 Found 1003 hits with Last Name = 'schmidt' and Initial = 'sj'
Found 1003 hits with Last Name = 'schmidt' and Initial = 'sj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora B ATP binding site by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Caspase-1
(Homo sapiens (Human)) | BDBM50058526
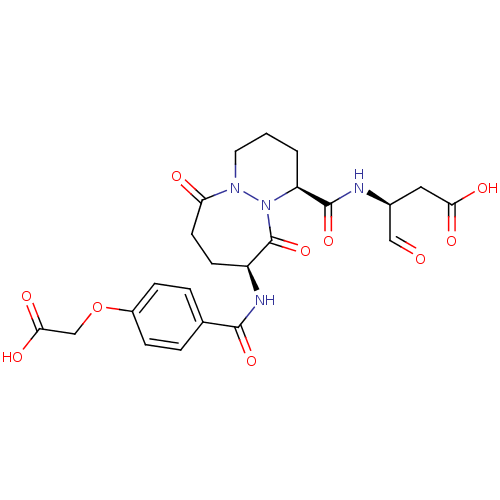
((S)-3-{[(1S,9S)-9-(4-Carboxymethoxy-benzoylamino)-...)Show SMILES OC(=O)COc1ccc(cc1)C(=O)N[C@H]1CCC(=O)N2CCC[C@H](N2C1=O)C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C23H26N4O10/c28-11-14(10-19(30)31)24-22(35)17-2-1-9-26-18(29)8-7-16(23(36)27(17)26)25-21(34)13-3-5-15(6-4-13)37-12-20(32)33/h3-6,11,14,16-17H,1-2,7-10,12H2,(H,24,35)(H,25,34)(H,30,31)(H,32,33)/t14-,16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Winthrop Inc.
Curated by ChEMBL
| Assay Description
Reversible Inhibition constants of the compound against IL-1 beta converting enzyme (ICE) |
J Med Chem 40: 1941-6 (1997)
Article DOI: 10.1021/jm9701637
BindingDB Entry DOI: 10.7270/Q2CC0ZSQ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50058530
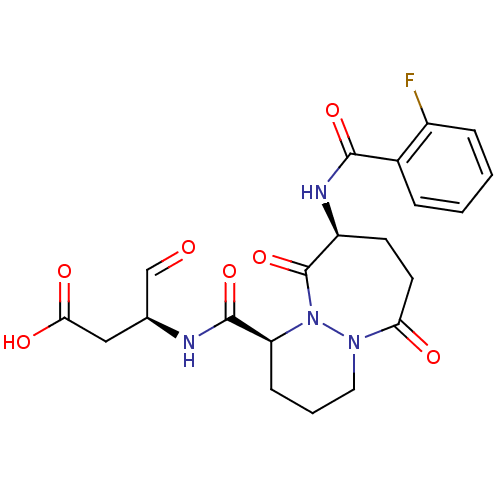
((S)-3-{[(1S,9S)-9-(2-Fluoro-benzoylamino)-6,10-dio...)Show SMILES OC(=O)C[C@H](NC(=O)[C@@H]1CCCN2N1C(=O)[C@H](CCC2=O)NC(=O)c1ccccc1F)C=O Show InChI InChI=1S/C21H23FN4O7/c22-14-5-2-1-4-13(14)19(31)24-15-7-8-17(28)25-9-3-6-16(26(25)21(15)33)20(32)23-12(11-27)10-18(29)30/h1-2,4-5,11-12,15-16H,3,6-10H2,(H,23,32)(H,24,31)(H,29,30)/t12-,15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Winthrop Inc.
Curated by ChEMBL
| Assay Description
Reversible Inhibition constants of the compound against IL-1 beta converting enzyme (ICE) |
J Med Chem 40: 1941-6 (1997)
Article DOI: 10.1021/jm9701637
BindingDB Entry DOI: 10.7270/Q2CC0ZSQ |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora C ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
7-dehydrocholesterol reductase
(Rattus norvegicus) | BDBM50406621
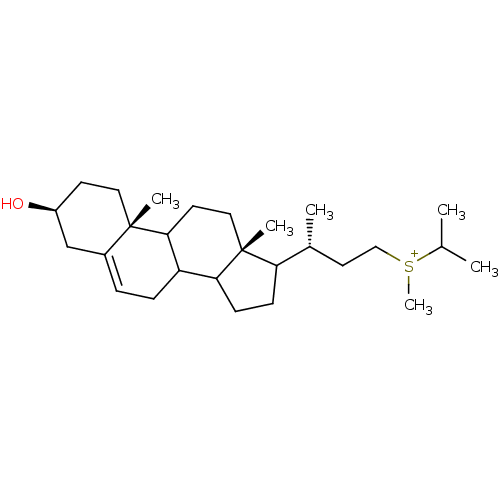
(CHEMBL9820)Show SMILES C[C@H](CC[S+](C)C(C)C)C1CCC2C3CC=C4C[C@@H](O)CC[C@]4(C)C3CC[C@]12C |t:15| Show InChI InChI=1S/C27H47OS/c1-18(2)29(6)16-13-19(3)23-9-10-24-22-8-7-20-17-21(28)11-14-26(20,4)25(22)12-15-27(23,24)5/h7,18-19,21-25,28H,8-17H2,1-6H3/q+1/t19-,21+,22?,23?,24?,25?,26+,27-,29?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Delta-(24)-sterol reductase |
J Med Chem 35: 100-6 (1992)
BindingDB Entry DOI: 10.7270/Q2C82BHD |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora C ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Caspase-1
(Homo sapiens (Human)) | BDBM50058528
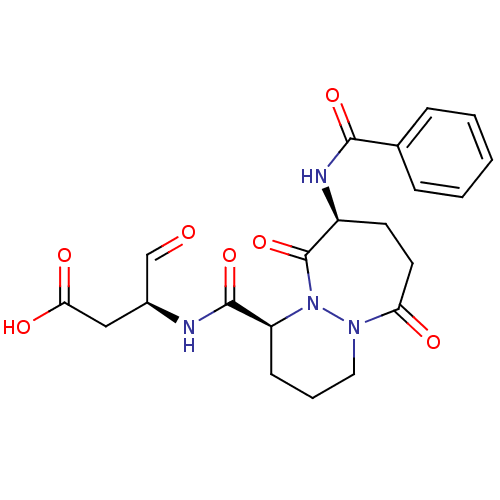
((S)-3-[((1S,9S)-9-Benzoylamino-6,10-dioxo-octahydr...)Show SMILES OC(=O)C[C@H](NC(=O)[C@@H]1CCCN2N1C(=O)[C@H](CCC2=O)NC(=O)c1ccccc1)C=O Show InChI InChI=1S/C21H24N4O7/c26-12-14(11-18(28)29)22-20(31)16-7-4-10-24-17(27)9-8-15(21(32)25(16)24)23-19(30)13-5-2-1-3-6-13/h1-3,5-6,12,14-16H,4,7-11H2,(H,22,31)(H,23,30)(H,28,29)/t14-,15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Winthrop Inc.
Curated by ChEMBL
| Assay Description
Reversible Inhibition constants of the compound against IL-1 beta converting enzyme (ICE) |
J Med Chem 40: 1941-6 (1997)
Article DOI: 10.1021/jm9701637
BindingDB Entry DOI: 10.7270/Q2CC0ZSQ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50058527
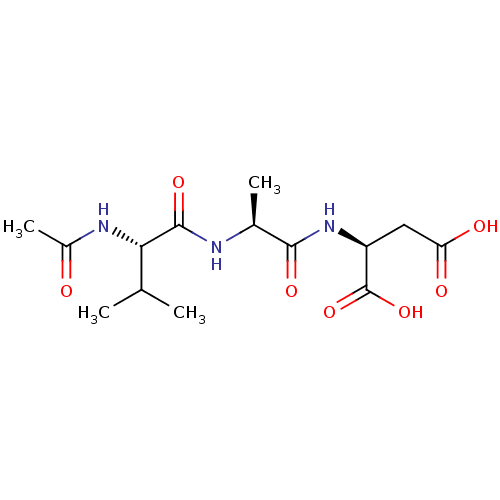
((S)-2-[(S)-2-((S)-2-Acetylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(O)=O Show InChI InChI=1S/C14H23N3O7/c1-6(2)11(16-8(4)18)13(22)15-7(3)12(21)17-9(14(23)24)5-10(19)20/h6-7,9,11H,5H2,1-4H3,(H,15,22)(H,16,18)(H,17,21)(H,19,20)(H,23,24)/t7-,9-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Winthrop Inc.
Curated by ChEMBL
| Assay Description
Reversible Inhibition constants of the compound against IL-1 beta converting enzyme (ICE) |
J Med Chem 40: 1941-6 (1997)
Article DOI: 10.1021/jm9701637
BindingDB Entry DOI: 10.7270/Q2CC0ZSQ |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Rattus norvegicus) | BDBM50406617
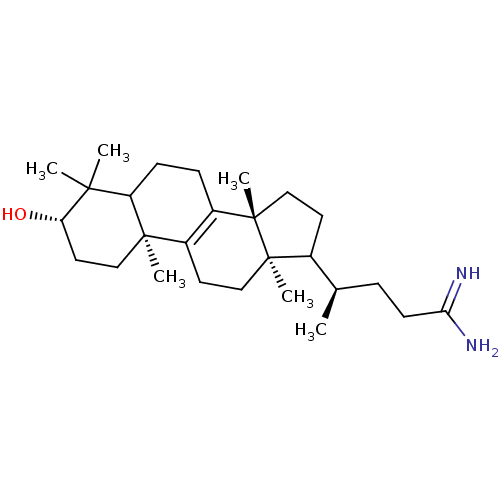
(CHEMBL276388)Show SMILES C[C@H](CCC(N)=N)C1CC[C@@]2(C)C3=C(CC[C@]12C)[C@@]1(C)CC[C@H](O)C(C)(C)C1CC3 |r,c:12| Show InChI InChI=1S/C27H46N2O/c1-17(7-10-23(28)29)18-11-15-27(6)20-8-9-21-24(2,3)22(30)13-14-25(21,4)19(20)12-16-26(18,27)5/h17-18,21-22,30H,7-16H2,1-6H3,(H3,28,29)/t17-,18?,21?,22+,25-,26-,27+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Delta-(24)-sterol reductase |
J Med Chem 35: 100-6 (1992)
BindingDB Entry DOI: 10.7270/Q2C82BHD |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Rattus norvegicus) | BDBM50406618

(CHEMBL9875)Show SMILES [#6]-[#6@H](-[#6]-[#6]\[#6](-[#7])=[#7+](/[#6])-[#6])-[#6]1-[#6]-[#6][C@@]2([#6])[#6]-3=[#6](-[#6]-[#6][C@]12[#6])[C@@]1([#6])[#6]-[#6]-[#6@H](-[#8])C([#6])([#6])[#6]1-[#6]-[#6]-3 |c:14| Show InChI InChI=1S/C29H50N2O/c1-19(9-12-25(30)31(7)8)20-13-17-29(6)22-10-11-23-26(2,3)24(32)15-16-27(23,4)21(22)14-18-28(20,29)5/h19-20,23-24,30,32H,9-18H2,1-8H3/p+1/t19-,20?,23?,24+,27-,28-,29+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of delta24-sterol reductase |
J Med Chem 35: 100-6 (1992)
BindingDB Entry DOI: 10.7270/Q2C82BHD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176519
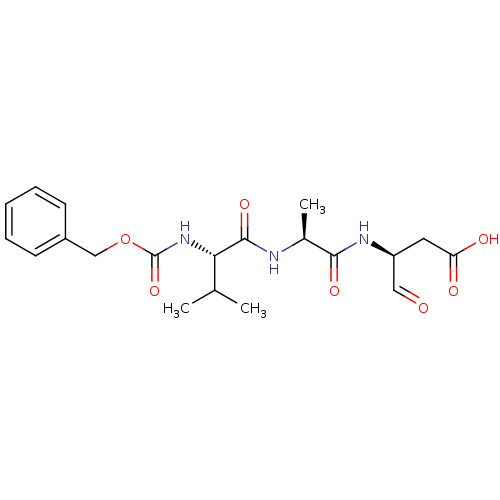
((S)-3-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methy...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C20H27N3O7/c1-12(2)17(23-20(29)30-11-14-7-5-4-6-8-14)19(28)21-13(3)18(27)22-15(10-24)9-16(25)26/h4-8,10,12-13,15,17H,9,11H2,1-3H3,(H,21,28)(H,22,27)(H,23,29)(H,25,26)/t13-,15-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against IL-1 beta converting enzyme |
Bioorg Med Chem Lett 5: 315-318 (1995)
Article DOI: 10.1016/0960-894X(95)00027-Q
BindingDB Entry DOI: 10.7270/Q2668D5S |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50286304
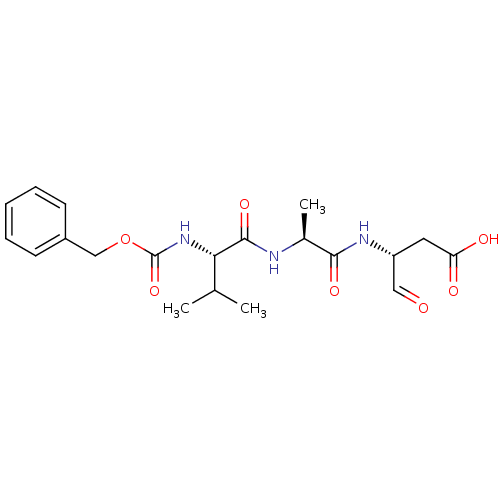
((R)-3-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methy...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@H](CC(O)=O)C=O Show InChI InChI=1S/C20H27N3O7/c1-12(2)17(23-20(29)30-11-14-7-5-4-6-8-14)19(28)21-13(3)18(27)22-15(10-24)9-16(25)26/h4-8,10,12-13,15,17H,9,11H2,1-3H3,(H,21,28)(H,22,27)(H,23,29)(H,25,26)/t13-,15+,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against IL-1 beta converting enzyme |
Bioorg Med Chem Lett 5: 315-318 (1995)
Article DOI: 10.1016/0960-894X(95)00027-Q
BindingDB Entry DOI: 10.7270/Q2668D5S |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50058529

((S)-2-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methy...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(O)=O Show InChI InChI=1S/C20H27N3O8/c1-11(2)16(23-20(30)31-10-13-7-5-4-6-8-13)18(27)21-12(3)17(26)22-14(19(28)29)9-15(24)25/h4-8,11-12,14,16H,9-10H2,1-3H3,(H,21,27)(H,22,26)(H,23,30)(H,24,25)(H,28,29)/t12-,14-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Winthrop Inc.
Curated by ChEMBL
| Assay Description
Reversible Inhibition constants of the compound against IL-1 beta converting enzyme (ICE) |
J Med Chem 40: 1941-6 (1997)
Article DOI: 10.1021/jm9701637
BindingDB Entry DOI: 10.7270/Q2CC0ZSQ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50058531
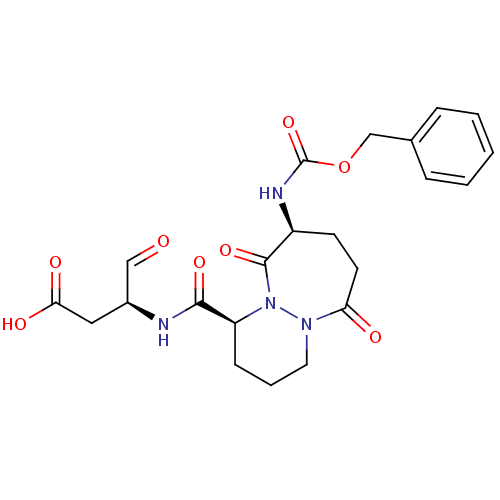
((S)-3-[((1S,9S)-9-Benzyloxycarbonylamino-6,10-diox...)Show SMILES OC(=O)C[C@H](NC(=O)[C@@H]1CCCN2N1C(=O)[C@H](CCC2=O)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C22H26N4O8/c27-12-15(11-19(29)30)23-20(31)17-7-4-10-25-18(28)9-8-16(21(32)26(17)25)24-22(33)34-13-14-5-2-1-3-6-14/h1-3,5-6,12,15-17H,4,7-11,13H2,(H,23,31)(H,24,33)(H,29,30)/t15-,16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Winthrop Inc.
Curated by ChEMBL
| Assay Description
Reversible Inhibition constants of the compound against IL-1 beta converting enzyme (ICE) |
J Med Chem 40: 1941-6 (1997)
Article DOI: 10.1021/jm9701637
BindingDB Entry DOI: 10.7270/Q2CC0ZSQ |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Rattus norvegicus) | BDBM50406620
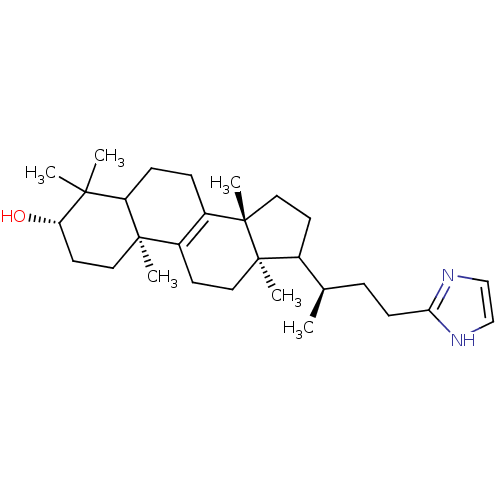
(CHEMBL9864)Show SMILES C[C@H](CCc1ncc[nH]1)C1CC[C@@]2(C)C3=C(CC[C@]12C)[C@@]1(C)CC[C@H](O)C(C)(C)C1CC3 |c:15| Show InChI InChI=1S/C29H46N2O/c1-19(7-10-25-30-17-18-31-25)20-11-15-29(6)22-8-9-23-26(2,3)24(32)13-14-27(23,4)21(22)12-16-28(20,29)5/h17-20,23-24,32H,7-16H2,1-6H3,(H,30,31)/t19-,20?,23?,24+,27-,28-,29+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of delta24-sterol reductase |
J Med Chem 35: 100-6 (1992)
BindingDB Entry DOI: 10.7270/Q2C82BHD |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Rattus norvegicus) | BDBM50406616
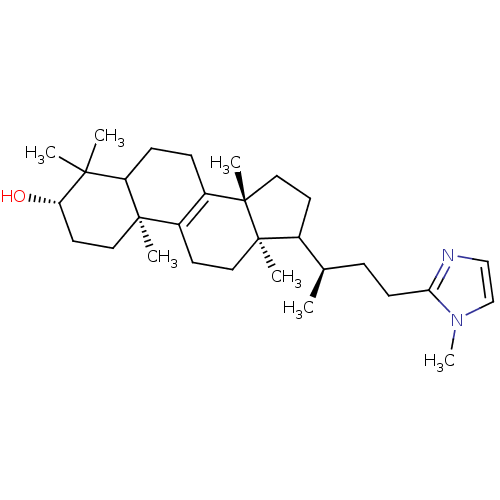
(CHEMBL268041)Show SMILES C[C@H](CCc1nccn1C)C1CC[C@@]2(C)C3=C(CC[C@]12C)[C@@]1(C)CC[C@H](O)C(C)(C)C1CC3 |c:16| Show InChI InChI=1S/C30H48N2O/c1-20(8-11-26-31-18-19-32(26)7)21-12-16-30(6)23-9-10-24-27(2,3)25(33)14-15-28(24,4)22(23)13-17-29(21,30)5/h18-21,24-25,33H,8-17H2,1-7H3/t20-,21?,24?,25+,28-,29-,30+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of delta24-sterol reductase |
J Med Chem 35: 100-6 (1992)
BindingDB Entry DOI: 10.7270/Q2C82BHD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50615186
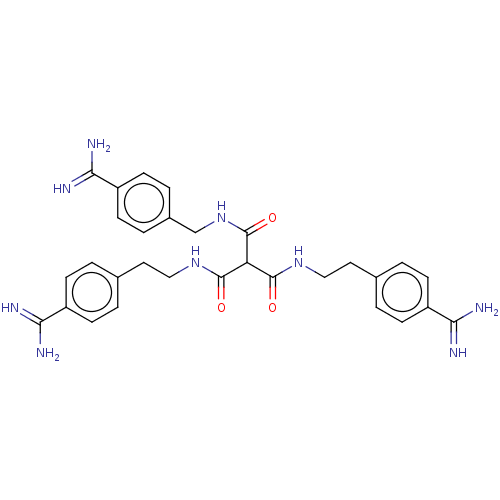
(CHEMBL5282514)Show SMILES NC(=N)c1ccc(CCNC(=O)C(C(=O)NCCc2ccc(cc2)C(N)=N)C(=O)NCc2ccc(cc2)C(N)=N)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 393 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50615190
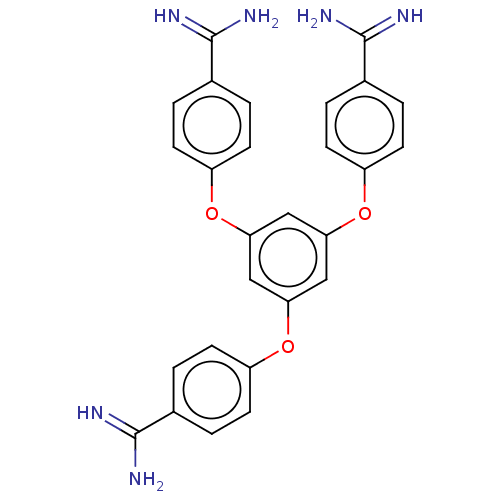
(CHEMBL5269464)Show SMILES NC(=N)c1ccc(Oc2cc(Oc3ccc(cc3)C(N)=N)cc(Oc3ccc(cc3)C(N)=N)c2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50615189

(CHEMBL5279198)Show SMILES NCc1ccc(Oc2cc(Oc3ccc(cc3)C(N)=N)cc(Oc3ccc(cc3)C(N)=N)c2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 459 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora A ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 492 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50615188
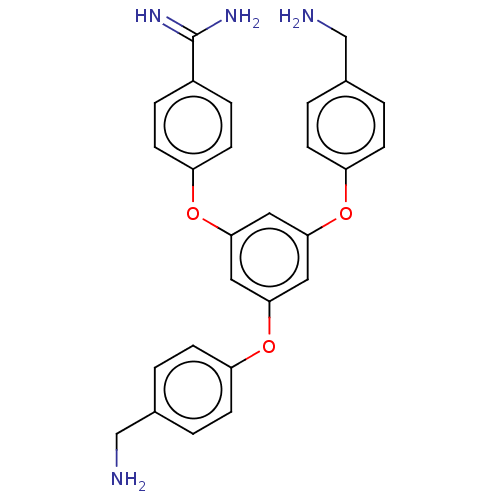
(CHEMBL5275339)Show SMILES NCc1ccc(Oc2cc(Oc3ccc(CN)cc3)cc(Oc3ccc(cc3)C(N)=N)c2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 775 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Rattus norvegicus) | BDBM50406619

(CHEMBL9808)Show SMILES CC(C)SCC[C@@H](C)C1CCC2C3CC=C4C[C@@H](O)CC[C@]4(C)C3CC[C@]12C |t:14| Show InChI InChI=1S/C26H44OS/c1-17(2)28-15-12-18(3)22-8-9-23-21-7-6-19-16-20(27)10-13-25(19,4)24(21)11-14-26(22,23)5/h6,17-18,20-24,27H,7-16H2,1-5H3/t18-,20+,21?,22?,23?,24?,25+,26-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Delta-(24)-sterol reductase |
J Med Chem 35: 100-6 (1992)
BindingDB Entry DOI: 10.7270/Q2C82BHD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50615185
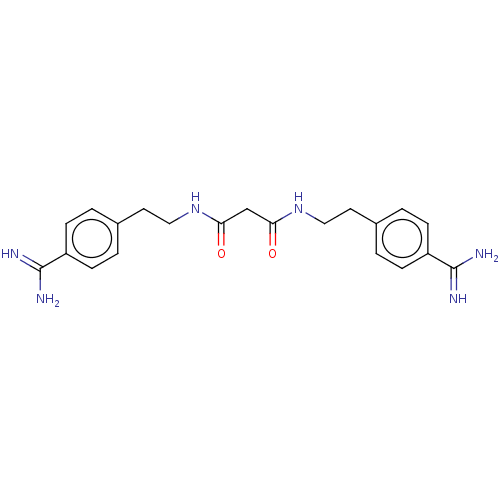
(CHEMBL5265971)Show SMILES NC(=N)c1ccc(CCNC(=O)CC(=O)NCCc2ccc(cc2)C(N)=N)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Dengue virus 2) | BDBM50572174
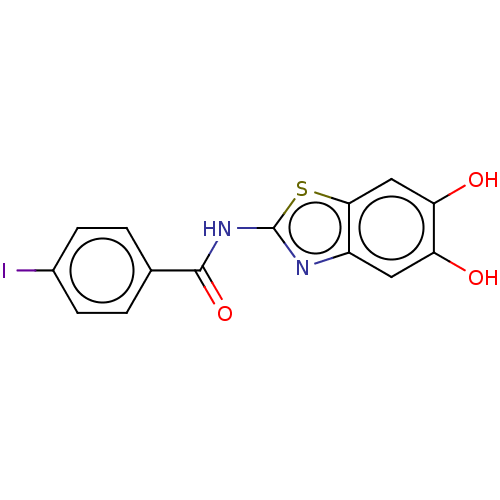
(CHEMBL4878316) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 7.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to DENV2 NS2B-NS3 protease by Dixon plot analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116392
BindingDB Entry DOI: 10.7270/Q2D50RR1 |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50615187
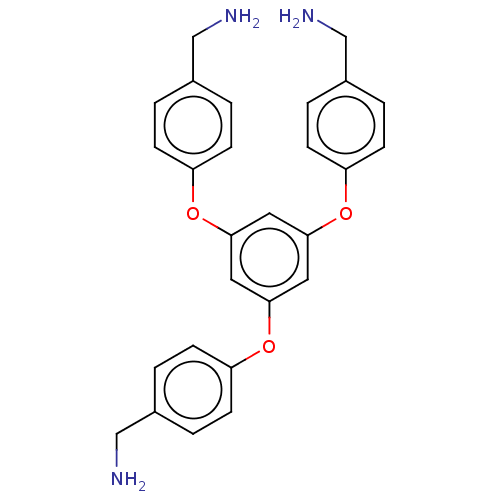
(CHEMBL5289898)Show SMILES NCc1ccc(Oc2cc(Oc3ccc(CN)cc3)cc(Oc3ccc(CN)cc3)c2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316497
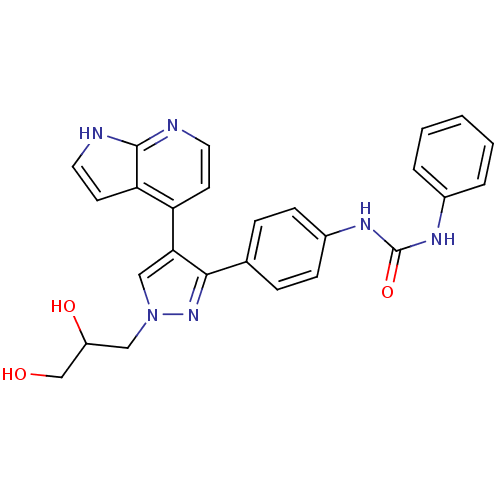
(CHEMBL1097106 | N-{4-[1-(2,3-Dihydroxypropyl)-4-(1...)Show SMILES OCC(O)Cn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C26H24N6O3/c33-16-20(34)14-32-15-23(21-10-12-27-25-22(21)11-13-28-25)24(31-32)17-6-8-19(9-7-17)30-26(35)29-18-4-2-1-3-5-18/h1-13,15,20,33-34H,14,16H2,(H,27,28)(H2,29,30,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50241089
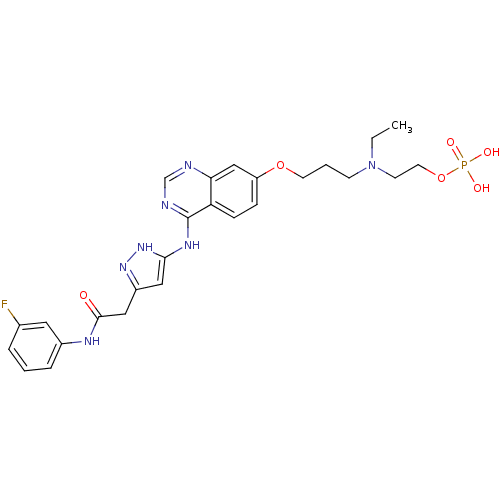
(2-(ethyl(3-(4-(5-(2-(3-fluorophenylamino)-2-oxoeth...)Show SMILES CCN(CCCOc1ccc2c(Nc3cc(CC(=O)Nc4cccc(F)c4)n[nH]3)ncnc2c1)CCOP(O)(O)=O Show InChI InChI=1S/C26H31FN7O6P/c1-2-34(10-12-40-41(36,37)38)9-4-11-39-21-7-8-22-23(16-21)28-17-29-26(22)31-24-14-20(32-33-24)15-25(35)30-19-6-3-5-18(27)13-19/h3,5-8,13-14,16-17H,2,4,9-12,15H2,1H3,(H,30,35)(H2,36,37,38)(H2,28,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316473
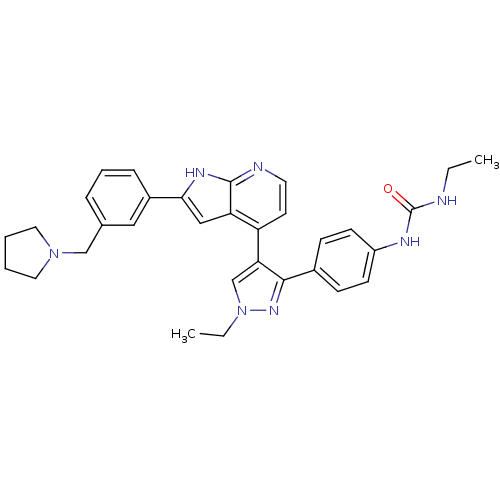
(4-[3-(4-N-Ethylcarbamylaminophenyl)-1-ethyl-1H-pyr...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1cccc(CN2CCCC2)c1 Show InChI InChI=1S/C32H35N7O/c1-3-33-32(40)35-25-12-10-23(11-13-25)30-28(21-39(4-2)37-30)26-14-15-34-31-27(26)19-29(36-31)24-9-7-8-22(18-24)20-38-16-5-6-17-38/h7-15,18-19,21H,3-6,16-17,20H2,1-2H3,(H,34,36)(H2,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316475
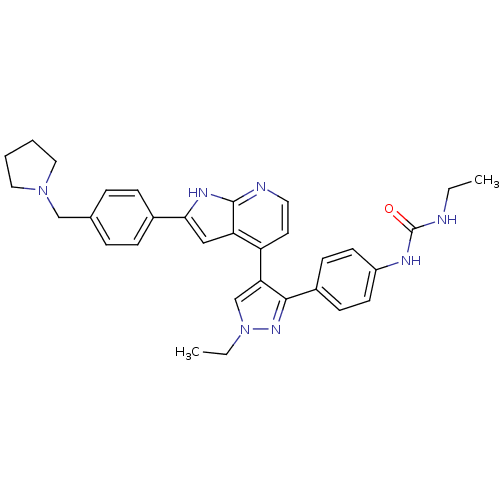
(CHEMBL1097191 | N-Ethyl-N'-[4-(1-ethyl-4-{2-[4-(1-...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1ccc(CN2CCCC2)cc1 Show InChI InChI=1S/C32H35N7O/c1-3-33-32(40)35-25-13-11-24(12-14-25)30-28(21-39(4-2)37-30)26-15-16-34-31-27(26)19-29(36-31)23-9-7-22(8-10-23)20-38-17-5-6-18-38/h7-16,19,21H,3-6,17-18,20H2,1-2H3,(H,34,36)(H2,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316471
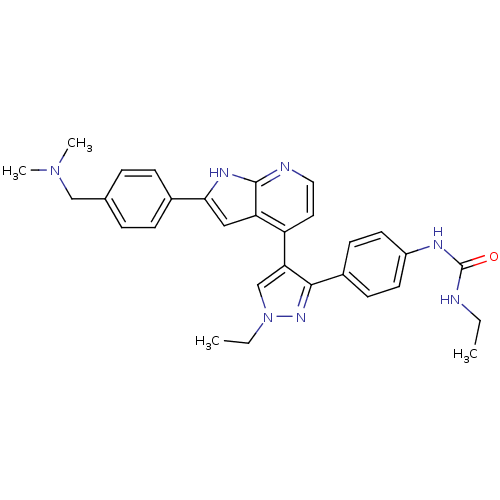
(4-[3-(4-N-Ethylcarbamylaminophenyl)-1-ethyl-1H-pyr...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1ccc(CN(C)C)cc1 Show InChI InChI=1S/C30H33N7O/c1-5-31-30(38)33-23-13-11-22(12-14-23)28-26(19-37(6-2)35-28)24-15-16-32-29-25(24)17-27(34-29)21-9-7-20(8-10-21)18-36(3)4/h7-17,19H,5-6,18H2,1-4H3,(H,32,34)(H2,31,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50091691
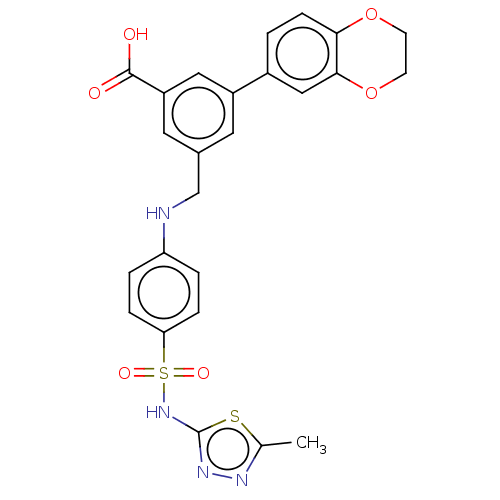
(CHEMBL3582356)Show SMILES Cc1nnc(NS(=O)(=O)c2ccc(NCc3cc(cc(c3)-c3ccc4OCCOc4c3)C(O)=O)cc2)s1 Show InChI InChI=1S/C25H22N4O6S2/c1-15-27-28-25(36-15)29-37(32,33)21-5-3-20(4-6-21)26-14-16-10-18(12-19(11-16)24(30)31)17-2-7-22-23(13-17)35-9-8-34-22/h2-7,10-13,26H,8-9,14H2,1H3,(H,28,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
ACS Med Chem Lett 6: 531-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00025
BindingDB Entry DOI: 10.7270/Q2BP04JF |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316492
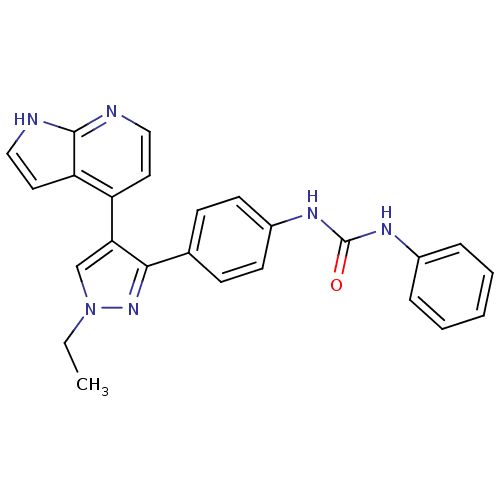
(1-(4-(1-ethyl-4-(1H-pyrrolo[2,3-b]pyridin-4-yl)-1H...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C25H22N6O/c1-2-31-16-22(20-12-14-26-24-21(20)13-15-27-24)23(30-31)17-8-10-19(11-9-17)29-25(32)28-18-6-4-3-5-7-18/h3-16H,2H2,1H3,(H,26,27)(H2,28,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316498
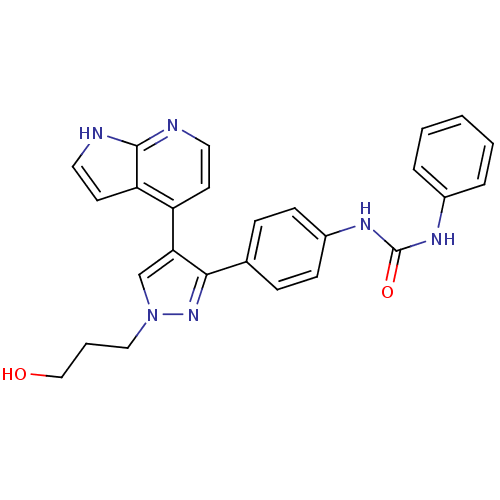
(CHEMBL1097454 | N-{4-[1-(3-Hydroxypropyl)-4-(1H-py...)Show SMILES OCCCn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C26H24N6O2/c33-16-4-15-32-17-23(21-11-13-27-25-22(21)12-14-28-25)24(31-32)18-7-9-20(10-8-18)30-26(34)29-19-5-2-1-3-6-19/h1-3,5-14,17,33H,4,15-16H2,(H,27,28)(H2,29,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316474
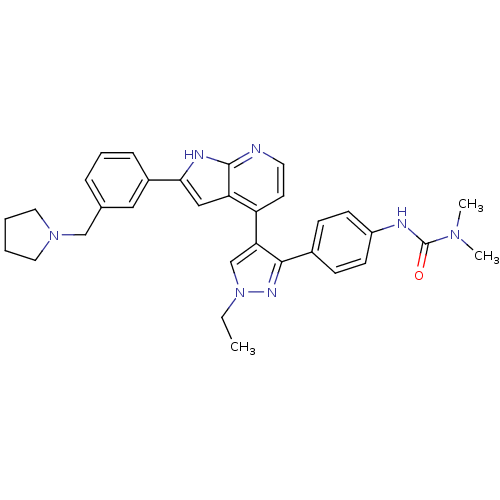
(4-[3-(4-N,N-Dimethylcarbamylaminophenyl)-1-ethyl-1...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN2CCCC2)c1 Show InChI InChI=1S/C32H35N7O/c1-4-39-21-28(30(36-39)23-10-12-25(13-11-23)34-32(40)37(2)3)26-14-15-33-31-27(26)19-29(35-31)24-9-7-8-22(18-24)20-38-16-5-6-17-38/h7-15,18-19,21H,4-6,16-17,20H2,1-3H3,(H,33,35)(H,34,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50091689
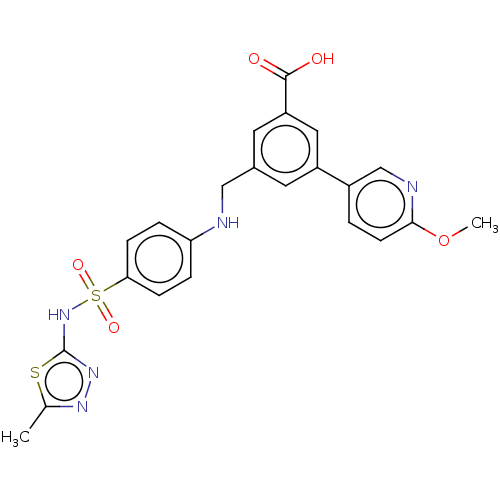
(CHEMBL3582354)Show SMILES COc1ccc(cn1)-c1cc(CNc2ccc(cc2)S(=O)(=O)Nc2nnc(C)s2)cc(c1)C(O)=O Show InChI InChI=1S/C23H21N5O5S2/c1-14-26-27-23(34-14)28-35(31,32)20-6-4-19(5-7-20)24-12-15-9-17(11-18(10-15)22(29)30)16-3-8-21(33-2)25-13-16/h3-11,13,24H,12H2,1-2H3,(H,27,28)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
ACS Med Chem Lett 6: 531-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00025
BindingDB Entry DOI: 10.7270/Q2BP04JF |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132G]
(Homo sapiens (Human)) | BDBM195601

(GSK321 | US11311527, Cpd ID GSK321 | US11376246, C...)Show SMILES C[C@H](O)c1cccc(NC(=O)c2nn(Cc3ccc(F)cc3)c3[C@H](C)CN(Cc23)C(=O)c2ccc[nH]2)c1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | 25 |
Albert Einstein College of Medicine
| Assay Description
RapidFire-MS/MS measurements of mutant IDH1 and IDH2 reactions were conducted at room temperature in 384-well Greiner polypropylene microtiter plates... |
Nat Chem Biol 11: 878-86 (2015)
Article DOI: 10.1038/nchembio.1930
BindingDB Entry DOI: 10.7270/Q2RR1X2G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316496
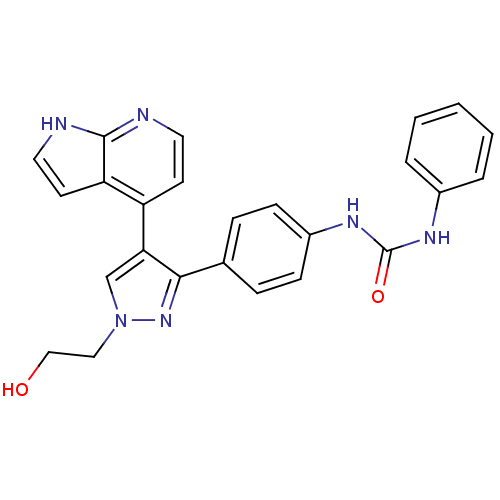
(CHEMBL1099010 | N-{4-[1-(2-Hydroxyethyl)-4-(1H-pyr...)Show SMILES OCCn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C25H22N6O2/c32-15-14-31-16-22(20-10-12-26-24-21(20)11-13-27-24)23(30-31)17-6-8-19(9-7-17)29-25(33)28-18-4-2-1-3-5-18/h1-13,16,32H,14-15H2,(H,26,27)(H2,28,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316470
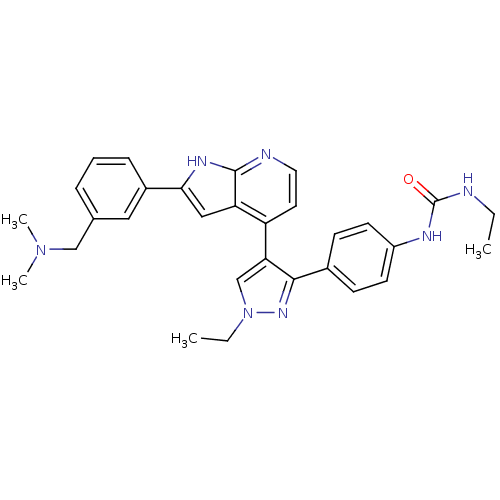
(4-[3-(4-N-Ethylcarbamylaminophenyl)-1-ethyl-1H-pyr...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-5-31-30(38)33-23-12-10-21(11-13-23)28-26(19-37(6-2)35-28)24-14-15-32-29-25(24)17-27(34-29)22-9-7-8-20(16-22)18-36(3)4/h7-17,19H,5-6,18H2,1-4H3,(H,32,34)(H2,31,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316472
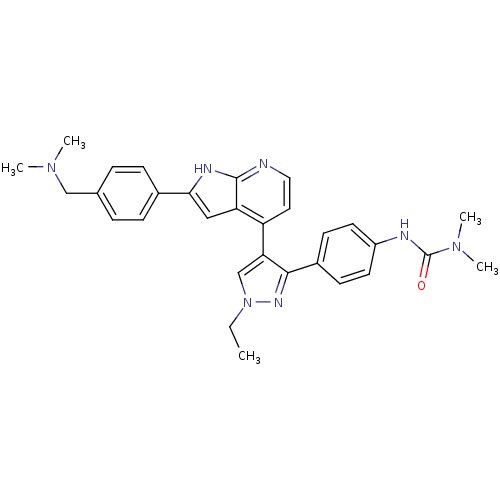
(4-[3-(4-N,N-Dimethylcarbamylaminophenyl)-1-ethyl-1...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1ccc(CN(C)C)cc1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)22-11-13-23(14-12-22)32-30(38)36(4)5)24-15-16-31-29-25(24)17-27(33-29)21-9-7-20(8-10-21)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132C]
(Homo sapiens (Human)) | BDBM195601

(GSK321 | US11311527, Cpd ID GSK321 | US11376246, C...)Show SMILES C[C@H](O)c1cccc(NC(=O)c2nn(Cc3ccc(F)cc3)c3[C@H](C)CN(Cc23)C(=O)c2ccc[nH]2)c1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | 25 |
Albert Einstein College of Medicine
| Assay Description
RapidFire-MS/MS measurements of mutant IDH1 and IDH2 reactions were conducted at room temperature in 384-well Greiner polypropylene microtiter plates... |
Nat Chem Biol 11: 878-86 (2015)
Article DOI: 10.1038/nchembio.1930
BindingDB Entry DOI: 10.7270/Q2RR1X2G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM31093

(4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...)Show SMILES OC(=O)c1ccc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2F)cc1 |c:13| Show InChI InChI=1S/C25H15ClF2N4O2/c26-15-6-9-17-18(10-15)23(21-19(27)2-1-3-20(21)28)29-11-14-12-30-25(32-22(14)17)31-16-7-4-13(5-8-16)24(33)34/h1-10,12H,11H2,(H,33,34)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50091692

(CHEMBL3582357)Show SMILES Cc1nnc(NS(=O)(=O)c2ccc(NCc3cc(cc(c3)-c3ccc4OCOc4c3)C(O)=O)cc2)s1 Show InChI InChI=1S/C24H20N4O6S2/c1-14-26-27-24(35-14)28-36(31,32)20-5-3-19(4-6-20)25-12-15-8-17(10-18(9-15)23(29)30)16-2-7-21-22(11-16)34-13-33-21/h2-11,25H,12-13H2,1H3,(H,27,28)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
ACS Med Chem Lett 6: 531-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00025
BindingDB Entry DOI: 10.7270/Q2BP04JF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50091696
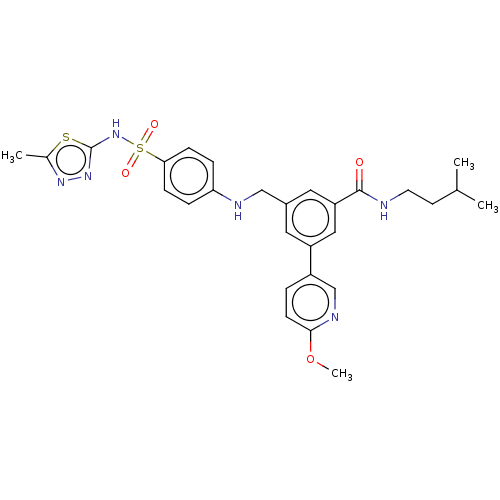
(CHEMBL3582351)Show SMILES COc1ccc(cn1)-c1cc(CNc2ccc(cc2)S(=O)(=O)Nc2nnc(C)s2)cc(c1)C(=O)NCCC(C)C Show InChI InChI=1S/C28H32N6O4S2/c1-18(2)11-12-29-27(35)23-14-20(13-22(15-23)21-5-10-26(38-4)31-17-21)16-30-24-6-8-25(9-7-24)40(36,37)34-28-33-32-19(3)39-28/h5-10,13-15,17-18,30H,11-12,16H2,1-4H3,(H,29,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
ACS Med Chem Lett 6: 531-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00025
BindingDB Entry DOI: 10.7270/Q2BP04JF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50091695
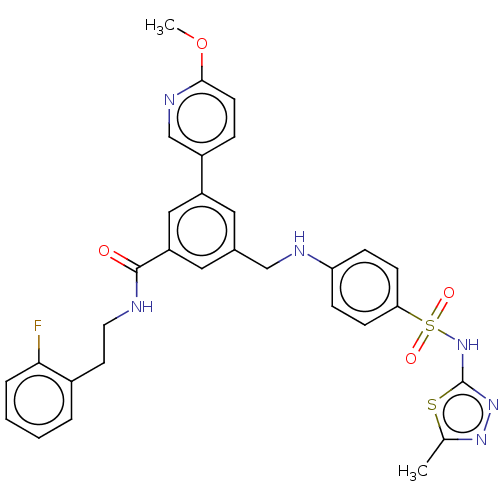
(CHEMBL3582350)Show SMILES COc1ccc(cn1)-c1cc(CNc2ccc(cc2)S(=O)(=O)Nc2nnc(C)s2)cc(c1)C(=O)NCCc1ccccc1F Show InChI InChI=1S/C31H29FN6O4S2/c1-20-36-37-31(43-20)38-44(40,41)27-10-8-26(9-11-27)34-18-21-15-24(23-7-12-29(42-2)35-19-23)17-25(16-21)30(39)33-14-13-22-5-3-4-6-28(22)32/h3-12,15-17,19,34H,13-14,18H2,1-2H3,(H,33,39)(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
ACS Med Chem Lett 6: 531-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00025
BindingDB Entry DOI: 10.7270/Q2BP04JF |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316476
(CHEMBL1097521 | N'-[4-(1-Ethyl-4-{2-[4-(1-pyrrolid...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1ccc(CN2CCCC2)cc1 Show InChI InChI=1S/C32H35N7O/c1-4-39-21-28(30(36-39)24-11-13-25(14-12-24)34-32(40)37(2)3)26-15-16-33-31-27(26)19-29(35-31)23-9-7-22(8-10-23)20-38-17-5-6-18-38/h7-16,19,21H,4-6,17-18,20H2,1-3H3,(H,33,35)(H,34,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM195601

(GSK321 | US11311527, Cpd ID GSK321 | US11376246, C...)Show SMILES C[C@H](O)c1cccc(NC(=O)c2nn(Cc3ccc(F)cc3)c3[C@H](C)CN(Cc23)C(=O)c2ccc[nH]2)c1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Albert Einstein College of Medicine
| Assay Description
RapidFire-MS/MS measurements of mutant IDH1 and IDH2 reactions were conducted at room temperature in 384-well Greiner polypropylene microtiter plates... |
Nat Chem Biol 11: 878-86 (2015)
Article DOI: 10.1038/nchembio.1930
BindingDB Entry DOI: 10.7270/Q2RR1X2G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data