 Found 254 hits with Last Name = 'chang' and Initial = 'sp'
Found 254 hits with Last Name = 'chang' and Initial = 'sp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352059
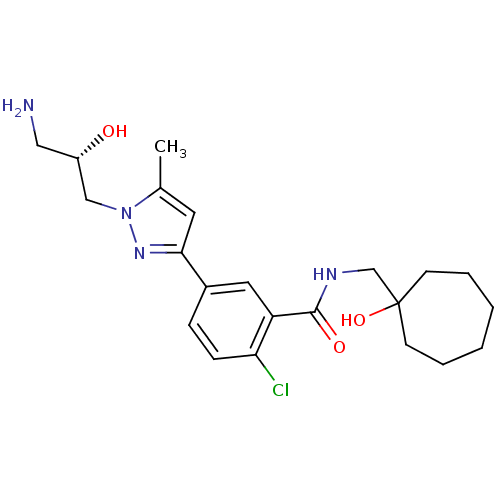
(CHEMBL1824026)Show SMILES Cc1cc(nn1C[C@@H](O)CN)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 |r| Show InChI InChI=1S/C22H31ClN4O3/c1-15-10-20(26-27(15)13-17(28)12-24)16-6-7-19(23)18(11-16)21(29)25-14-22(30)8-4-2-3-5-9-22/h6-7,10-11,17,28,30H,2-5,8-9,12-14,24H2,1H3,(H,25,29)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced YO-PRO-1 uptake |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163809
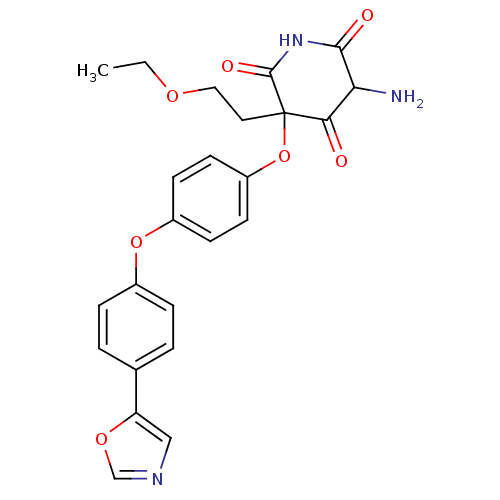
(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(4-oxazol-5-yl-phe...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3cnco3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C24H23N3O7/c1-2-31-12-11-24(21(28)20(25)22(29)27-23(24)30)34-18-9-7-17(8-10-18)33-16-5-3-15(4-6-16)19-13-26-14-32-19/h3-10,13-14,20H,2,11-12,25H2,1H3,(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163808

(5-Amino-3-(2-ethoxy-ethyl)-3-{4-[4-(1H-pyrazol-4-y...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3cn[nH]c3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C24H24N4O6/c1-2-32-12-11-24(21(29)20(25)22(30)28-23(24)31)34-19-9-7-18(8-10-19)33-17-5-3-15(4-6-17)16-13-26-27-14-16/h3-10,13-14,20H,2,11-12,25H2,1H3,(H,26,27)(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50344738
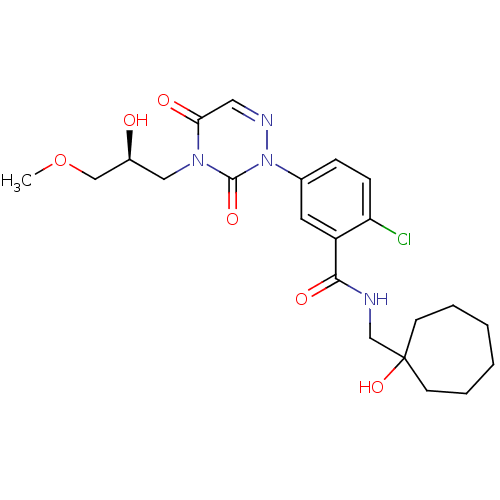
((S)-2-chloro-5-(4-(2-hydroxy-3-methoxypropyl)-3,5-...)Show SMILES COC[C@@H](O)Cn1c(=O)cnn(-c2ccc(Cl)c(c2)C(=O)NCC2(O)CCCCCC2)c1=O |r| Show InChI InChI=1S/C22H29ClN4O6/c1-33-13-16(28)12-26-19(29)11-25-27(21(26)31)15-6-7-18(23)17(10-15)20(30)24-14-22(32)8-4-2-3-5-9-22/h6-7,10-11,16,28,32H,2-5,8-9,12-14H2,1H3,(H,24,30)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor in human LPS-stimulated monocytes assessed as inhibition of ATP-induced IL-1beta release by ELISA in presence of... |
Bioorg Med Chem Lett 21: 3708-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.077
BindingDB Entry DOI: 10.7270/Q2B27VMG |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163807
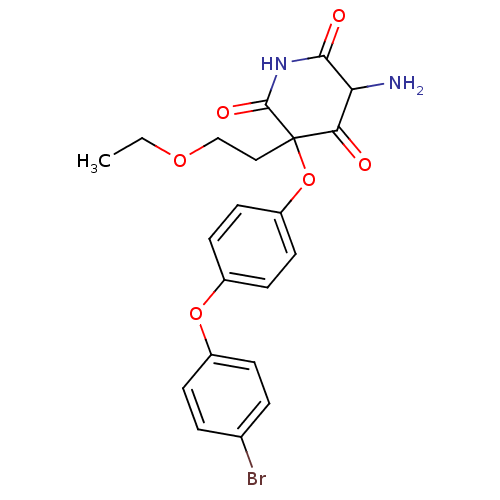
(5-Amino-3-[4-(4-bromo-phenoxy)-phenoxy]-3-(2-ethox...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(Br)cc3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C21H21BrN2O6/c1-2-28-12-11-21(18(25)17(23)19(26)24-20(21)27)30-16-9-7-15(8-10-16)29-14-5-3-13(22)4-6-14/h3-10,17H,2,11-12,23H2,1H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163812
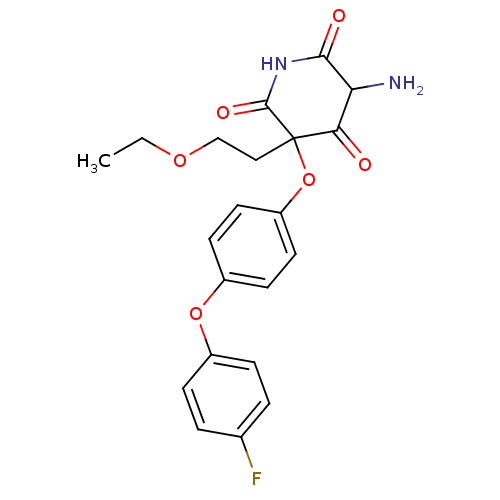
(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(4-fluoro-phenoxy)...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(F)cc3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C21H21FN2O6/c1-2-28-12-11-21(18(25)17(23)19(26)24-20(21)27)30-16-9-7-15(8-10-16)29-14-5-3-13(22)4-6-14/h3-10,17H,2,11-12,23H2,1H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163824
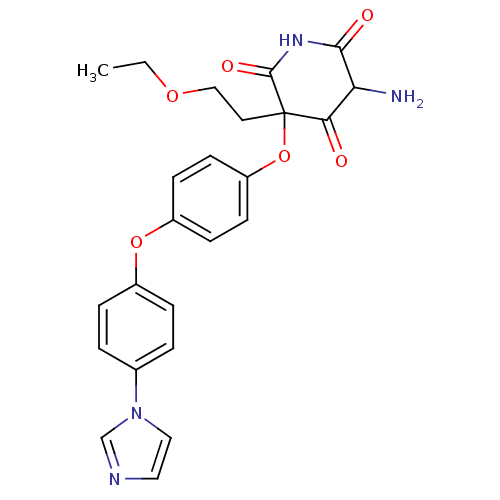
(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(4-imidazol-1-yl-p...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-n3ccnc3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C24H24N4O6/c1-2-32-14-11-24(21(29)20(25)22(30)27-23(24)31)34-19-9-7-18(8-10-19)33-17-5-3-16(4-6-17)28-13-12-26-15-28/h3-10,12-13,15,20H,2,11,14,25H2,1H3,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163799

(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(4-[1,3,4]oxadiazo...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3nnco3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C23H22N4O7/c1-2-31-12-11-23(19(28)18(24)20(29)26-22(23)30)34-17-9-7-16(8-10-17)33-15-5-3-14(4-6-15)21-27-25-13-32-21/h3-10,13,18H,2,11-12,24H2,1H3,(H,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352039

(CHEMBL1823817)Show SMILES COC[C@H](O)Cn1c(=O)cnn(-c2ccc(Cl)c(c2)C(=O)NCC2(O)CCCCCC2)c1=O |r| Show InChI InChI=1S/C22H29ClN4O6/c1-33-13-16(28)12-26-19(29)11-25-27(21(26)31)15-6-7-18(23)17(10-15)20(30)24-14-22(32)8-4-2-3-5-9-22/h6-7,10-11,16,28,32H,2-5,8-9,12-14H2,1H3,(H,24,30)/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-activated human monocytes assessed as inhibition of ATP-induced IL-1beta release in presence of low ser... |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50344738

((S)-2-chloro-5-(4-(2-hydroxy-3-methoxypropyl)-3,5-...)Show SMILES COC[C@@H](O)Cn1c(=O)cnn(-c2ccc(Cl)c(c2)C(=O)NCC2(O)CCCCCC2)c1=O |r| Show InChI InChI=1S/C22H29ClN4O6/c1-33-13-16(28)12-26-19(29)11-25-27(21(26)31)15-6-7-18(23)17(10-15)20(30)24-14-22(32)8-4-2-3-5-9-22/h6-7,10-11,16,28,32H,2-5,8-9,12-14H2,1H3,(H,24,30)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor in human LPS-stimulated monocytes assessed as inhibition of ATP-induced IL1-beta release by ELISA |
Bioorg Med Chem Lett 21: 3708-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.077
BindingDB Entry DOI: 10.7270/Q2B27VMG |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163796
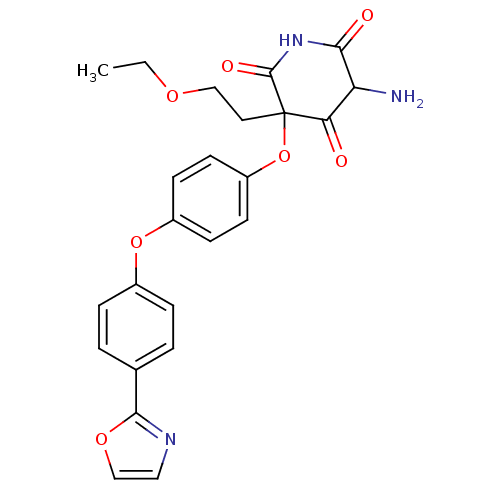
(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(4-oxazol-2-yl-phe...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3ncco3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C24H23N3O7/c1-2-31-13-11-24(20(28)19(25)21(29)27-23(24)30)34-18-9-7-17(8-10-18)33-16-5-3-15(4-6-16)22-26-12-14-32-22/h3-10,12,14,19H,2,11,13,25H2,1H3,(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163811

(5-Amino-3-[4-(4-chloro-phenoxy)-phenoxy]-3-(2-etho...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(Cl)cc3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C21H21ClN2O6/c1-2-28-12-11-21(18(25)17(23)19(26)24-20(21)27)30-16-9-7-15(8-10-16)29-14-5-3-13(22)4-6-14/h3-10,17H,2,11-12,23H2,1H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163802

(5-Amino-3-[4-(biphenyl-4-yloxy)-phenoxy]-3-(2-etho...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3ccccc3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C27H26N2O6/c1-2-33-17-16-27(24(30)23(28)25(31)29-26(27)32)35-22-14-12-21(13-15-22)34-20-10-8-19(9-11-20)18-6-4-3-5-7-18/h3-15,23H,2,16-17,28H2,1H3,(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163828
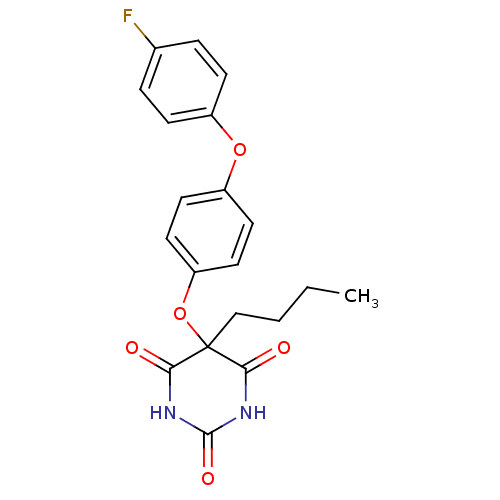
(5-Butyl-5-[4-(4-fluoro-phenoxy)-phenoxy]-pyrimidin...)Show SMILES CCCCC1(Oc2ccc(Oc3ccc(F)cc3)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C20H19FN2O5/c1-2-3-12-20(17(24)22-19(26)23-18(20)25)28-16-10-8-15(9-11-16)27-14-6-4-13(21)5-7-14/h4-11H,2-3,12H2,1H3,(H2,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352059

(CHEMBL1824026)Show SMILES Cc1cc(nn1C[C@@H](O)CN)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 |r| Show InChI InChI=1S/C22H31ClN4O3/c1-15-10-20(26-27(15)13-17(28)12-24)16-6-7-19(23)18(11-16)21(29)25-14-22(30)8-4-2-3-5-9-22/h6-7,10-11,17,28,30H,2-5,8-9,12-14,24H2,1H3,(H,25,29)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-activated human monocytes assessed as inhibition of ATP-induced IL-1beta release in presence of low ser... |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163797

(5-Amino-3-(2-ethoxy-ethyl)-3-(4-p-tolyloxy-phenoxy...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(C)cc3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C22H24N2O6/c1-3-28-13-12-22(19(25)18(23)20(26)24-21(22)27)30-17-10-8-16(9-11-17)29-15-6-4-14(2)5-7-15/h4-11,18H,3,12-13,23H2,1-2H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352059

(CHEMBL1824026)Show SMILES Cc1cc(nn1C[C@@H](O)CN)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 |r| Show InChI InChI=1S/C22H31ClN4O3/c1-15-10-20(26-27(15)13-17(28)12-24)16-6-7-19(23)18(11-16)21(29)25-14-22(30)8-4-2-3-5-9-22/h6-7,10-11,17,28,30H,2-5,8-9,12-14,24H2,1H3,(H,25,29)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-stimulated human whole blood assessed as inhibition of ATP-induced IL-1beta release |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50163809

(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(4-oxazol-5-yl-phe...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3cnco3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C24H23N3O7/c1-2-31-12-11-24(21(28)20(25)22(29)27-23(24)30)34-18-9-7-17(8-10-18)33-16-5-3-15(4-6-16)19-13-26-14-32-19/h3-10,13-14,20H,2,11-12,25H2,1H3,(H,27,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 14 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352058
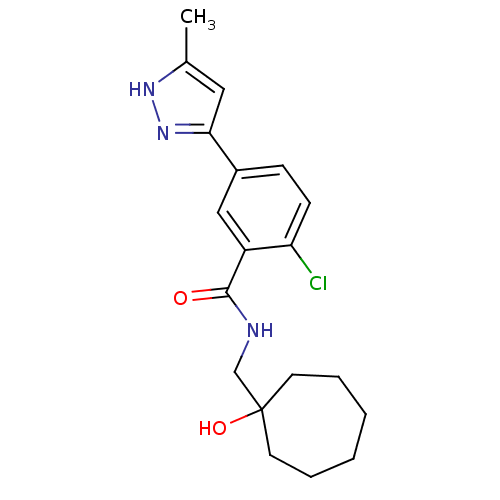
(CHEMBL560241)Show SMILES Cc1cc(n[nH]1)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 Show InChI InChI=1S/C19H24ClN3O2/c1-13-10-17(23-22-13)14-6-7-16(20)15(11-14)18(24)21-12-19(25)8-4-2-3-5-9-19/h6-7,10-11,25H,2-5,8-9,12H2,1H3,(H,21,24)(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced YO-PRO-1 uptake |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352060

(CHEMBL1824027)Show SMILES Cc1cc(nn1C[C@H](O)CN)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 |r| Show InChI InChI=1S/C22H31ClN4O3/c1-15-10-20(26-27(15)13-17(28)12-24)16-6-7-19(23)18(11-16)21(29)25-14-22(30)8-4-2-3-5-9-22/h6-7,10-11,17,28,30H,2-5,8-9,12-14,24H2,1H3,(H,25,29)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced YO-PRO-1 uptake |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163821
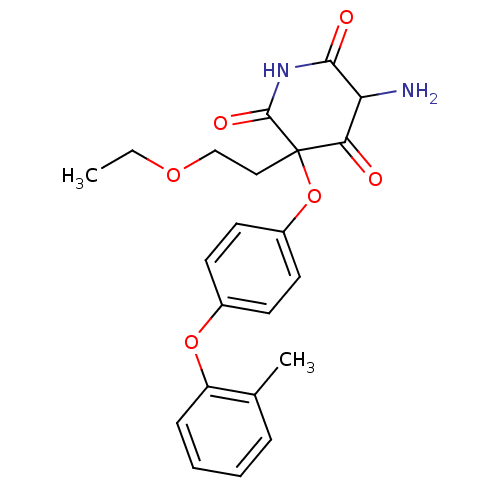
(5-Amino-3-(2-ethoxy-ethyl)-3-(4-o-tolyloxy-phenoxy...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccccc3C)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C22H24N2O6/c1-3-28-13-12-22(19(25)18(23)20(26)24-21(22)27)30-16-10-8-15(9-11-16)29-17-7-5-4-6-14(17)2/h4-11,18H,3,12-13,23H2,1-2H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163810

(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(2-fluoro-phenoxy)...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccccc3F)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C21H21FN2O6/c1-2-28-12-11-21(18(25)17(23)19(26)24-20(21)27)30-14-9-7-13(8-10-14)29-16-6-4-3-5-15(16)22/h3-10,17H,2,11-12,23H2,1H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163798
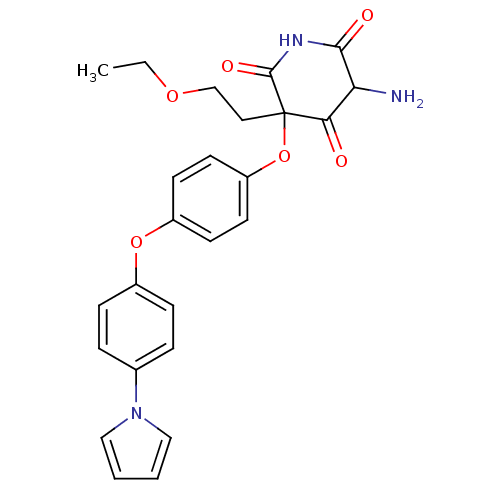
(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(4-pyrrol-1-yl-phe...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-n3cccc3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C25H25N3O6/c1-2-32-16-13-25(22(29)21(26)23(30)27-24(25)31)34-20-11-9-19(10-12-20)33-18-7-5-17(6-8-18)28-14-3-4-15-28/h3-12,14-15,21H,2,13,16,26H2,1H3,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163819
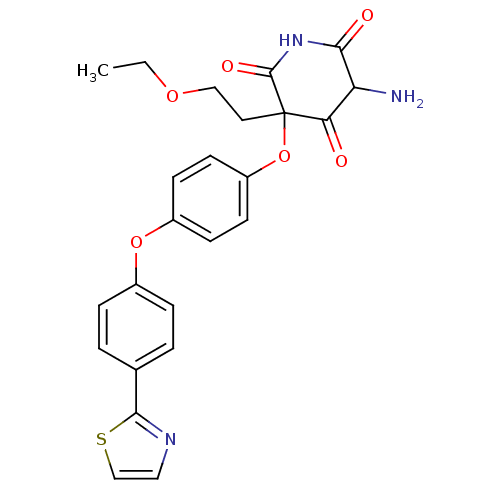
(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(4-thiazol-2-yl-ph...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3nccs3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C24H23N3O6S/c1-2-31-13-11-24(20(28)19(25)21(29)27-23(24)30)33-18-9-7-17(8-10-18)32-16-5-3-15(4-6-16)22-26-12-14-34-22/h3-10,12,14,19H,2,11,13,25H2,1H3,(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163814

(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(4-thiazol-4-yl-ph...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3cscn3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C24H23N3O6S/c1-2-31-12-11-24(21(28)20(25)22(29)27-23(24)30)33-18-9-7-17(8-10-18)32-16-5-3-15(4-6-16)19-13-34-14-26-19/h3-10,13-14,20H,2,11-12,25H2,1H3,(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163803
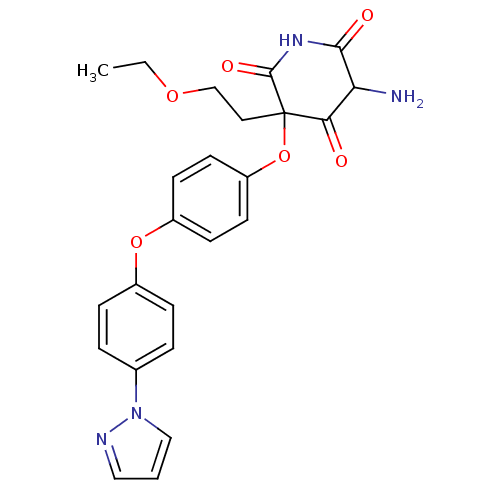
(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(4-pyrazol-1-yl-ph...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-n3cccn3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C24H24N4O6/c1-2-32-15-12-24(21(29)20(25)22(30)27-23(24)31)34-19-10-8-18(9-11-19)33-17-6-4-16(5-7-17)28-14-3-13-26-28/h3-11,13-14,20H,2,12,15,25H2,1H3,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50410149
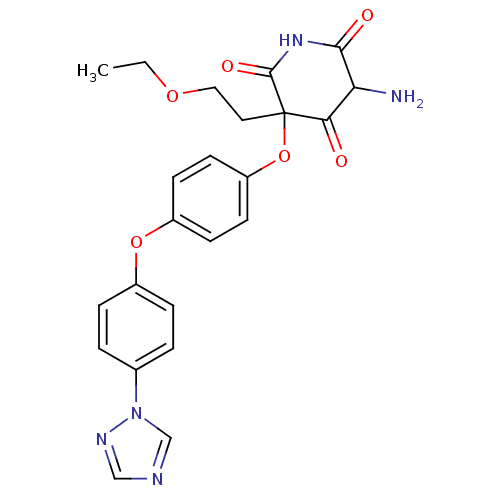
(CHEMBL2096775)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-n3cncn3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C23H23N5O6/c1-2-32-12-11-23(20(29)19(24)21(30)27-22(23)31)34-18-9-7-17(8-10-18)33-16-5-3-15(4-6-16)28-14-25-13-26-28/h3-10,13-14,19H,2,11-12,24H2,1H3,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50344738

((S)-2-chloro-5-(4-(2-hydroxy-3-methoxypropyl)-3,5-...)Show SMILES COC[C@@H](O)Cn1c(=O)cnn(-c2ccc(Cl)c(c2)C(=O)NCC2(O)CCCCCC2)c1=O |r| Show InChI InChI=1S/C22H29ClN4O6/c1-33-13-16(28)12-26-19(29)11-25-27(21(26)31)15-6-7-18(23)17(10-15)20(30)24-14-22(32)8-4-2-3-5-9-22/h6-7,10-11,16,28,32H,2-5,8-9,12-14H2,1H3,(H,24,30)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor expressed in HEK293 cells assessed as inhibition of ATP-induced YOPRO-1 uptake |
Bioorg Med Chem Lett 21: 3708-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.077
BindingDB Entry DOI: 10.7270/Q2B27VMG |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163801

(5-Amino-3-(2-ethoxy-ethyl)-3-{4-[4-(2H-pyrazol-3-y...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3cc[nH]n3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C24H24N4O6/c1-2-32-14-12-24(21(29)20(25)22(30)27-23(24)31)34-18-9-7-17(8-10-18)33-16-5-3-15(4-6-16)19-11-13-26-28-19/h3-11,13,20H,2,12,14,25H2,1H3,(H,26,28)(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163826

(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(4-trifluoromethyl...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)C(F)(F)F)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C22H21F3N2O6/c1-2-31-12-11-21(18(28)17(26)19(29)27-20(21)30)33-16-9-7-15(8-10-16)32-14-5-3-13(4-6-14)22(23,24)25/h3-10,17H,2,11-12,26H2,1H3,(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163815

(4-{4-[5-Amino-3-(2-ethoxy-ethyl)-2,4,6-trioxo-pipe...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)C#N)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C22H21N3O6/c1-2-29-12-11-22(19(26)18(24)20(27)25-21(22)28)31-17-9-7-16(8-10-17)30-15-5-3-14(13-23)4-6-15/h3-10,18H,2,11-12,24H2,1H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352052

(CHEMBL1824020)Show SMILES Cc1nc(cs1)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 Show InChI InChI=1S/C19H23ClN2O2S/c1-13-22-17(11-25-13)14-6-7-16(20)15(10-14)18(23)21-12-19(24)8-4-2-3-5-9-19/h6-7,10-11,24H,2-5,8-9,12H2,1H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced YO-PRO-1 uptake |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163817
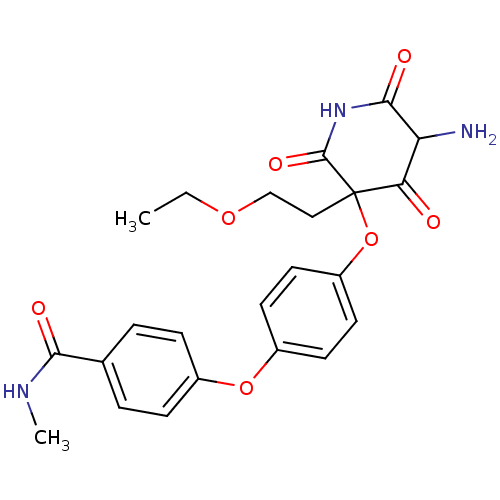
(4-{4-[5-Amino-3-(2-ethoxy-ethyl)-2,4,6-trioxo-pipe...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)C(=O)NC)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C23H25N3O7/c1-3-31-13-12-23(19(27)18(24)21(29)26-22(23)30)33-17-10-8-16(9-11-17)32-15-6-4-14(5-7-15)20(28)25-2/h4-11,18H,3,12-13,24H2,1-2H3,(H,25,28)(H,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352060

(CHEMBL1824027)Show SMILES Cc1cc(nn1C[C@H](O)CN)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 |r| Show InChI InChI=1S/C22H31ClN4O3/c1-15-10-20(26-27(15)13-17(28)12-24)16-6-7-19(23)18(11-16)21(29)25-14-22(30)8-4-2-3-5-9-22/h6-7,10-11,17,28,30H,2-5,8-9,12-14,24H2,1H3,(H,25,29)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-activated human monocytes assessed as inhibition of ATP-induced IL-1beta release in presence of low ser... |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352058

(CHEMBL560241)Show SMILES Cc1cc(n[nH]1)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 Show InChI InChI=1S/C19H24ClN3O2/c1-13-10-17(23-22-13)14-6-7-16(20)15(11-14)18(24)21-12-19(25)8-4-2-3-5-9-19/h6-7,10-11,25H,2-5,8-9,12H2,1H3,(H,21,24)(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-activated human monocytes assessed as inhibition of ATP-induced IL-1beta release in presence of low ser... |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163813

(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(4-[1,2,4]triazol-...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-n3cnnc3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C23H23N5O6/c1-2-32-12-11-23(20(29)19(24)21(30)27-22(23)31)34-18-9-7-17(8-10-18)33-16-5-3-15(4-6-16)28-13-25-26-14-28/h3-10,13-14,19H,2,11-12,24H2,1H3,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163827
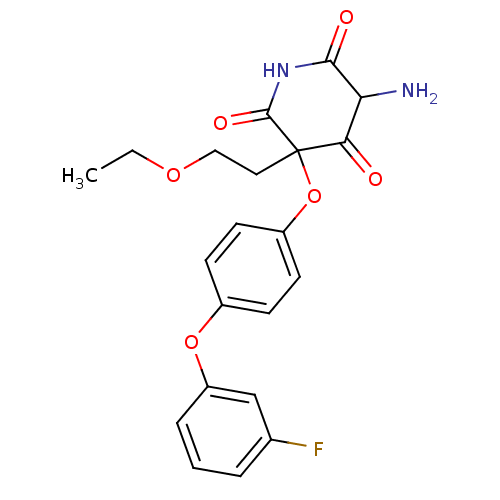
(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(3-fluoro-phenoxy)...)Show SMILES CCOCCC1(Oc2ccc(Oc3cccc(F)c3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C21H21FN2O6/c1-2-28-11-10-21(18(25)17(23)19(26)24-20(21)27)30-15-8-6-14(7-9-15)29-16-5-3-4-13(22)12-16/h3-9,12,17H,2,10-11,23H2,1H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352060

(CHEMBL1824027)Show SMILES Cc1cc(nn1C[C@H](O)CN)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 |r| Show InChI InChI=1S/C22H31ClN4O3/c1-15-10-20(26-27(15)13-17(28)12-24)16-6-7-19(23)18(11-16)21(29)25-14-22(30)8-4-2-3-5-9-22/h6-7,10-11,17,28,30H,2-5,8-9,12-14,24H2,1H3,(H,25,29)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-stimulated human whole blood assessed as inhibition of ATP-induced IL-1beta release |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50163808

(5-Amino-3-(2-ethoxy-ethyl)-3-{4-[4-(1H-pyrazol-4-y...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3cn[nH]c3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C24H24N4O6/c1-2-32-12-11-24(21(29)20(25)22(30)28-23(24)31)34-19-9-7-18(8-10-19)33-17-5-3-15(4-6-17)16-13-26-27-14-16/h3-10,13-14,20H,2,11-12,25H2,1H3,(H,26,27)(H,28,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 14 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11694
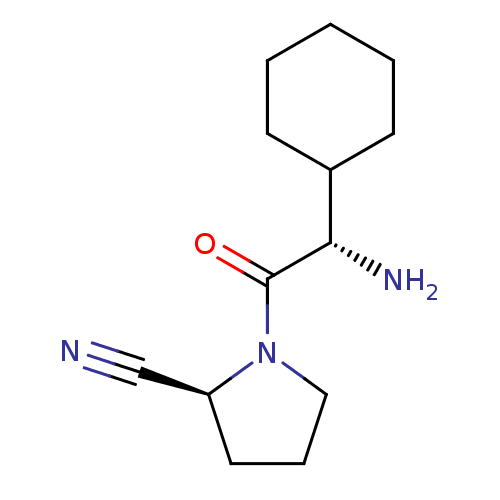
((2S)-1-[(2S)-2-amino-2-cyclohexylacetyl]pyrrolidin...)Show InChI InChI=1S/C13H21N3O/c14-9-11-7-4-8-16(11)13(17)12(15)10-5-2-1-3-6-10/h10-12H,1-8,15H2/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
| Assay Description
The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... |
Bioorg Med Chem Lett 15: 687-91 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.023
BindingDB Entry DOI: 10.7270/Q2XG9PDW |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11694

((2S)-1-[(2S)-2-amino-2-cyclohexylacetyl]pyrrolidin...)Show InChI InChI=1S/C13H21N3O/c14-9-11-7-4-8-16(11)13(17)12(15)10-5-2-1-3-6-10/h10-12H,1-8,15H2/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Dipeptidyl-peptidase IV [DPP-IV] |
Bioorg Med Chem Lett 15: 3271-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.051
BindingDB Entry DOI: 10.7270/Q2RF5TJX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352055

(CHEMBL549618)Show InChI InChI=1S/C18H22ClN3O2/c19-15-6-5-13(16-7-10-21-22-16)11-14(15)17(23)20-12-18(24)8-3-1-2-4-9-18/h5-7,10-11,24H,1-4,8-9,12H2,(H,20,23)(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced YO-PRO-1 uptake |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM12195

(2-amino-4-{4-[bis(4-fluorophenyl)methyl]piperazin-...)Show SMILES NC(CC(=O)N1CCN(CC1)C(c1ccc(F)cc1)c1ccc(F)cc1)C(=O)N1Cc2ccccc2C1 Show InChI InChI=1S/C29H30F2N4O2/c30-24-9-5-20(6-10-24)28(21-7-11-25(31)12-8-21)34-15-13-33(14-16-34)27(36)17-26(32)29(37)35-18-22-3-1-2-4-23(22)19-35/h1-12,26,28H,13-19,32H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes
| Assay Description
The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... |
Bioorg Med Chem Lett 15: 687-91 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.023
BindingDB Entry DOI: 10.7270/Q2XG9PDW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163820
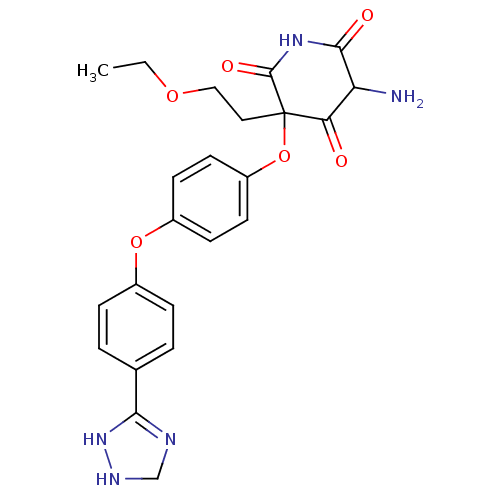
(5-Amino-3-{4-[4-(3,4-dihydro-2H-[1,2,4]triazol-3-y...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)C3=NCNN3)cc2)C(=O)NC(=O)C(N)C1=O |t:19| Show InChI InChI=1S/C23H25N5O6/c1-2-32-12-11-23(19(29)18(24)21(30)27-22(23)31)34-17-9-7-16(8-10-17)33-15-5-3-14(4-6-15)20-25-13-26-28-20/h3-10,18,26H,2,11-13,24H2,1H3,(H,25,28)(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50344734

(2-chloro-5-(3,5-dioxo-4,5-dihydro-1,2,4-triazin-2(...)Show SMILES OC1(CNC(=O)c2cc(ccc2Cl)-n2ncc(=O)[nH]c2=O)CCCCCC1 Show InChI InChI=1S/C18H21ClN4O4/c19-14-6-5-12(23-17(26)22-15(24)10-21-23)9-13(14)16(25)20-11-18(27)7-3-1-2-4-8-18/h5-6,9-10,27H,1-4,7-8,11H2,(H,20,25)(H,22,24,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor expressed in HEK293 cells assessed as inhibition of ATP-induced YOPRO-1 uptake |
Bioorg Med Chem Lett 21: 3708-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.077
BindingDB Entry DOI: 10.7270/Q2B27VMG |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50163810

(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(2-fluoro-phenoxy)...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccccc3F)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C21H21FN2O6/c1-2-28-12-11-21(18(25)17(23)19(26)24-20(21)27)30-14-9-7-13(8-10-14)29-16-6-4-3-5-15(16)22/h3-10,17H,2,11-12,23H2,1H3,(H,24,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 14 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM12183
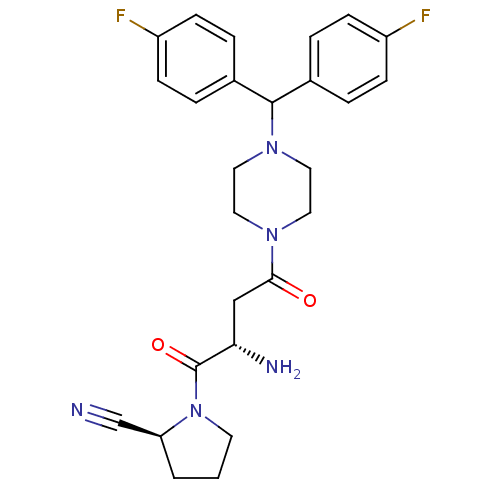
((2S)-1-[(2S)-2-amino-4-{4-[bis(4-fluorophenyl)meth...)Show SMILES N[C@@H](CC(=O)N1CCN(CC1)C(c1ccc(F)cc1)c1ccc(F)cc1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C26H29F2N5O2/c27-20-7-3-18(4-8-20)25(19-5-9-21(28)10-6-19)32-14-12-31(13-15-32)24(34)16-23(30)26(35)33-11-1-2-22(33)17-29/h3-10,22-23,25H,1-2,11-16,30H2/t22-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Dipeptidyl-peptidase 8 |
Bioorg Med Chem Lett 15: 3271-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.051
BindingDB Entry DOI: 10.7270/Q2RF5TJX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM12183

((2S)-1-[(2S)-2-amino-4-{4-[bis(4-fluorophenyl)meth...)Show SMILES N[C@@H](CC(=O)N1CCN(CC1)C(c1ccc(F)cc1)c1ccc(F)cc1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C26H29F2N5O2/c27-20-7-3-18(4-8-20)25(19-5-9-21(28)10-6-19)32-14-12-31(13-15-32)24(34)16-23(30)26(35)33-11-1-2-22(33)17-29/h3-10,22-23,25H,1-2,11-16,30H2/t22-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes
| Assay Description
The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... |
Bioorg Med Chem Lett 15: 687-91 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.023
BindingDB Entry DOI: 10.7270/Q2XG9PDW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163822
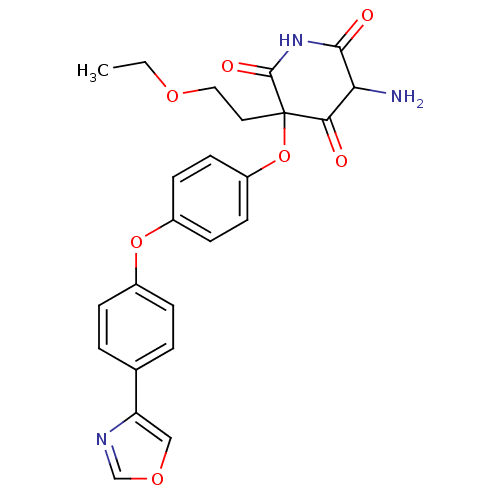
(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(4-oxazol-4-yl-phe...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3cocn3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C24H23N3O7/c1-2-31-12-11-24(21(28)20(25)22(29)27-23(24)30)34-18-9-7-17(8-10-18)33-16-5-3-15(4-6-16)19-13-32-14-26-19/h3-10,13-14,20H,2,11-12,25H2,1H3,(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50163824

(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(4-imidazol-1-yl-p...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-n3ccnc3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C24H24N4O6/c1-2-32-14-11-24(21(29)20(25)22(30)27-23(24)31)34-19-9-7-18(8-10-19)33-17-5-3-16(4-6-17)28-13-12-26-15-28/h3-10,12-13,15,20H,2,11,14,25H2,1H3,(H,27,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 14 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data