 Found 1474 hits with Last Name = 'lo' and Initial = 'sm'
Found 1474 hits with Last Name = 'lo' and Initial = 'sm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066647

(CHEMBL113724 | {(S)-3-Methyl-1-[3-oxo-1-(4-phenoxy...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C31H35N3O7S/c1-22(2)19-28(33-31(37)40-21-23-9-5-3-6-10-23)30(36)32-27-17-18-34(20-29(27)35)42(38,39)26-15-13-25(14-16-26)41-24-11-7-4-8-12-24/h3-16,22,27-28H,17-21H2,1-2H3,(H,32,36)(H,33,37)/t27?,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19808
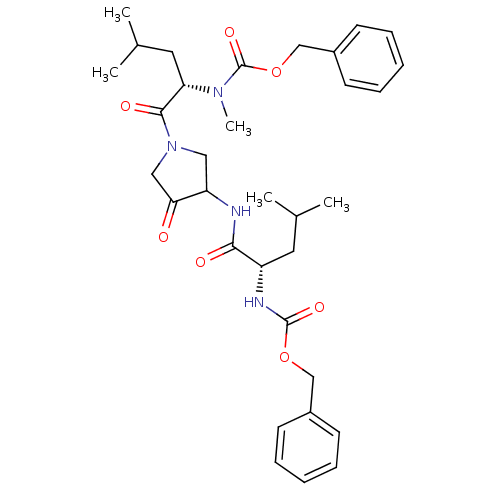
(benzyl N-[(1S)-1-({1-[(2S)-2-{[(benzyloxy)carbonyl...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)N(C)C(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C33H44N4O7/c1-22(2)16-26(35-32(41)43-20-24-12-8-6-9-13-24)30(39)34-27-18-37(19-29(27)38)31(40)28(17-23(3)4)36(5)33(42)44-21-25-14-10-7-11-15-25/h6-15,22-23,26-28H,16-21H2,1-5H3,(H,34,39)(H,35,41)/t26-,27?,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | -52.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
J Med Chem 44: 725-36 (2001)
Article DOI: 10.1021/jm000320t
BindingDB Entry DOI: 10.7270/Q2J67F6C |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19813
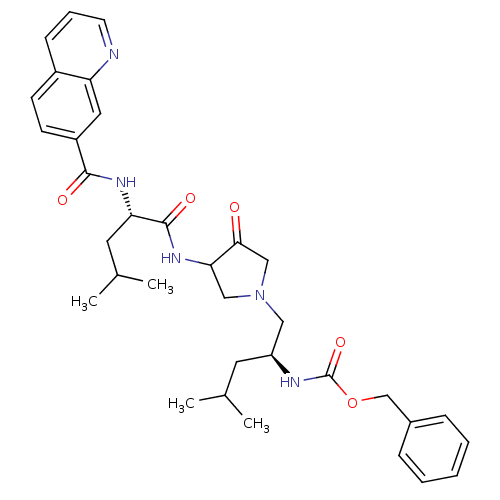
(benzyl N-[(2S)-4-methyl-1-{3-[(2S)-4-methyl-2-(qui...)Show SMILES CC(C)C[C@@H](CN1CC(NC(=O)[C@H](CC(C)C)NC(=O)c2ccc3cccnc3c2)C(=O)C1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C34H43N5O5/c1-22(2)15-27(36-34(43)44-21-24-9-6-5-7-10-24)18-39-19-30(31(40)20-39)38-33(42)29(16-23(3)4)37-32(41)26-13-12-25-11-8-14-35-28(25)17-26/h5-14,17,22-23,27,29-30H,15-16,18-21H2,1-4H3,(H,36,43)(H,37,41)(H,38,42)/t27-,29-,30?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | -49.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
J Med Chem 44: 725-36 (2001)
Article DOI: 10.1021/jm000320t
BindingDB Entry DOI: 10.7270/Q2J67F6C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098580
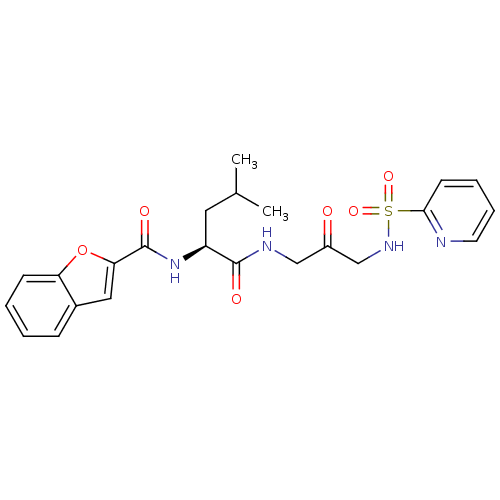
(Benzofuran-2-carboxylic acid {3-methyl-1-[2-oxo-3-...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)NCC(=O)CNS(=O)(=O)c1ccccn1 Show InChI InChI=1S/C23H26N4O6S/c1-15(2)11-18(27-23(30)20-12-16-7-3-4-8-19(16)33-20)22(29)25-13-17(28)14-26-34(31,32)21-9-5-6-10-24-21/h3-10,12,15,18,26H,11,13-14H2,1-2H3,(H,25,29)(H,27,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19811
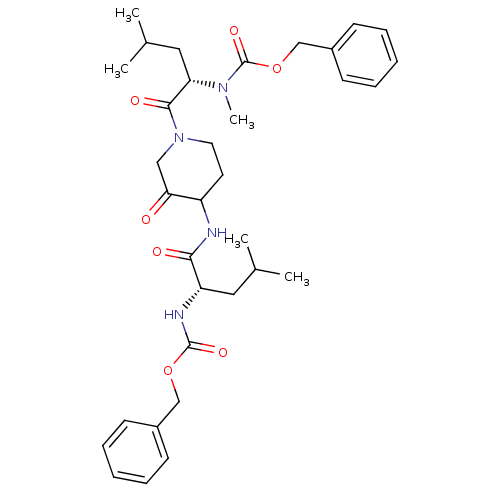
(benzyl N-[(1S)-1-({1-[(2S)-2-{[(benzyloxy)carbonyl...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)N(C)C(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C34H46N4O7/c1-23(2)18-28(36-33(42)44-21-25-12-8-6-9-13-25)31(40)35-27-16-17-38(20-30(27)39)32(41)29(19-24(3)4)37(5)34(43)45-22-26-14-10-7-11-15-26/h6-15,23-24,27-29H,16-22H2,1-5H3,(H,35,40)(H,36,42)/t27?,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | -49.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
J Med Chem 44: 725-36 (2001)
Article DOI: 10.1021/jm000320t
BindingDB Entry DOI: 10.7270/Q2J67F6C |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50417494
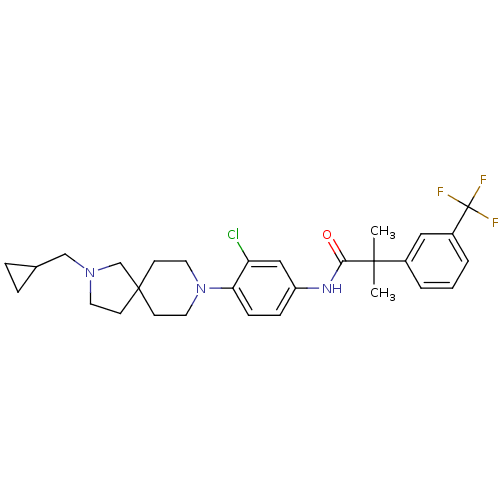
(CHEMBL1288285)Show SMILES CC(C)(C(=O)Nc1ccc(N2CCC3(CCN(CC4CC4)C3)CC2)c(Cl)c1)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C29H35ClF3N3O/c1-27(2,21-4-3-5-22(16-21)29(31,32)33)26(37)34-23-8-9-25(24(30)17-23)36-14-11-28(12-15-36)10-13-35(19-28)18-20-6-7-20/h3-5,8-9,16-17,20H,6-7,10-15,18-19H2,1-2H3,(H,34,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y2 receptor in KAN-TS cells by [35]GTPgammaS assay |
Bioorg Med Chem Lett 20: 7341-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.065
BindingDB Entry DOI: 10.7270/Q2BV7HWV |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098584
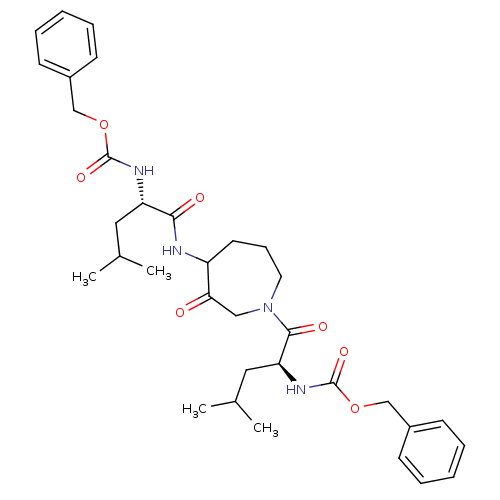
(CHEMBL31947 | {1-[4-(2-Benzyloxycarbonylamino-4-me...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C34H46N4O7/c1-23(2)18-28(36-33(42)44-21-25-12-7-5-8-13-25)31(40)35-27-16-11-17-38(20-30(27)39)32(41)29(19-24(3)4)37-34(43)45-22-26-14-9-6-10-15-26/h5-10,12-15,23-24,27-29H,11,16-22H2,1-4H3,(H,35,40)(H,36,42)(H,37,43)/t27?,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Procathepsin L
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19807
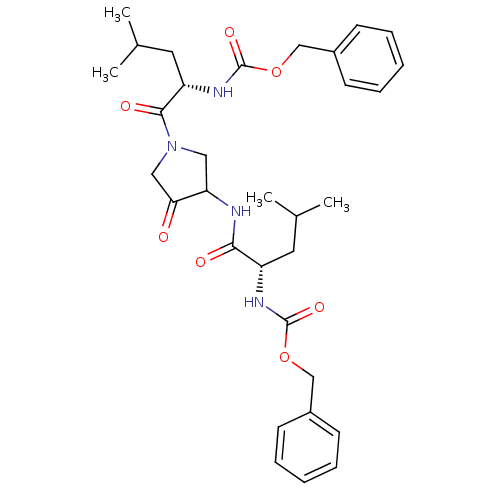
(CHEMBL100563 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C32H42N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-27H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.30 | -48.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
J Med Chem 44: 725-36 (2001)
Article DOI: 10.1021/jm000320t
BindingDB Entry DOI: 10.7270/Q2J67F6C |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19807

(CHEMBL100563 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C32H42N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-27H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098579
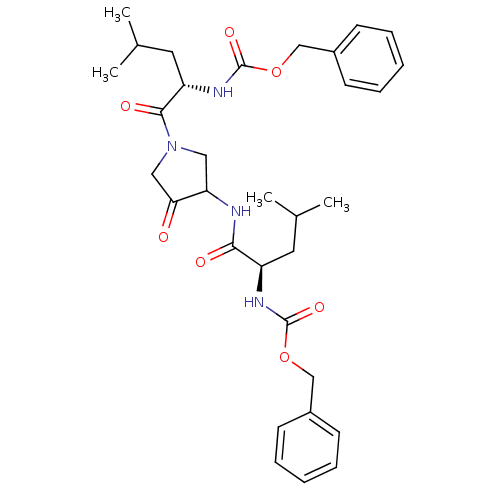
(CHEMBL29483 | {1-[3-(2-Benzyloxycarbonylamino-4-me...)Show SMILES CC(C)C[C@@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C32H42N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-27H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26+,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470323
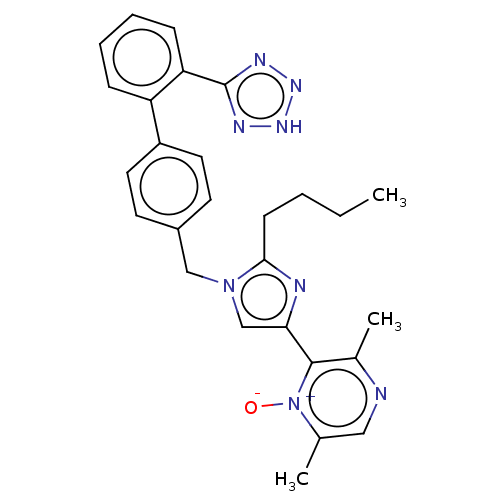
(CHEMBL327505)Show SMILES CCCCc1nc(cn1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-c1c(C)ncc(C)[n+]1[O-] Show InChI InChI=1S/C27H28N8O/c1-4-5-10-25-29-24(26-19(3)28-15-18(2)35(26)36)17-34(25)16-20-11-13-21(14-12-20)22-8-6-7-9-23(22)27-30-32-33-31-27/h6-9,11-15,17H,4-5,10,16H2,1-3H3,(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 |
J Med Chem 38: 2925-37 (1995)
Article DOI: 10.1021/jm00015a015
BindingDB Entry DOI: 10.7270/Q2697689 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50414466

(CHEMBL550583)Show SMILES COc1ccccc1C(=O)N1CCN(CC1)c1ccc(NC(=O)C(C)(C)c2ccccc2)cc1Cl Show InChI InChI=1S/C28H30ClN3O3/c1-28(2,20-9-5-4-6-10-20)27(34)30-21-13-14-24(23(29)19-21)31-15-17-32(18-16-31)26(33)22-11-7-8-12-25(22)35-3/h4-14,19H,15-18H2,1-3H3,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human neuropeptide Y2 receptor in KAN-TS cells by [35]GTPgammaS assay |
Bioorg Med Chem Lett 19: 4022-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.035
BindingDB Entry DOI: 10.7270/Q2B27VBW |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098575
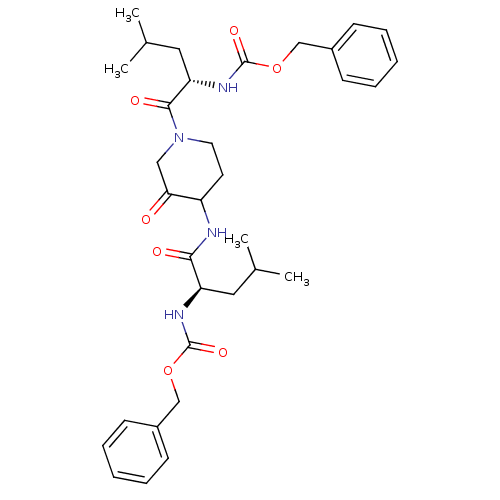
(CHEMBL281086 | {1-[4-(2-Benzyloxycarbonylamino-4-m...)Show SMILES CC(C)C[C@@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19810
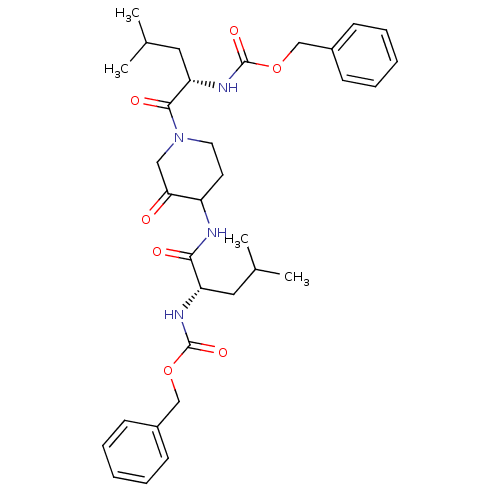
(CHEMBL118449 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | -48.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
J Med Chem 44: 725-36 (2001)
Article DOI: 10.1021/jm000320t
BindingDB Entry DOI: 10.7270/Q2J67F6C |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19810

(CHEMBL118449 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066648
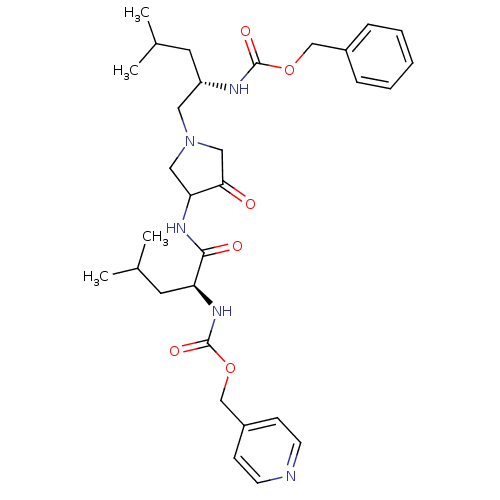
(((S)-3-Methyl-1-{3-[(S)-4-methyl-2-(pyridin-4-ylme...)Show SMILES CC(C)C[C@@H](CN1CC(NC(=O)[C@H](CC(C)C)NC(=O)OCc2ccncc2)C(=O)C1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H43N5O6/c1-21(2)14-25(33-30(39)41-19-23-8-6-5-7-9-23)16-36-17-27(28(37)18-36)34-29(38)26(15-22(3)4)35-31(40)42-20-24-10-12-32-13-11-24/h5-13,21-22,25-27H,14-20H2,1-4H3,(H,33,39)(H,34,38)(H,35,40)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50414465

(CHEMBL550559)Show SMILES CC(C)(C(=O)Nc1ccc(N2CCN(CC2)C(=O)c2ccccc2C(F)(F)F)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C28H27ClF3N3O2/c1-27(2,19-8-4-3-5-9-19)26(37)33-20-12-13-24(23(29)18-20)34-14-16-35(17-15-34)25(36)21-10-6-7-11-22(21)28(30,31)32/h3-13,18H,14-17H2,1-2H3,(H,33,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human neuropeptide Y2 receptor in KAN-TS cells by [35]GTPgammaS assay |
Bioorg Med Chem Lett 19: 4022-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.035
BindingDB Entry DOI: 10.7270/Q2B27VBW |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin S |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470328
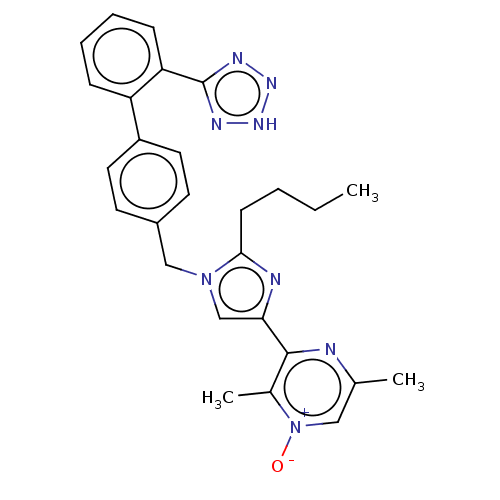
(CHEMBL317300)Show SMILES CCCCc1nc(cn1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-c1nc(C)c[n+]([O-])c1C Show InChI InChI=1S/C27H28N8O/c1-4-5-10-25-29-24(26-19(3)35(36)15-18(2)28-26)17-34(25)16-20-11-13-21(14-12-20)22-8-6-7-9-23(22)27-30-32-33-31-27/h6-9,11-15,17H,4-5,10,16H2,1-3H3,(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 |
J Med Chem 38: 2925-37 (1995)
Article DOI: 10.1021/jm00015a015
BindingDB Entry DOI: 10.7270/Q2697689 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470310
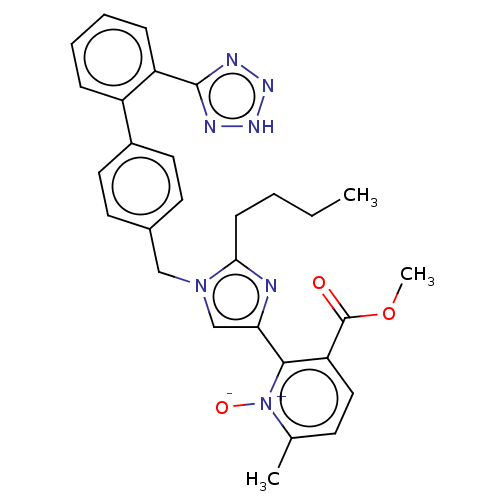
(CHEMBL98592)Show SMILES CCCCc1nc(cn1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-c1c(ccc(C)[n+]1[O-])C(=O)OC Show InChI InChI=1S/C29H29N7O3/c1-4-5-10-26-30-25(27-24(29(37)39-3)16-11-19(2)36(27)38)18-35(26)17-20-12-14-21(15-13-20)22-8-6-7-9-23(22)28-31-33-34-32-28/h6-9,11-16,18H,4-5,10,17H2,1-3H3,(H,31,32,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 |
J Med Chem 38: 2925-37 (1995)
Article DOI: 10.1021/jm00015a015
BindingDB Entry DOI: 10.7270/Q2697689 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098581

(5-(2-Morpholin-4-yl-ethoxy)-benzofuran-2-carboxyli...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Rattus norvegicus) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Rat cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50414470

(CHEMBL551311)Show SMILES Cc1cccc(c1)C(C)(C)C(=O)Nc1ccc(N2CCN(CC2)C(=O)c2ccccc2)c(Cl)c1 Show InChI InChI=1S/C28H30ClN3O2/c1-20-8-7-11-22(18-20)28(2,3)27(34)30-23-12-13-25(24(29)19-23)31-14-16-32(17-15-31)26(33)21-9-5-4-6-10-21/h4-13,18-19H,14-17H2,1-3H3,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human neuropeptide Y2 receptor in KAN-TS cells by [35]GTPgammaS assay |
Bioorg Med Chem Lett 19: 4022-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.035
BindingDB Entry DOI: 10.7270/Q2B27VBW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50432672
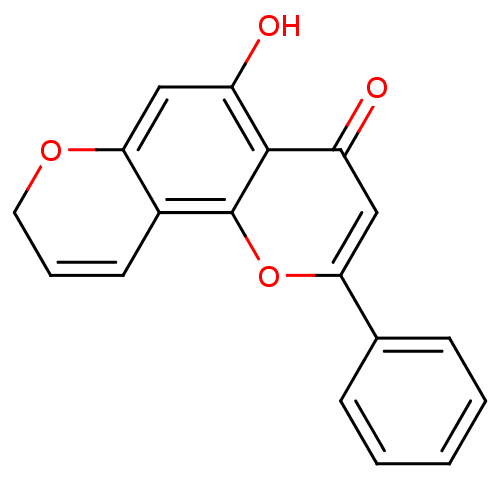
(CHEMBL2347912)Show SMILES Oc1cc2OCC=Cc2c2oc(cc(=O)c12)-c1ccccc1 |c:6| Show InChI InChI=1S/C18H12O4/c19-13-9-15(11-5-2-1-3-6-11)22-18-12-7-4-8-21-16(12)10-14(20)17(13)18/h1-7,9-10,20H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana
Curated by ChEMBL
| Assay Description
Inhibition of human microsomal CYP1B1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... |
J Med Chem 58: 6481-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00494
BindingDB Entry DOI: 10.7270/Q2183895 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50084137
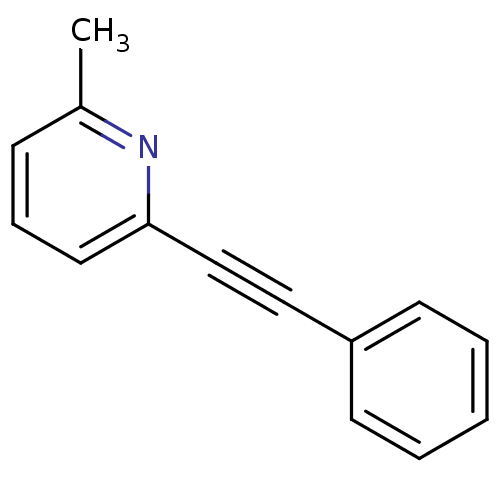
(2-Methyl-6-(phenylethynyl)pyridine | 2-Methyl-6-ph...)Show InChI InChI=1S/C14H11N/c1-12-6-5-9-14(15-12)11-10-13-7-3-2-4-8-13/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEP from mGlu5 receptor in rat brain |
Bioorg Med Chem Lett 17: 4415-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.030
BindingDB Entry DOI: 10.7270/Q28G8KDC |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50414458
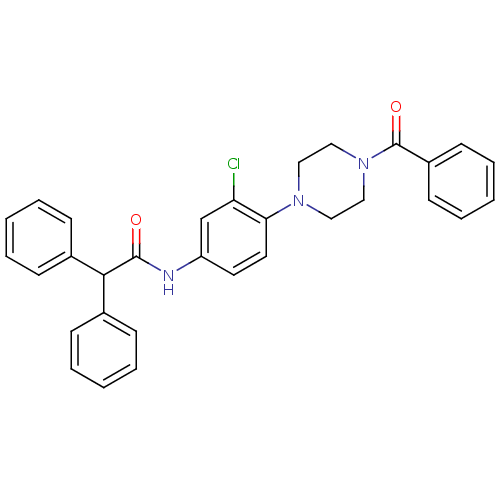
(CHEMBL562725)Show SMILES Clc1cc(NC(=O)C(c2ccccc2)c2ccccc2)ccc1N1CCN(CC1)C(=O)c1ccccc1 Show InChI InChI=1S/C31H28ClN3O2/c32-27-22-26(33-30(36)29(23-10-4-1-5-11-23)24-12-6-2-7-13-24)16-17-28(27)34-18-20-35(21-19-34)31(37)25-14-8-3-9-15-25/h1-17,22,29H,18-21H2,(H,33,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human neuropeptide Y2 receptor in KAN-TS cells by [35]GTPgammaS assay |
Bioorg Med Chem Lett 19: 4022-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.035
BindingDB Entry DOI: 10.7270/Q2B27VBW |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19782
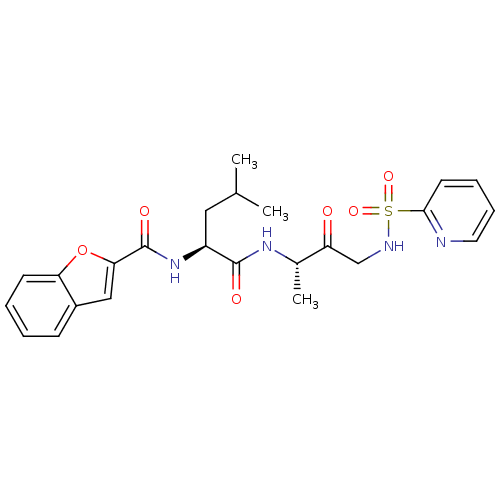
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(2...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@@H](C)C(=O)CNS(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C24H28N4O6S/c1-15(2)12-18(28-24(31)21-13-17-8-4-5-9-20(17)34-21)23(30)27-16(3)19(29)14-26-35(32,33)22-10-6-7-11-25-22/h4-11,13,15-16,18,26H,12,14H2,1-3H3,(H,27,30)(H,28,31)/t16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470321

(CHEMBL99008)Show SMILES CCCCc1nc(cn1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-c1nc(C)cnc1C Show InChI InChI=1S/C27H28N8/c1-4-5-10-25-30-24(26-19(3)28-15-18(2)29-26)17-35(25)16-20-11-13-21(14-12-20)22-8-6-7-9-23(22)27-31-33-34-32-27/h6-9,11-15,17H,4-5,10,16H2,1-3H3,(H,31,32,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 |
J Med Chem 38: 2925-37 (1995)
Article DOI: 10.1021/jm00015a015
BindingDB Entry DOI: 10.7270/Q2697689 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50240692
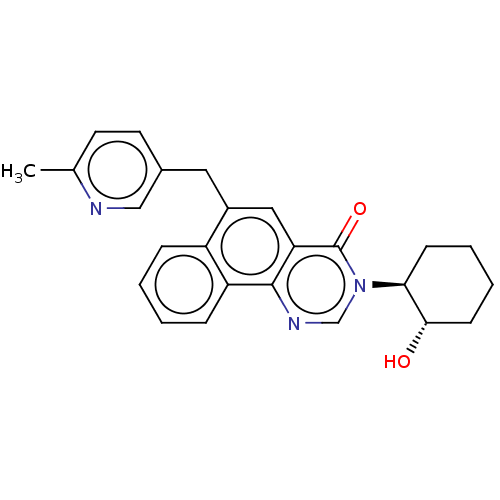
(CHEMBL4078588)Show SMILES Cc1ccc(Cc2cc3c(ncn([C@H]4CCCC[C@@H]4O)c3=O)c3ccccc23)cn1 |r| Show InChI InChI=1S/C25H25N3O2/c1-16-10-11-17(14-26-16)12-18-13-21-24(20-7-3-2-6-19(18)20)27-15-28(25(21)30)22-8-4-5-9-23(22)29/h2-3,6-7,10-11,13-15,22-23,29H,4-5,8-9,12H2,1H3/t22-,23-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PT-1284 human muscarinic acetylcholine receptor M1 expressed in CHO cell membranes after 90 mins scintillation counting method |
J Med Chem 60: 6649-6663 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00597
BindingDB Entry DOI: 10.7270/Q2ZK5JS0 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19810

(CHEMBL118449 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
J Med Chem 44: 725-36 (2001)
Article DOI: 10.1021/jm000320t
BindingDB Entry DOI: 10.7270/Q2J67F6C |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470340
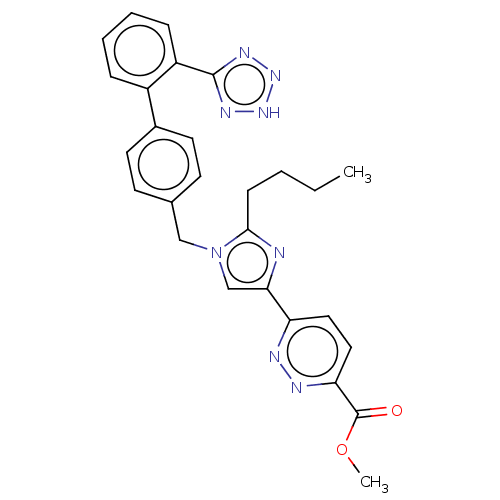
(CHEMBL98370)Show SMILES CCCCc1nc(cn1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-c1ccc(nn1)C(=O)OC Show InChI InChI=1S/C27H26N8O2/c1-3-4-9-25-28-24(22-14-15-23(30-29-22)27(36)37-2)17-35(25)16-18-10-12-19(13-11-18)20-7-5-6-8-21(20)26-31-33-34-32-26/h5-8,10-15,17H,3-4,9,16H2,1-2H3,(H,31,32,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 |
J Med Chem 38: 2925-37 (1995)
Article DOI: 10.1021/jm00015a015
BindingDB Entry DOI: 10.7270/Q2697689 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470326

(CHEMBL319990)Show SMILES CCCCc1nc(cn1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-c1c(C(=O)OC)c(C)cc(C)[n+]1[O-] Show InChI InChI=1S/C30H31N7O3/c1-5-6-11-26-31-25(28-27(30(38)40-4)19(2)16-20(3)37(28)39)18-36(26)17-21-12-14-22(15-13-21)23-9-7-8-10-24(23)29-32-34-35-33-29/h7-10,12-16,18H,5-6,11,17H2,1-4H3,(H,32,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 |
J Med Chem 38: 2925-37 (1995)
Article DOI: 10.1021/jm00015a015
BindingDB Entry DOI: 10.7270/Q2697689 |
More data for this
Ligand-Target Pair | |
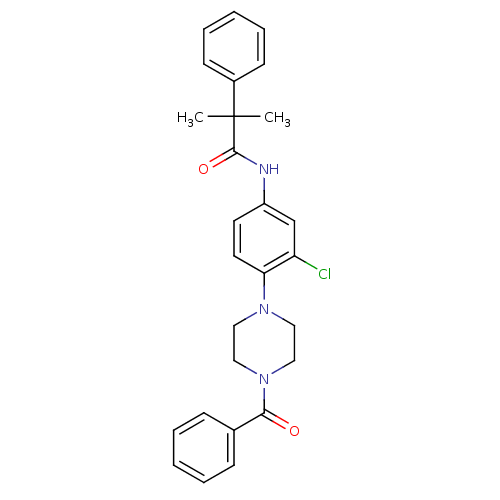
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50414459
(CHEMBL549771)Show SMILES CC(C)(C(=O)Nc1ccc(N2CCN(CC2)C(=O)c2ccccc2)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C27H28ClN3O2/c1-27(2,21-11-7-4-8-12-21)26(33)29-22-13-14-24(23(28)19-22)30-15-17-31(18-16-30)25(32)20-9-5-3-6-10-20/h3-14,19H,15-18H2,1-2H3,(H,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human neuropeptide Y2 receptor in KAN-TS cells by [35]GTPgammaS assay |
Bioorg Med Chem Lett 19: 4022-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.035
BindingDB Entry DOI: 10.7270/Q2B27VBW |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19815
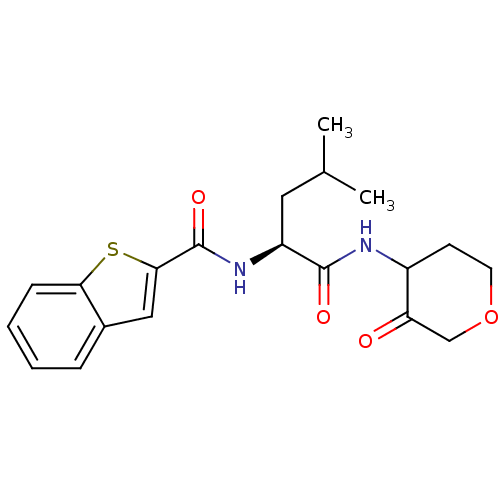
((2S)-2-(1-benzothiophen-2-ylformamido)-4-methyl-N-...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2s1)C(=O)NC1CCOCC1=O |r| Show InChI InChI=1S/C20H24N2O4S/c1-12(2)9-15(19(24)21-14-7-8-26-11-16(14)23)22-20(25)18-10-13-5-3-4-6-17(13)27-18/h3-6,10,12,14-15H,7-9,11H2,1-2H3,(H,21,24)(H,22,25)/t14?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
J Med Chem 44: 725-36 (2001)
Article DOI: 10.1021/jm000320t
BindingDB Entry DOI: 10.7270/Q2J67F6C |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470330
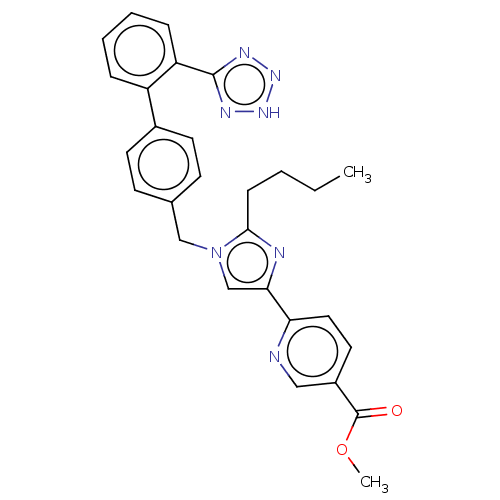
(CHEMBL318194)Show SMILES CCCCc1nc(cn1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-c1ccc(cn1)C(=O)OC Show InChI InChI=1S/C28H27N7O2/c1-3-4-9-26-30-25(24-15-14-21(16-29-24)28(36)37-2)18-35(26)17-19-10-12-20(13-11-19)22-7-5-6-8-23(22)27-31-33-34-32-27/h5-8,10-16,18H,3-4,9,17H2,1-2H3,(H,31,32,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 |
J Med Chem 38: 2925-37 (1995)
Article DOI: 10.1021/jm00015a015
BindingDB Entry DOI: 10.7270/Q2697689 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470313

(CHEMBL441640)Show SMILES CCCCc1nc(cn1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-c1ccc(C)nn1 Show InChI InChI=1S/C26H26N8/c1-3-4-9-25-27-24(23-15-10-18(2)28-29-23)17-34(25)16-19-11-13-20(14-12-19)21-7-5-6-8-22(21)26-30-32-33-31-26/h5-8,10-15,17H,3-4,9,16H2,1-2H3,(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 |
J Med Chem 38: 2925-37 (1995)
Article DOI: 10.1021/jm00015a015
BindingDB Entry DOI: 10.7270/Q2697689 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50414467
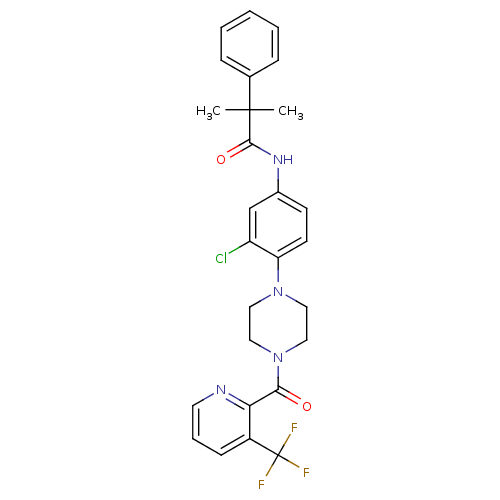
(CHEMBL552112)Show SMILES CC(C)(C(=O)Nc1ccc(N2CCN(CC2)C(=O)c2ncccc2C(F)(F)F)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C27H26ClF3N4O2/c1-26(2,18-7-4-3-5-8-18)25(37)33-19-10-11-22(21(28)17-19)34-13-15-35(16-14-34)24(36)23-20(27(29,30)31)9-6-12-32-23/h3-12,17H,13-16H2,1-2H3,(H,33,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human neuropeptide Y2 receptor in KAN-TS cells by [35]GTPgammaS assay |
Bioorg Med Chem Lett 19: 4022-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.035
BindingDB Entry DOI: 10.7270/Q2B27VBW |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50414461
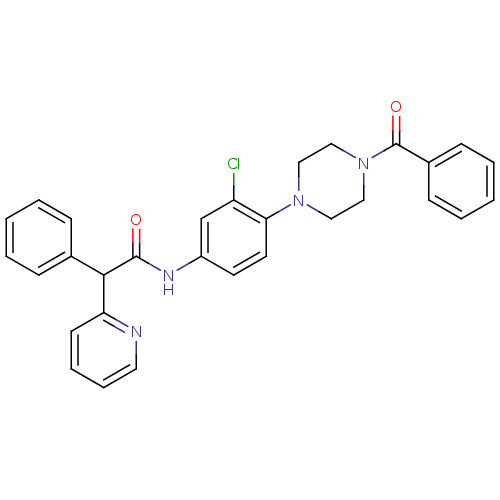
(CHEMBL556481)Show SMILES Clc1cc(NC(=O)C(c2ccccc2)c2ccccn2)ccc1N1CCN(CC1)C(=O)c1ccccc1 Show InChI InChI=1S/C30H27ClN4O2/c31-25-21-24(33-29(36)28(22-9-3-1-4-10-22)26-13-7-8-16-32-26)14-15-27(25)34-17-19-35(20-18-34)30(37)23-11-5-2-6-12-23/h1-16,21,28H,17-20H2,(H,33,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human neuropeptide Y2 receptor in KAN-TS cells by [35]GTPgammaS assay |
Bioorg Med Chem Lett 19: 4022-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.035
BindingDB Entry DOI: 10.7270/Q2B27VBW |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin S |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50432672

(CHEMBL2347912)Show SMILES Oc1cc2OCC=Cc2c2oc(cc(=O)c12)-c1ccccc1 |c:6| Show InChI InChI=1S/C18H12O4/c19-13-9-15(11-5-2-1-3-6-11)22-18-12-7-4-8-21-16(12)10-14(20)17(13)18/h1-7,9-10,20H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana
Curated by ChEMBL
| Assay Description
Inhibition of human microsomal CYP1A2-dependent methoxyresorufin-O-demethylase activity by spectrofluorimetric analysis in presence of NADPH regenera... |
J Med Chem 58: 6481-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00494
BindingDB Entry DOI: 10.7270/Q2183895 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098578
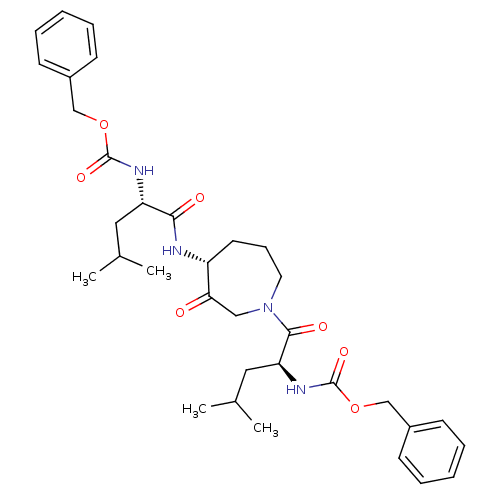
(CHEMBL286034 | {1-[4-(2-Benzyloxycarbonylamino-4-m...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H]1CCCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C34H46N4O7/c1-23(2)18-28(36-33(42)44-21-25-12-7-5-8-13-25)31(40)35-27-16-11-17-38(20-30(27)39)32(41)29(19-24(3)4)37-34(43)45-22-26-14-9-6-10-15-26/h5-10,12-15,23-24,27-29H,11,16-22H2,1-4H3,(H,35,40)(H,36,42)(H,37,43)/t27-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50417479

(CHEMBL1288078)Show SMILES CC(C)(C(=O)Nc1ccc(N2CCC(CC2)N2CCCCC2)c(Cl)c1)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C27H33ClF3N3O/c1-26(2,19-7-6-8-20(17-19)27(29,30)31)25(35)32-21-9-10-24(23(28)18-21)34-15-11-22(12-16-34)33-13-4-3-5-14-33/h6-10,17-18,22H,3-5,11-16H2,1-2H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y2 receptor in KAN-TS cells by [35]GTPgammaS assay |
Bioorg Med Chem Lett 20: 7341-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.065
BindingDB Entry DOI: 10.7270/Q2BV7HWV |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50417493
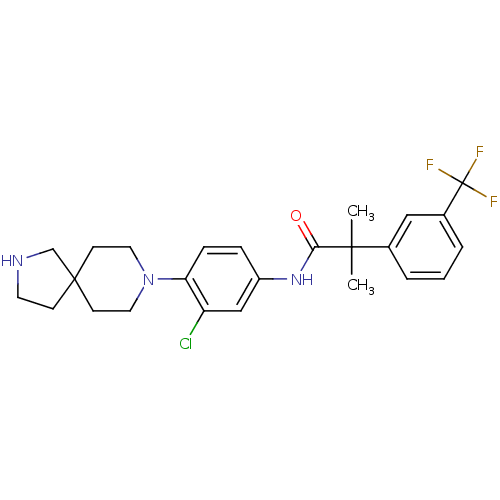
(CHEMBL1288284)Show SMILES CC(C)(C(=O)Nc1ccc(N2CCC3(CCNC3)CC2)c(Cl)c1)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C25H29ClF3N3O/c1-23(2,17-4-3-5-18(14-17)25(27,28)29)22(33)31-19-6-7-21(20(26)15-19)32-12-9-24(10-13-32)8-11-30-16-24/h3-7,14-15,30H,8-13,16H2,1-2H3,(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y2 receptor in KAN-TS cells by [35]GTPgammaS assay |
Bioorg Med Chem Lett 20: 7341-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.065
BindingDB Entry DOI: 10.7270/Q2BV7HWV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50240694
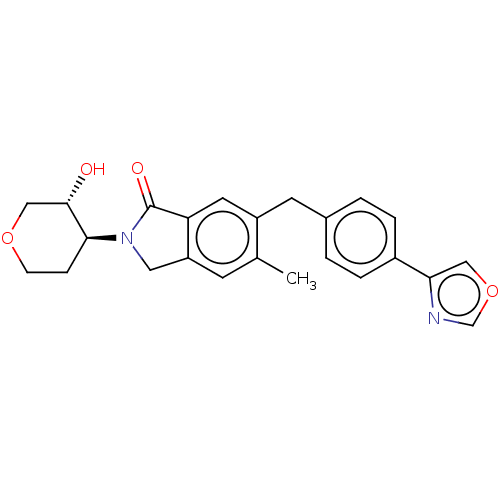
(CHEMBL4067722)Show SMILES Cc1cc2CN([C@H]3CCOC[C@@H]3O)C(=O)c2cc1Cc1ccc(cc1)-c1cocn1 |r| Show InChI InChI=1S/C24H24N2O4/c1-15-8-19-11-26(22-6-7-29-13-23(22)27)24(28)20(19)10-18(15)9-16-2-4-17(5-3-16)21-12-30-14-25-21/h2-5,8,10,12,14,22-23,27H,6-7,9,11,13H2,1H3/t22-,23-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PT-1284 human muscarinic acetylcholine receptor M1 expressed in CHO cell membranes after 90 mins scintillation counting method |
J Med Chem 60: 6649-6663 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00597
BindingDB Entry DOI: 10.7270/Q2ZK5JS0 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19810

(CHEMBL118449 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
J Med Chem 44: 725-36 (2001)
Article DOI: 10.1021/jm000320t
BindingDB Entry DOI: 10.7270/Q2J67F6C |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19810

(CHEMBL118449 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data