22,2.61,;-2.22,1.07,;-3.55,.3,;-3.55,-1.24,;-2.22,-2.01,;-.88,-1.24,;.49,-1.84,;1.22,-.35,;2.76,-.35,;3.53,.98,;3.53,-1.69,;4.83,-1.69,;5.6,-.35,;7.14,-.35,;7.91,-1.69,;7.14,-3.02,;5.6,-3.02,;7.91,-4.35,;7.14,-5.69,;9.45,-4.35,;10.22,-5.69,;.45,1.07,;.45,2.61,;-.88,3.38,;-.88,4.92,;-.88,.3,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50032885
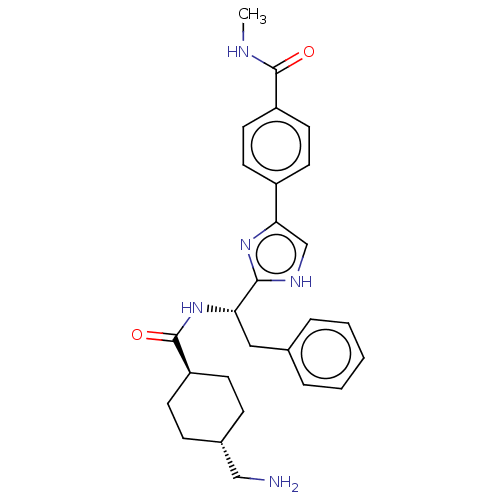
(CHEMBL3355672)Show SMILES OC(=O)C(F)(F)F.CNC(=O)c1ccc(cc1)-c1c[nH]c(n1)[C@H](Cc1ccccc1)NC(=O)[C@H]1CC[C@H](CN)CC1 |r,wU:22.23,33.34,wD:36.38,(24.46,-10.74,;25.79,-11.51,;25.79,-13.05,;27.12,-10.74,;28.46,-11.51,;28.45,-9.95,;27.12,-9.2,;27.89,-18.72,;28.51,-17.31,;27.6,-16.07,;28.22,-14.66,;26.07,-16.23,;25.15,-14.99,;23.63,-15.15,;23.01,-16.56,;23.91,-17.8,;25.44,-17.64,;21.48,-16.71,;20.71,-18.05,;19.21,-17.73,;19.05,-16.2,;20.45,-15.57,;17.72,-15.43,;17.72,-13.89,;19.05,-13.12,;20.38,-13.89,;21.72,-13.12,;21.72,-11.58,;20.37,-10.81,;19.05,-11.58,;16.38,-16.2,;15.05,-15.43,;15.05,-13.89,;13.72,-16.2,;13.72,-17.74,;12.38,-18.51,;11.04,-17.73,;9.7,-18.5,;8.38,-17.72,;11.05,-16.19,;12.39,-15.43,)| Show InChI InChI=1S/C27H33N5O2.C2HF3O2/c1-29-26(33)21-13-11-20(12-14-21)24-17-30-25(31-24)23(15-18-5-3-2-4-6-18)32-27(34)22-9-7-19(16-28)8-10-22;3-2(4,5)1(6)7/h2-6,11-14,17,19,22-23H,7-10,15-16,28H2,1H3,(H,29,33)(H,30,31)(H,32,34);(H,6,7)/t19-,22-,23-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032865

(CHEMBL3355665)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1ccc(cc1)C#N |r,wU:18.18,12.14,wD:9.7,(25.97,-5.14,;27.3,-5.91,;27.3,-7.45,;28.64,-5.14,;29.97,-5.91,;29.96,-4.35,;28.64,-3.6,;10.28,-12.71,;11.61,-13.48,;12.94,-12.72,;14.28,-13.49,;15.62,-12.73,;15.62,-11.18,;14.29,-10.41,;12.95,-11.18,;16.95,-10.41,;16.95,-8.87,;18.28,-11.18,;19.62,-10.41,;19.62,-8.87,;20.96,-8.1,;22.29,-8.88,;23.62,-8.11,;23.62,-6.57,;22.28,-5.8,;20.95,-6.57,;20.95,-11.18,;22.36,-10.56,;23.39,-11.7,;22.62,-13.04,;21.11,-12.71,;24.92,-11.54,;25.54,-10.13,;27.06,-9.97,;27.98,-11.22,;27.35,-12.63,;25.82,-12.79,;29.5,-11.05,;31.03,-10.89,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032880
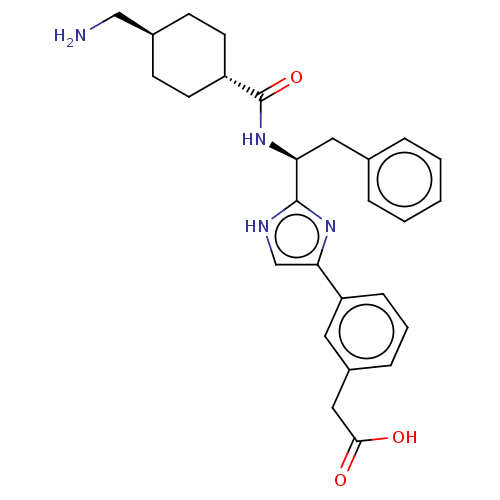
(CHEMBL3355677)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1cccc(CC(O)=O)c1 |r,wU:18.18,12.14,wD:9.7,(29.77,-17.68,;31.11,-18.45,;31.11,-19.99,;32.44,-17.68,;33.78,-18.45,;33.77,-16.89,;32.44,-16.14,;13.69,-24.66,;15.02,-25.44,;16.36,-24.67,;17.7,-25.45,;19.04,-24.68,;19.03,-23.14,;17.7,-22.37,;16.36,-23.13,;20.36,-22.37,;20.36,-20.83,;21.69,-23.14,;23.03,-22.37,;23.03,-20.83,;24.37,-20.06,;25.7,-20.83,;27.03,-20.07,;27.03,-18.52,;25.69,-17.75,;24.36,-18.53,;24.36,-23.14,;25.77,-22.51,;26.8,-23.65,;26.03,-24.99,;24.52,-24.67,;28.32,-23.5,;28.94,-22.09,;30.47,-21.93,;31.39,-23.18,;30.76,-24.58,;31.67,-25.83,;31.04,-27.24,;29.51,-27.41,;31.95,-28.49,;29.23,-24.74,)| Show InChI InChI=1S/C27H32N4O3.C2HF3O2/c28-16-19-9-11-21(12-10-19)27(34)31-23(14-18-5-2-1-3-6-18)26-29-17-24(30-26)22-8-4-7-20(13-22)15-25(32)33;3-2(4,5)1(6)7/h1-8,13,17,19,21,23H,9-12,14-16,28H2,(H,29,30)(H,31,34)(H,32,33);(H,6,7)/t19-,21-,23-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 280 | -37.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50398711
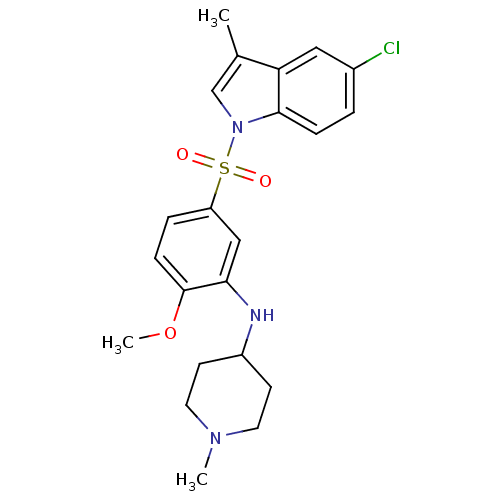
(CHEMBL2179217)Show SMILES COc1ccc(cc1NC1CCN(C)CC1)S(=O)(=O)n1cc(C)c2cc(Cl)ccc12 Show InChI InChI=1S/C22H26ClN3O3S/c1-15-14-26(21-6-4-16(23)12-19(15)21)30(27,28)18-5-7-22(29-3)20(13-18)24-17-8-10-25(2)11-9-17/h4-7,12-14,17,24H,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 303 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D2 receptor |
J Med Chem 55: 9255-69 (2012)
Article DOI: 10.1021/jm300955x
BindingDB Entry DOI: 10.7270/Q21G0ND0 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50032866

(CHEMBL3355664)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1ccccc1 |r,wU:18.18,12.14,wD:9.7,(25.97,-5.14,;27.3,-5.91,;27.3,-7.45,;28.64,-5.14,;29.97,-5.91,;29.96,-4.35,;28.64,-3.6,;10.28,-12.71,;11.61,-13.48,;12.94,-12.72,;14.28,-13.49,;15.62,-12.73,;15.62,-11.18,;14.29,-10.41,;12.95,-11.18,;16.95,-10.41,;16.95,-8.87,;18.28,-11.18,;19.62,-10.41,;19.62,-8.87,;20.96,-8.1,;22.29,-8.88,;23.62,-8.11,;23.62,-6.57,;22.28,-5.8,;20.95,-6.57,;20.95,-11.18,;22.36,-10.56,;23.39,-11.7,;22.62,-13.04,;21.11,-12.71,;24.92,-11.54,;25.54,-10.13,;27.06,-9.97,;27.98,-11.22,;27.35,-12.63,;25.82,-12.79,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50448583

(CHEMBL3127491)Show SMILES CC(C)CC(=O)Nc1cc(cc(c1)-c1ccc(cc1C(O)=O)C(N)=O)[C@@H]1C[C@](C)(c2ccccc2)c2cc(ccc2N1)C(N)=N |r| Show InChI InChI=1S/C36H37N5O4/c1-20(2)13-32(42)40-26-15-23(27-11-9-22(34(39)43)17-28(27)35(44)45)14-24(16-26)31-19-36(3,25-7-5-4-6-8-25)29-18-21(33(37)38)10-12-30(29)41-31/h4-12,14-18,20,31,41H,13,19H2,1-3H3,(H3,37,38)(H2,39,43)(H,40,42)(H,44,45)/t31-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using N-benzoyl-Ile-Glu-(OH, OMe)-Gly-Arg-pNA as substrate by spectrophotometric analysis |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032885

(CHEMBL3355672)Show SMILES OC(=O)C(F)(F)F.CNC(=O)c1ccc(cc1)-c1c[nH]c(n1)[C@H](Cc1ccccc1)NC(=O)[C@H]1CC[C@H](CN)CC1 |r,wU:22.23,33.34,wD:36.38,(24.46,-10.74,;25.79,-11.51,;25.79,-13.05,;27.12,-10.74,;28.46,-11.51,;28.45,-9.95,;27.12,-9.2,;27.89,-18.72,;28.51,-17.31,;27.6,-16.07,;28.22,-14.66,;26.07,-16.23,;25.15,-14.99,;23.63,-15.15,;23.01,-16.56,;23.91,-17.8,;25.44,-17.64,;21.48,-16.71,;20.71,-18.05,;19.21,-17.73,;19.05,-16.2,;20.45,-15.57,;17.72,-15.43,;17.72,-13.89,;19.05,-13.12,;20.38,-13.89,;21.72,-13.12,;21.72,-11.58,;20.37,-10.81,;19.05,-11.58,;16.38,-16.2,;15.05,-15.43,;15.05,-13.89,;13.72,-16.2,;13.72,-17.74,;12.38,-18.51,;11.04,-17.73,;9.7,-18.5,;8.38,-17.72,;11.05,-16.19,;12.39,-15.43,)| Show InChI InChI=1S/C27H33N5O2.C2HF3O2/c1-29-26(33)21-13-11-20(12-14-21)24-17-30-25(31-24)23(15-18-5-3-2-4-6-18)32-27(34)22-9-7-19(16-28)8-10-22;3-2(4,5)1(6)7/h2-6,11-14,17,19,22-23H,7-10,15-16,28H2,1H3,(H,29,33)(H,30,31)(H,32,34);(H,6,7)/t19-,22-,23-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467845

((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-(6-phenox...)Show SMILES C=CC(=O)N1CCC[C@H](C1)NC(=O)c1sc2nccc3n(-c4ccc(Oc5ccccc5)nc4)c(=O)[nH]c1c23 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 354 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032882
(CHEMBL3355675)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1ccc(CC(N)=O)cc1 |r,wU:18.18,12.14,wD:9.7,(29.77,-17.68,;31.11,-18.45,;31.11,-19.99,;32.44,-17.68,;33.77,-18.45,;33.77,-16.89,;32.44,-16.14,;13.69,-24.66,;15.02,-25.44,;16.35,-24.67,;17.7,-25.45,;19.03,-24.68,;19.03,-23.14,;17.7,-22.37,;16.36,-23.13,;20.36,-22.37,;20.36,-20.83,;21.69,-23.14,;23.03,-22.37,;23.03,-20.83,;24.37,-20.06,;25.7,-20.83,;27.03,-20.06,;27.03,-18.52,;25.69,-17.75,;24.36,-18.52,;24.36,-23.14,;25.76,-22.51,;26.8,-23.65,;26.03,-24.99,;24.52,-24.67,;28.32,-23.5,;28.94,-22.09,;30.47,-21.93,;31.38,-23.17,;32.92,-23.01,;33.82,-24.25,;33.2,-25.67,;35.36,-24.09,;30.76,-24.58,;29.23,-24.74,)| Show InChI InChI=1S/C27H33N5O2.C2HF3O2/c28-16-20-8-12-22(13-9-20)27(34)32-23(14-18-4-2-1-3-5-18)26-30-17-24(31-26)21-10-6-19(7-11-21)15-25(29)33;3-2(4,5)1(6)7/h1-7,10-11,17,20,22-23H,8-9,12-16,28H2,(H2,29,33)(H,30,31)(H,32,34);(H,6,7)/t20-,22-,23-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467852

((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-(5-phenox...)Show SMILES C=CC(=O)N1CCC[C@H](C1)NC(=O)c1sc2nccc3n(-c4ccc(Oc5ccccc5)cn4)c(=O)[nH]c1c23 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data