

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM232997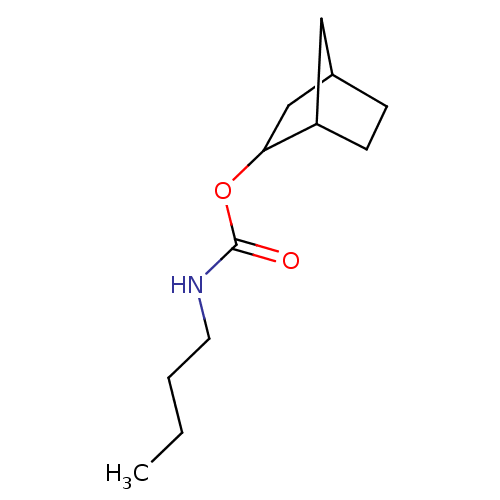 ((S)-(-)-endo-2-norbornyl-N-n-butylcarbamate) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Chung Shan Medical University Hospital | Assay Description BChE inhibitions by the carbamate inhibitors were assayed by the Ellman method [Ellman et al., Biochem. Pharm., 7:88-95]. BChE-catalyzed hydrolysis o... | J Enzyme Inhib Med Chem 25: 13-20 (2010) Article DOI: 10.3109/14756360902888200 BindingDB Entry DOI: 10.7270/Q2C8286J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50005429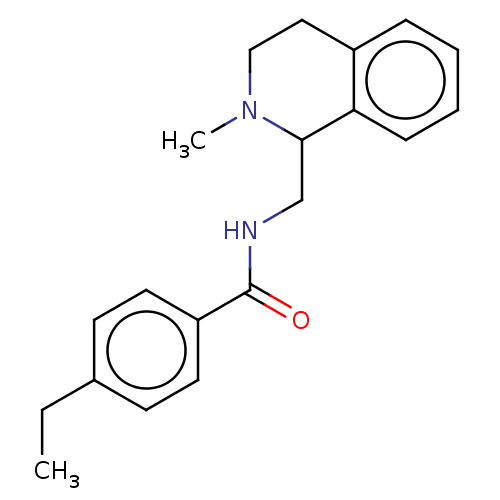 (CHEMBL4070288) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM232998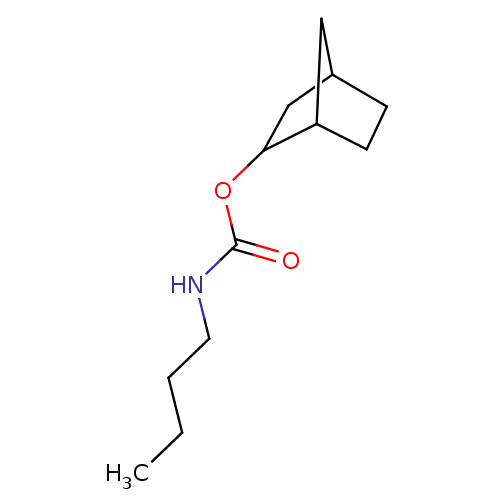 ((R)-(+)-endo-2-norbornyl-N-n-butylcarbamate | rac-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Chung Shan Medical University Hospital | Assay Description BChE inhibitions by the carbamate inhibitors were assayed by the Ellman method [Ellman et al., Biochem. Pharm., 7:88-95]. BChE-catalyzed hydrolysis o... | J Enzyme Inhib Med Chem 25: 13-20 (2010) Article DOI: 10.3109/14756360902888200 BindingDB Entry DOI: 10.7270/Q2C8286J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50007985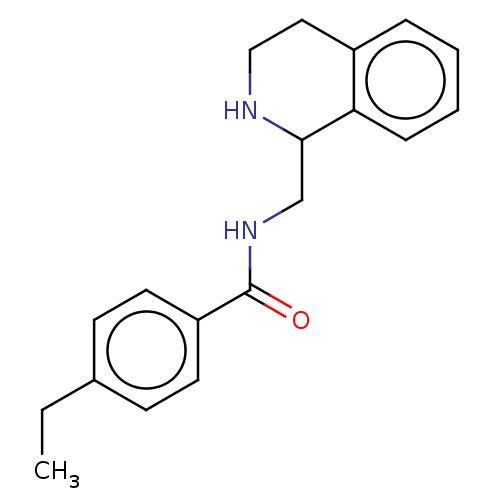 (CHEMBL4097865) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM232998 ((R)-(+)-endo-2-norbornyl-N-n-butylcarbamate | rac-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Chung Shan Medical University Hospital | Assay Description BChE inhibitions by the carbamate inhibitors were assayed by the Ellman method [Ellman et al., Biochem. Pharm., 7:88-95]. BChE-catalyzed hydrolysis o... | J Enzyme Inhib Med Chem 25: 13-20 (2010) Article DOI: 10.3109/14756360902888200 BindingDB Entry DOI: 10.7270/Q2C8286J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50042064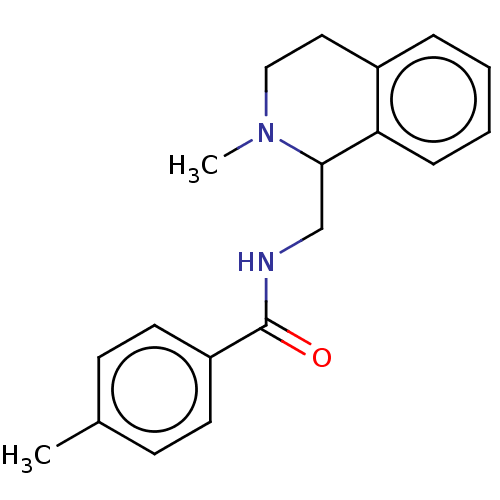 (CHEMBL4097466) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015426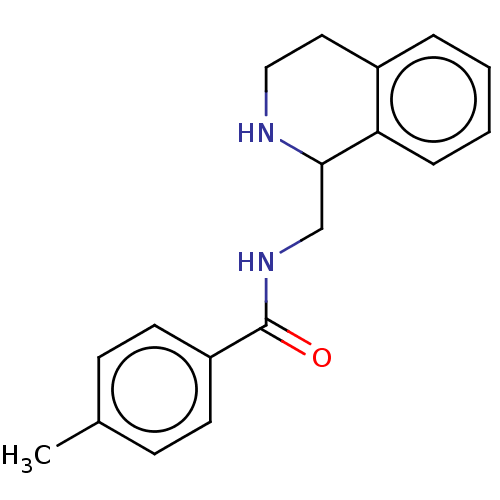 (CHEMBL4070750) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50009239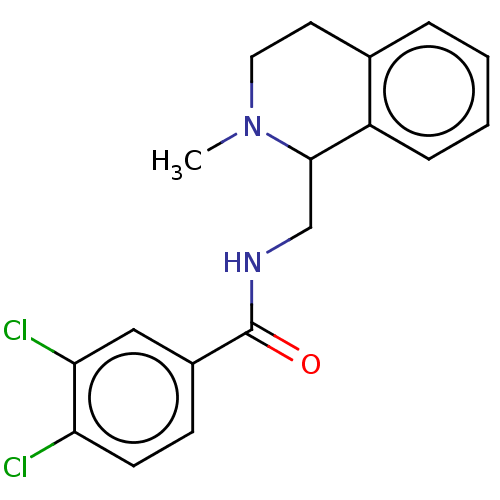 (CHEMBL4092125) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50234418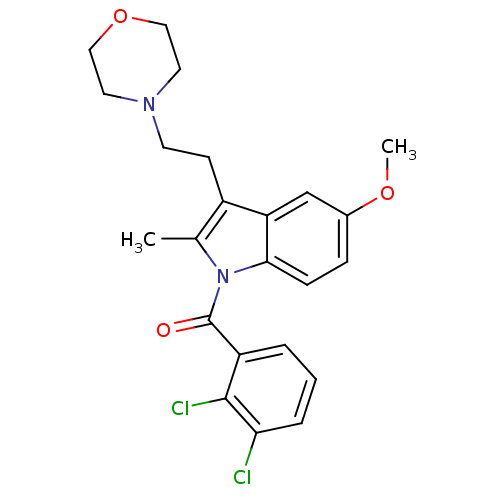 ((2,3-Dichloro-phenyl)-[5-methoxy-2-methyl-3-(2-mor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM232995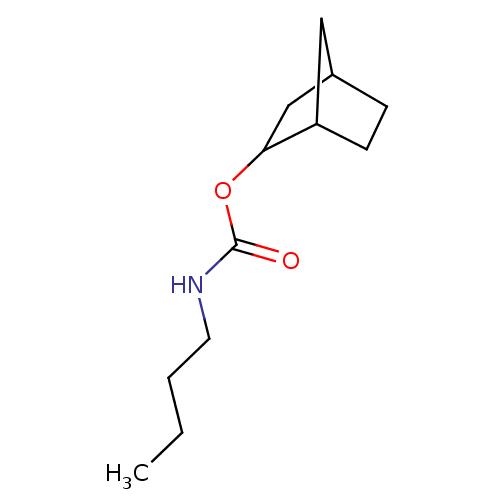 ((R)-(+)-exo-2-norbonyl-N-n-butylcarbamate) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Chung Shan Medical University Hospital | Assay Description BChE inhibitions by the carbamate inhibitors were assayed by the Ellman method [Ellman et al., Biochem. Pharm., 7:88-95]. BChE-catalyzed hydrolysis o... | J Enzyme Inhib Med Chem 25: 13-20 (2010) Article DOI: 10.3109/14756360902888200 BindingDB Entry DOI: 10.7270/Q2C8286J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50193788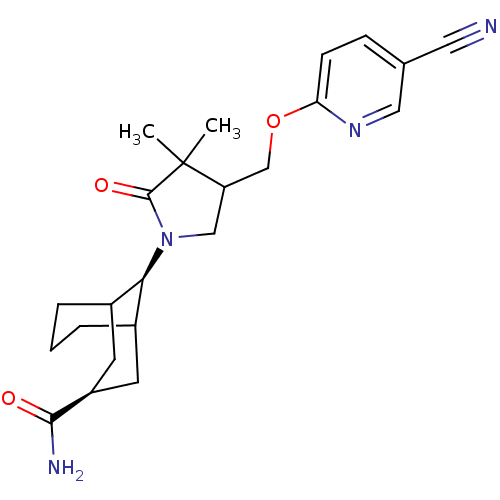 ((3s,9s)-9-(4-((5-cyanopyridin-2-yloxy)methyl)-3,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 | Bioorg Med Chem Lett 16: 5555-60 (2006) Article DOI: 10.1016/j.bmcl.2006.08.034 BindingDB Entry DOI: 10.7270/Q25X28K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50193788 ((3s,9s)-9-(4-((5-cyanopyridin-2-yloxy)methyl)-3,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 16: 5555-60 (2006) Article DOI: 10.1016/j.bmcl.2006.08.034 BindingDB Entry DOI: 10.7270/Q25X28K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM232999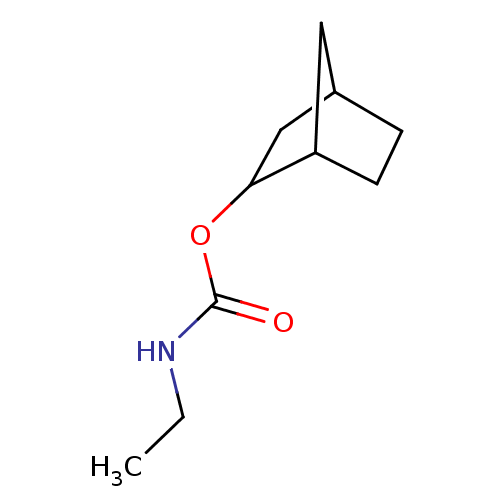 (rac-(±)-exo-2-norbornyl carbamate) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Chung Shan Medical University Hospital | Assay Description BChE inhibitions by the carbamate inhibitors were assayed by the Ellman method [Ellman et al., Biochem. Pharm., 7:88-95]. BChE-catalyzed hydrolysis o... | J Enzyme Inhib Med Chem 25: 13-20 (2010) Article DOI: 10.3109/14756360902888200 BindingDB Entry DOI: 10.7270/Q2C8286J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008523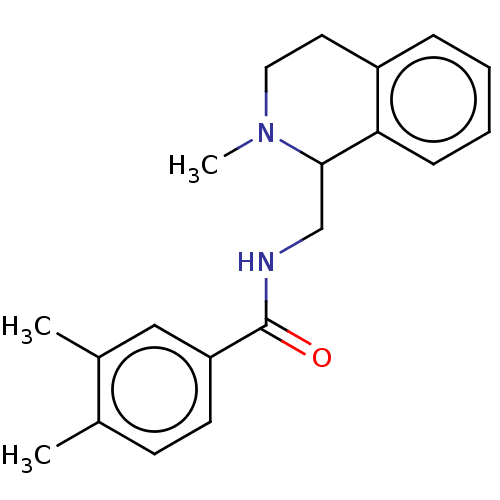 (CHEMBL4071332) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015433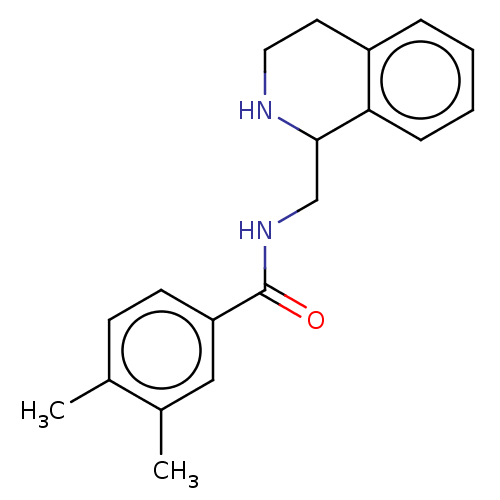 (CHEMBL4105599) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM232996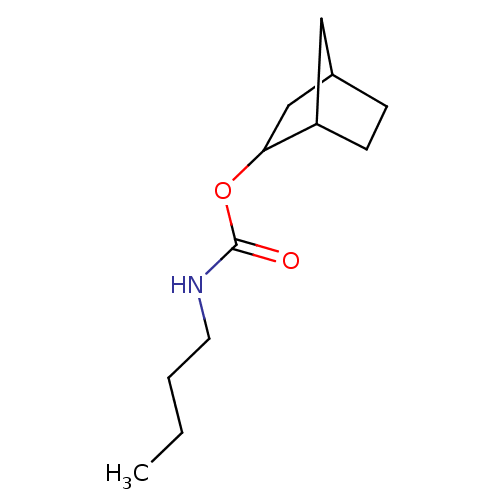 ((S)-(-)-exo-2-norbonyl-N-n-butylcarbamate) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | -41.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Chung Shan Medical University Hospital | Assay Description BChE inhibitions by the carbamate inhibitors were assayed by the Ellman method [Ellman et al., Biochem. Pharm., 7:88-95]. BChE-catalyzed hydrolysis o... | J Enzyme Inhib Med Chem 25: 13-20 (2010) Article DOI: 10.3109/14756360902888200 BindingDB Entry DOI: 10.7270/Q2C8286J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50193783 ((1R,7S,8r)-ethyl 4-(4-((5-cyanopyridin-2-yloxy)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 16: 5555-60 (2006) Article DOI: 10.1016/j.bmcl.2006.08.034 BindingDB Entry DOI: 10.7270/Q25X28K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008003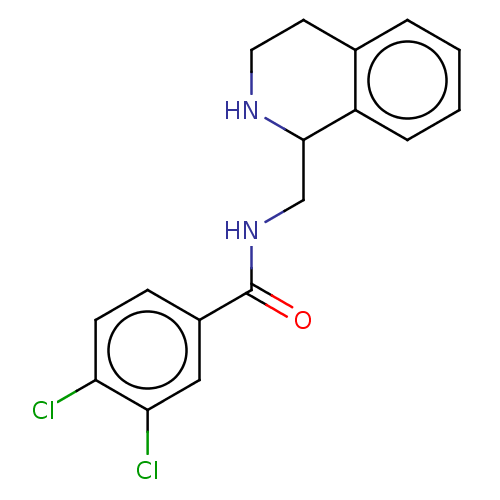 (CHEMBL4065924) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146461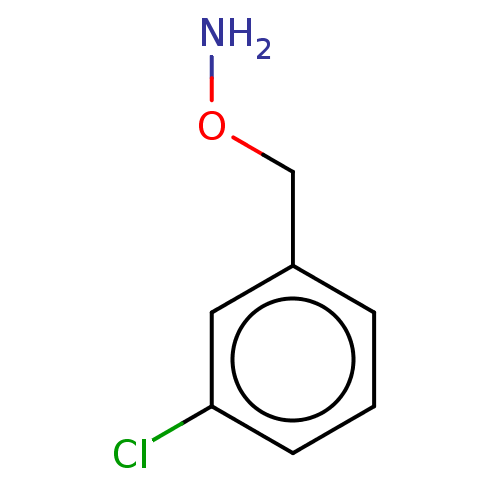 (CHEMBL3765807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by Michaelis-Menton... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146460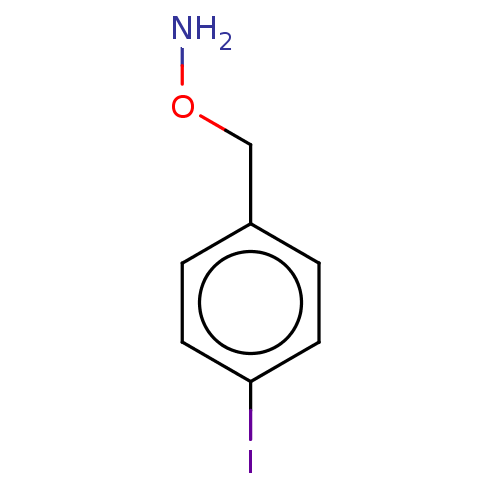 (CHEMBL3763688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by Michaelis-Menton... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50193800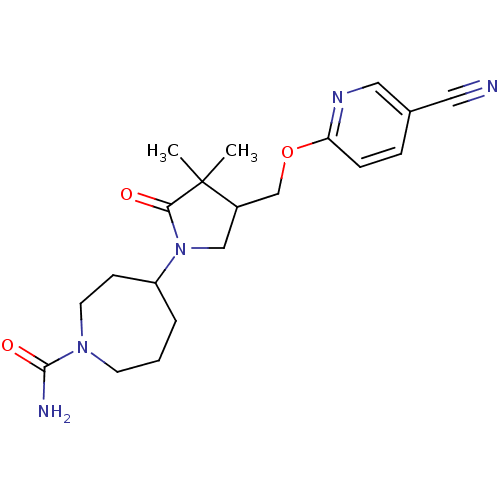 (4-(4-((5-cyanopyridin-2-yloxy)methyl)-3,3-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 16: 5555-60 (2006) Article DOI: 10.1016/j.bmcl.2006.08.034 BindingDB Entry DOI: 10.7270/Q25X28K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50193800 (4-(4-((5-cyanopyridin-2-yloxy)methyl)-3,3-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 | Bioorg Med Chem Lett 16: 5555-60 (2006) Article DOI: 10.1016/j.bmcl.2006.08.034 BindingDB Entry DOI: 10.7270/Q25X28K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50193783 ((1R,7S,8r)-ethyl 4-(4-((5-cyanopyridin-2-yloxy)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 | Bioorg Med Chem Lett 16: 5555-60 (2006) Article DOI: 10.1016/j.bmcl.2006.08.034 BindingDB Entry DOI: 10.7270/Q25X28K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055967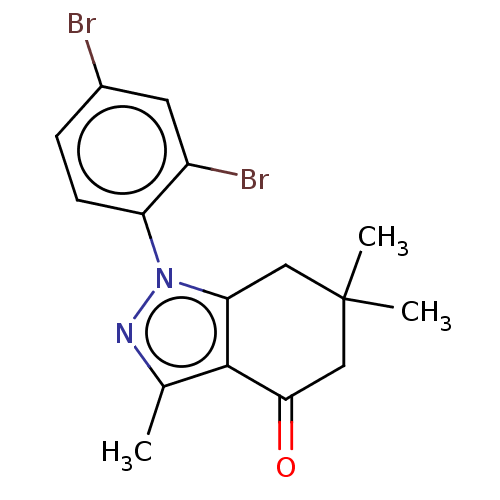 (CHEMBL3325714) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055966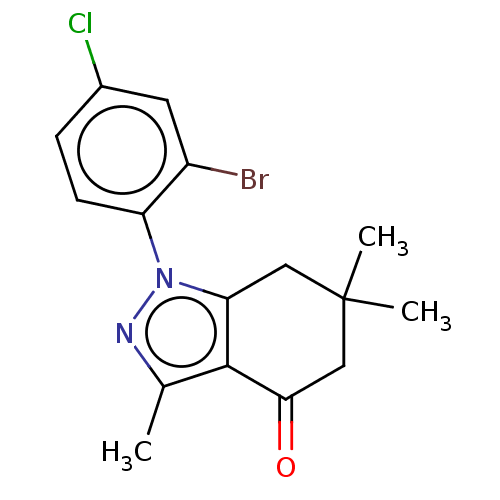 (CHEMBL3325715) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055965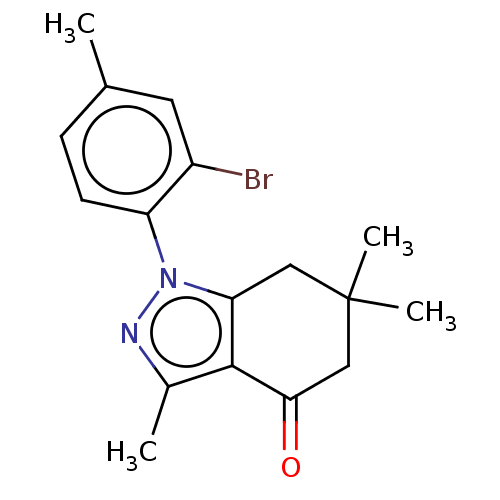 (CHEMBL3325716) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055969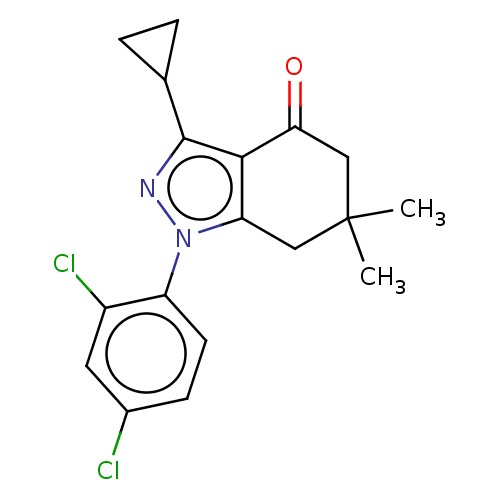 (CHEMBL3325719) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50234418 ((2,3-Dichloro-phenyl)-[5-methoxy-2-methyl-3-(2-mor...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055973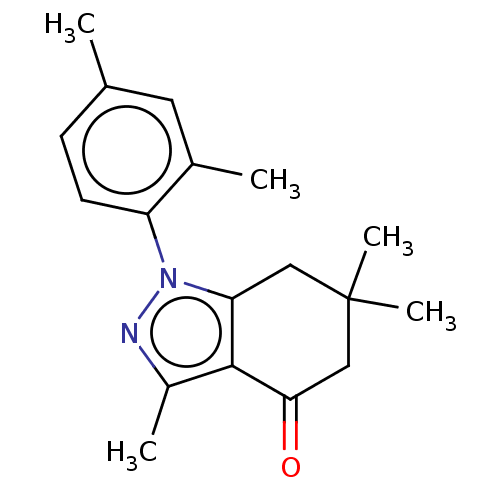 (CHEMBL3325707) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055972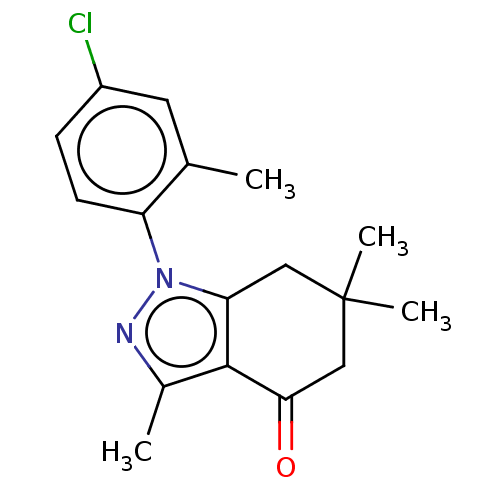 (CHEMBL3325710) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055964 (CHEMBL3325841) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341928 (CHEMBL1765160 | cis-(4-chlorophenyl)((4R,4aS,8aR)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21008 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human DOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50171552 ((S)-2-(S)-Amino-1-(2-boron-dihydroxide-pyrrolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences Curated by ChEMBL | Assay Description Inhibitory concentration against human dipeptidylpeptidase 4 | Bioorg Med Chem Lett 15: 4256-60 (2005) Article DOI: 10.1016/j.bmcl.2005.06.076 BindingDB Entry DOI: 10.7270/Q2RR1XSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins b... | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340312 ((2,3-dichlorophenyl)(1-((4,4-difluoropiperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050517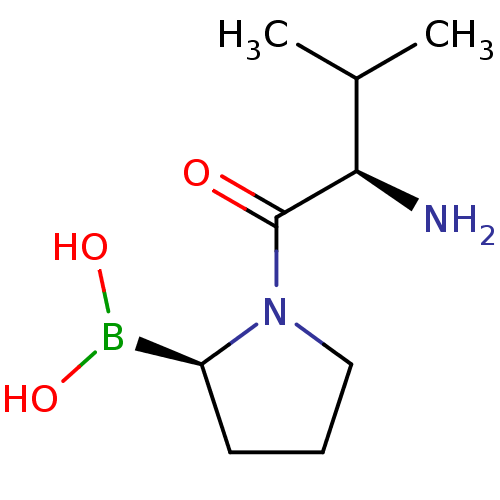 (Boronic acid derivative | CHEMBL305170 | N-alkyl G...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human DPP4 using Gly-Pro-AMC in fluorometric assay | Bioorg Med Chem Lett 15: 4239-42 (2005) Article DOI: 10.1016/j.bmcl.2005.06.075 BindingDB Entry DOI: 10.7270/Q2CJ8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50171546 ((S)-2,4-Diamino-N-((R)-1-boron-dihydroxide-pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences Curated by ChEMBL | Assay Description Inhibitory concentration against human dipeptidylpeptidase 7 | Bioorg Med Chem Lett 15: 4256-60 (2005) Article DOI: 10.1016/j.bmcl.2005.06.076 BindingDB Entry DOI: 10.7270/Q2RR1XSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341934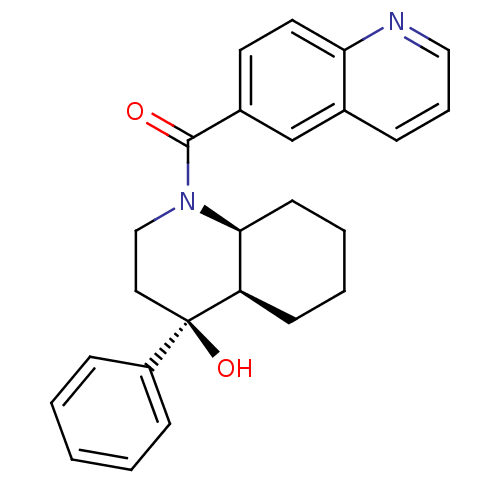 (CHEMBL1765259 | cis-rac-((4S,4aR,8aS)-4-hydroxy-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341920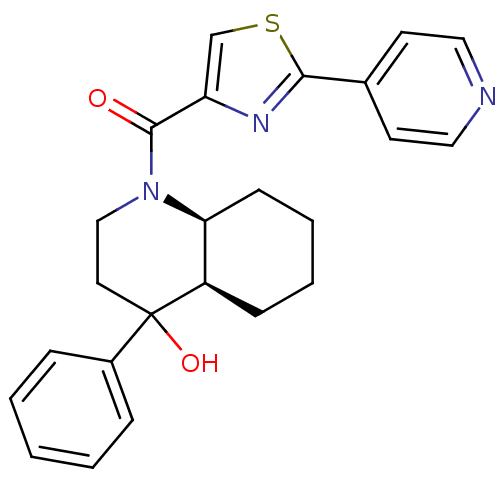 (((4aR,8aS)-4-hydroxy-4-phenyloctahydroquinolin-1(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341920 (((4aR,8aS)-4-hydroxy-4-phenyloctahydroquinolin-1(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341930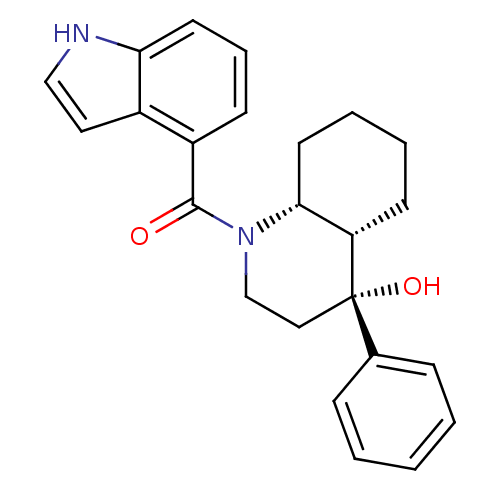 (CHEMBL1765246 | cis-((4R,4aS,8aR)-4-hydroxy-4-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340311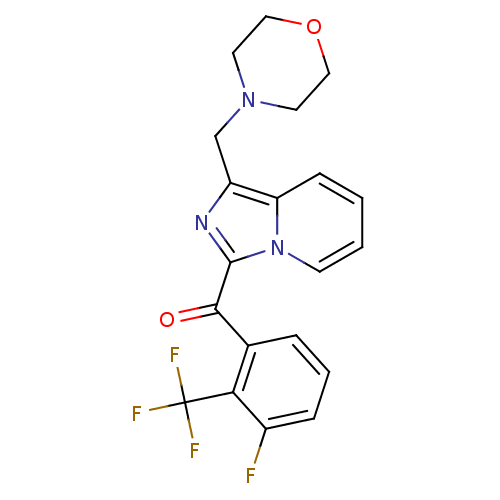 ((3-fluoro-2-(trifluoromethyl)phenyl)(1-(morpholino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50171555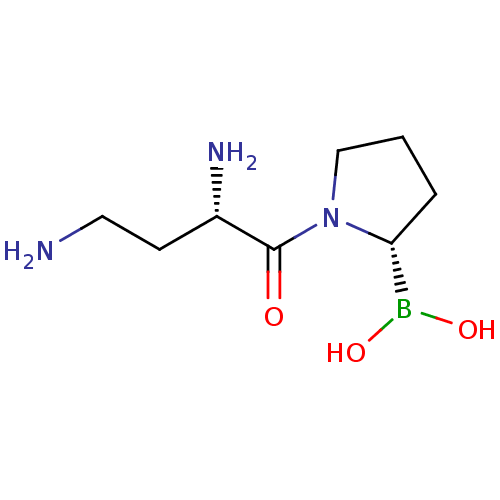 ((S)-2,4-Diamino-1-(2-boron-dihydroxide-pyrrolidin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences Curated by ChEMBL | Assay Description Inhibitory concentration against human dipeptidylpeptidase 7 | Bioorg Med Chem Lett 15: 4256-60 (2005) Article DOI: 10.1016/j.bmcl.2005.06.076 BindingDB Entry DOI: 10.7270/Q2RR1XSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50171653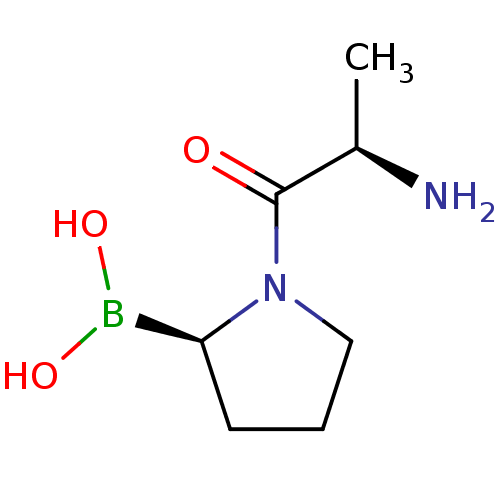 (CHEMBL196120 | N-alkyl Gly-boro-Pro derivative) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human DPP4 using Gly-Pro-AMC in fluorometric assay | Bioorg Med Chem Lett 15: 4239-42 (2005) Article DOI: 10.1016/j.bmcl.2005.06.075 BindingDB Entry DOI: 10.7270/Q2CJ8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341929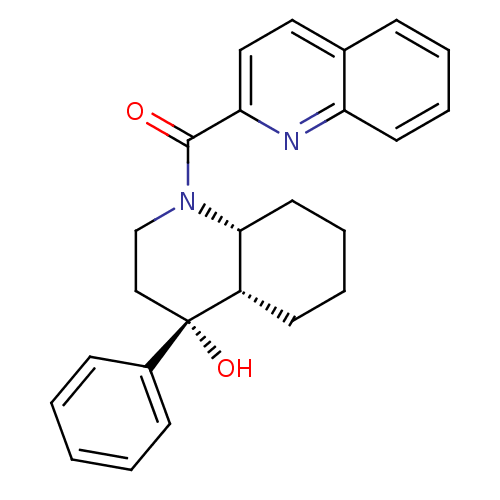 (CHEMBL1765161 | cis-((4R,4aS,8aR)-4-hydroxy-4-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50171556 ((R)-1-((S)-2-amino-3-methylbutanamido)ethylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences Curated by ChEMBL | Assay Description Inhibitory concentration against human dipeptidylpeptidase 4 | Bioorg Med Chem Lett 15: 4256-60 (2005) Article DOI: 10.1016/j.bmcl.2005.06.076 BindingDB Entry DOI: 10.7270/Q2RR1XSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human delta opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins b... | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1419 total ) | Next | Last >> |