

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357210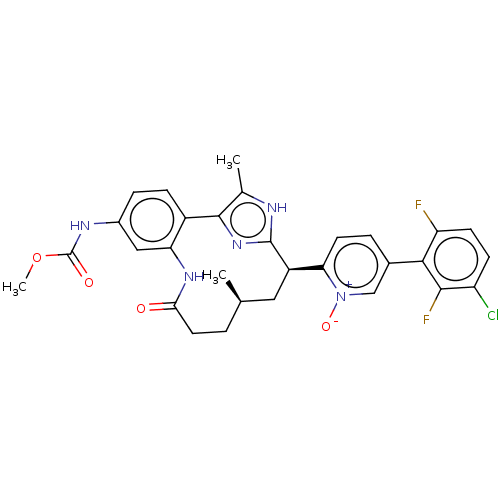 (US10214512, Example 151-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518241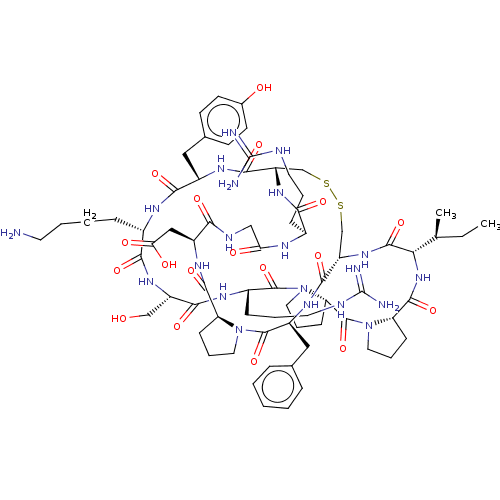 (CHEMBL4569923) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50122293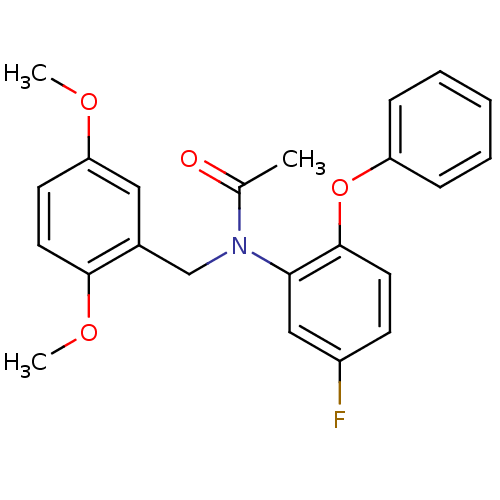 (CHEMBL401000 | CHEMBL63064 | N-(2,5-Dimethoxy-benz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description In vitro binding affinity for PBR (peripheral benzodiazepine receptor) in rat brain | Bioorg Med Chem Lett 13: 201-4 (2002) BindingDB Entry DOI: 10.7270/Q2PZ585X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518240 (CHEMBL4439523) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM50084033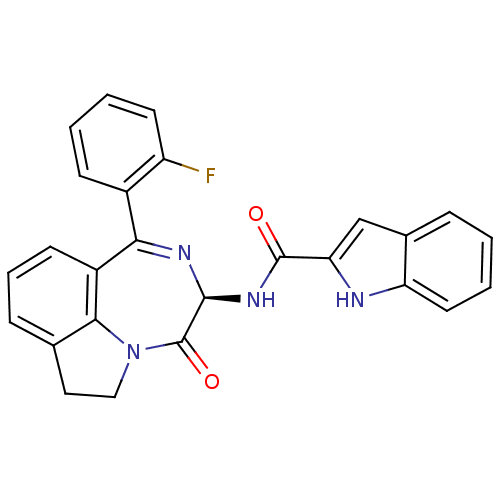 (1H-Indole-2-carboxylic acid [(R)-1-(2-fluoro-pheny...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 268: 571-5 (1994) BindingDB Entry DOI: 10.7270/Q2WQ029S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50185958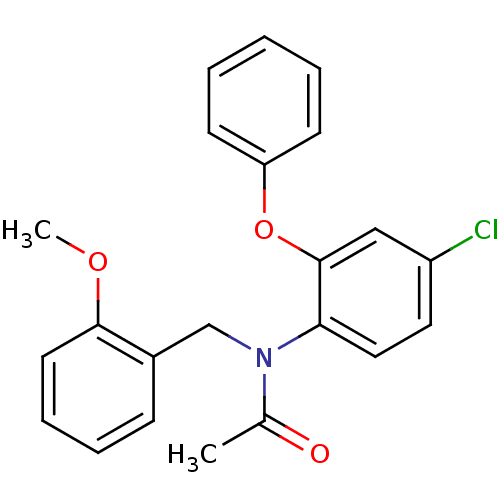 (CHEMBL205971 | N-(2-methoxybenzyl)-N-(4-chloro-2-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C](R)-PK1119 from Sprague-Dawley rat brain PBR | J Med Chem 49: 2735-42 (2006) Article DOI: 10.1021/jm060006k BindingDB Entry DOI: 10.7270/Q20K29CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50185958 (CHEMBL205971 | N-(2-methoxybenzyl)-N-(4-chloro-2-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C](R)-PK1119 from Sprague-Dawley rat brain PBR | J Med Chem 49: 2735-42 (2006) Article DOI: 10.1021/jm060006k BindingDB Entry DOI: 10.7270/Q20K29CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50122294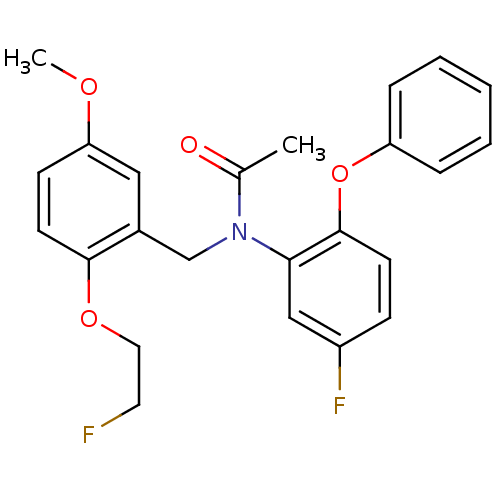 (CHEMBL292092 | N-(5-fluoro-2-phenoxyphenyl)-N-(2-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description In vitro inhibition of [11C]2 binding to Peripheral benzodiazepine receptor (PBR) in rat brain | J Med Chem 47: 2228-35 (2004) Article DOI: 10.1021/jm0304919 BindingDB Entry DOI: 10.7270/Q20G3KXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357161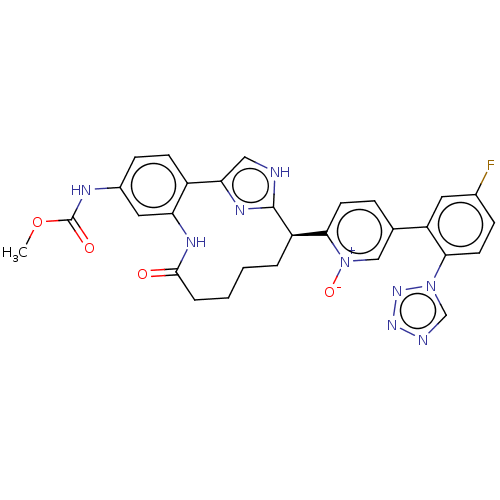 (US10214512, Example 117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357017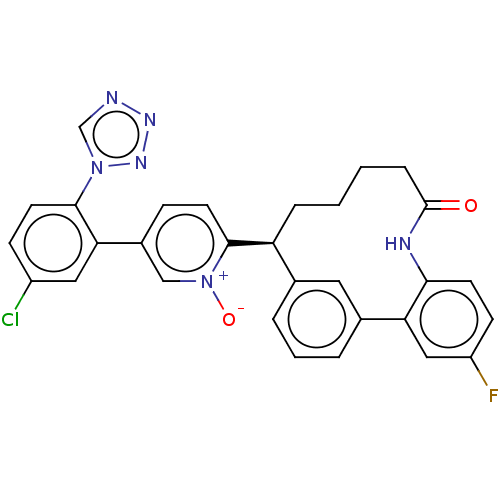 ((S) 5-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)-2-(25-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357214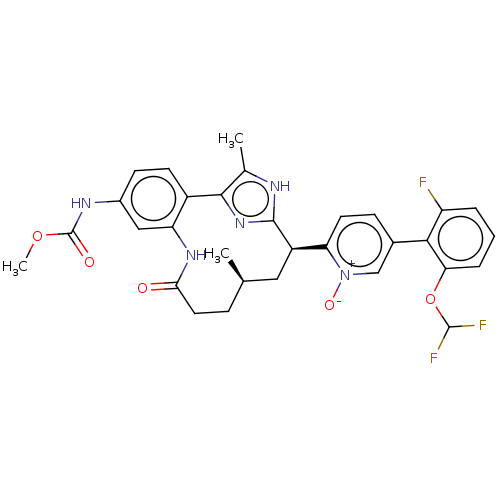 (US10214512, Example 152-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50185959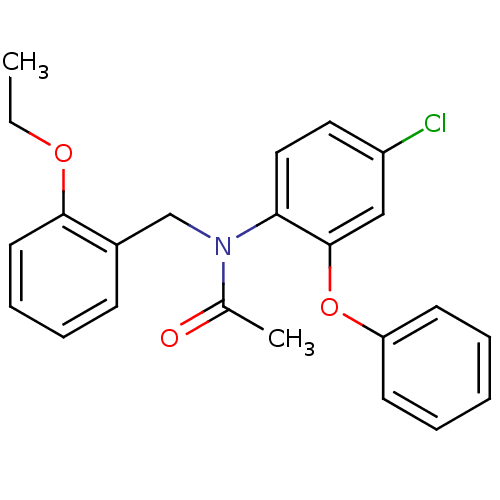 (CHEMBL381602 | N-(2-ethoxybenzyl)-N-(4-chloro-2-ph...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C](R)-PK1119 from Sprague-Dawley rat brain PBR | J Med Chem 49: 2735-42 (2006) Article DOI: 10.1021/jm060006k BindingDB Entry DOI: 10.7270/Q20K29CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357188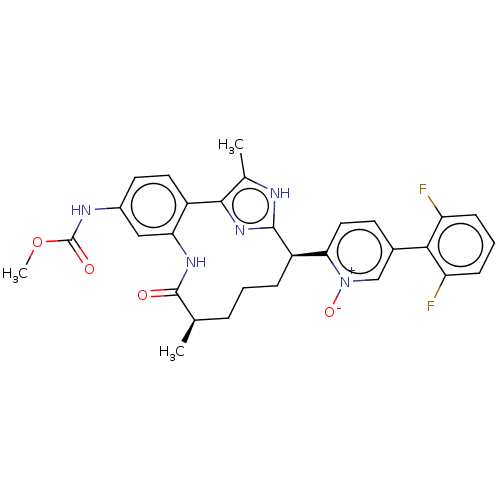 (US10214512, Example 143-b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50185959 (CHEMBL381602 | N-(2-ethoxybenzyl)-N-(4-chloro-2-ph...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C](R)-PK1119 from Sprague-Dawley rat brain PBR | J Med Chem 49: 2735-42 (2006) Article DOI: 10.1021/jm060006k BindingDB Entry DOI: 10.7270/Q20K29CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357026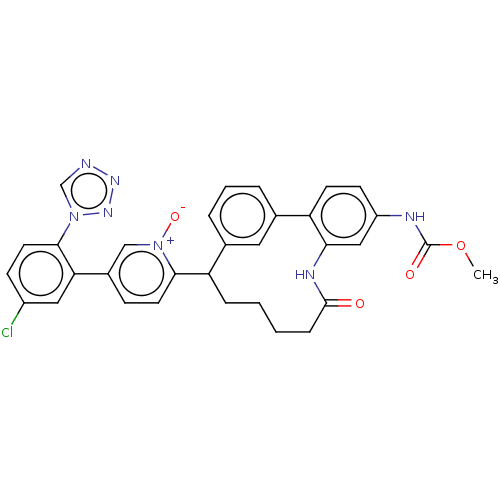 (5-(5-Chloro-2-(1H-tetrazol-1-yl)phenyl)-2-(24-((me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50122293 (CHEMBL401000 | CHEMBL63064 | N-(2,5-Dimethoxy-benz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C](R)-PK1119 from Sprague-Dawley rat brain PBR | J Med Chem 49: 2735-42 (2006) Article DOI: 10.1021/jm060006k BindingDB Entry DOI: 10.7270/Q20K29CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357058 (US10214512, Example 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357166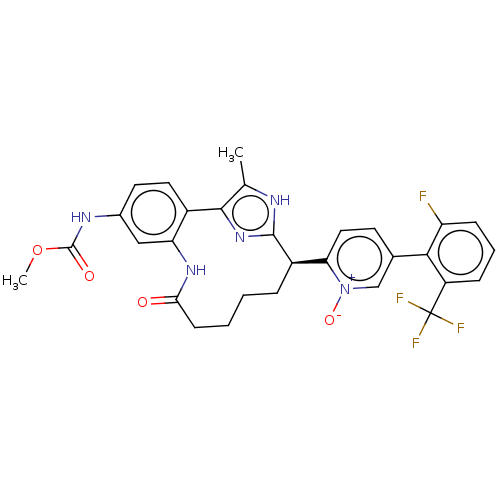 (US10214512, Example 122 | US10214512, Example 125) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357173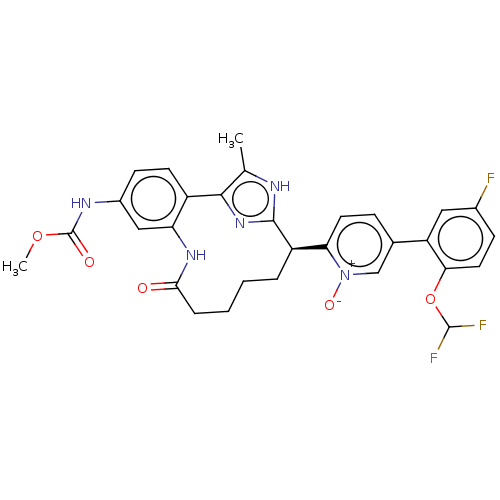 (US10214512, Example 129) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357153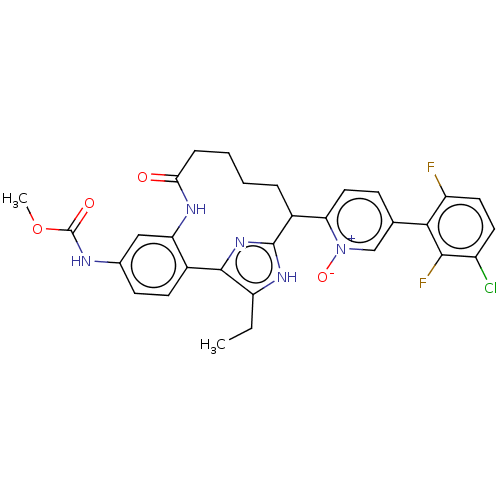 ((Z)-5-(3-Chloro-2,6-difluorophenyl)-2-(15-ethyl-24...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357206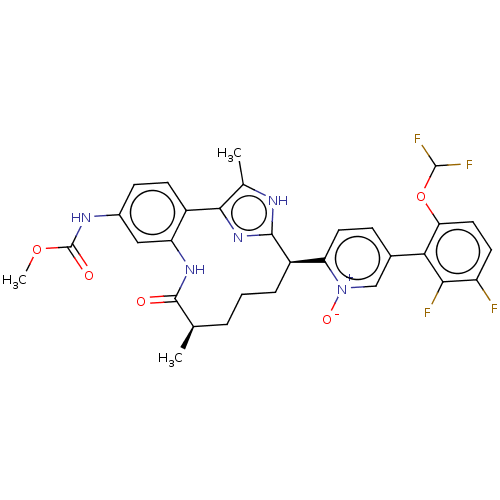 (US10214512, Example 150-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357057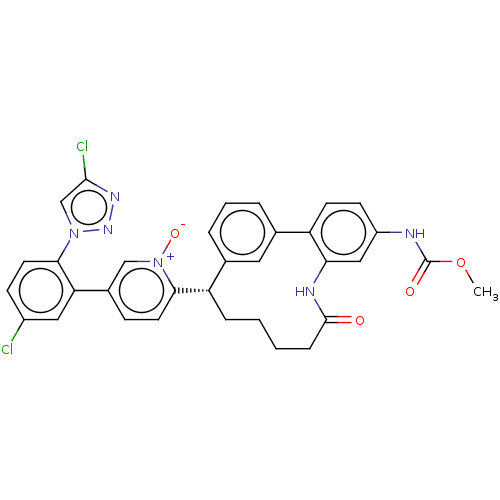 (US10214512, Example 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357025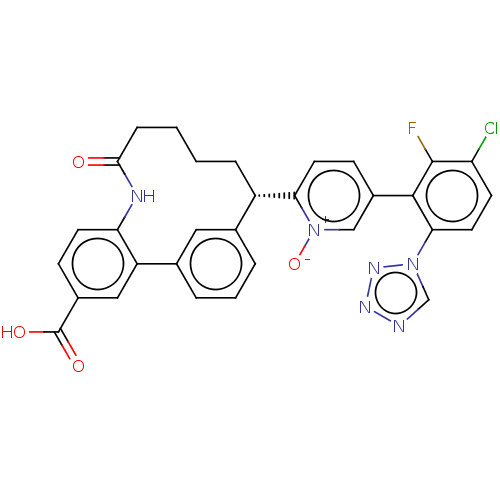 (2-(25-carboxy-4-oxo-3-aza-1(1,3),2(1,2)-dibenzenac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357172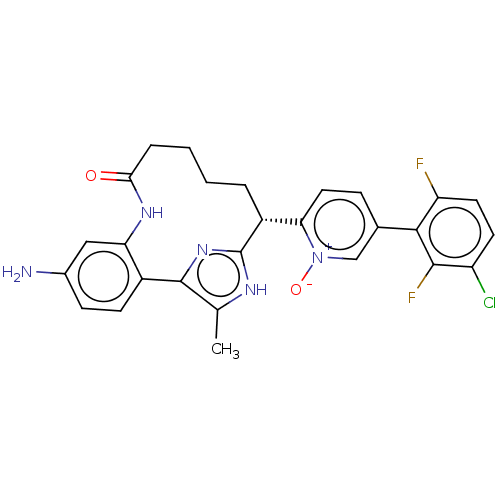 (US10214512, Example 128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357170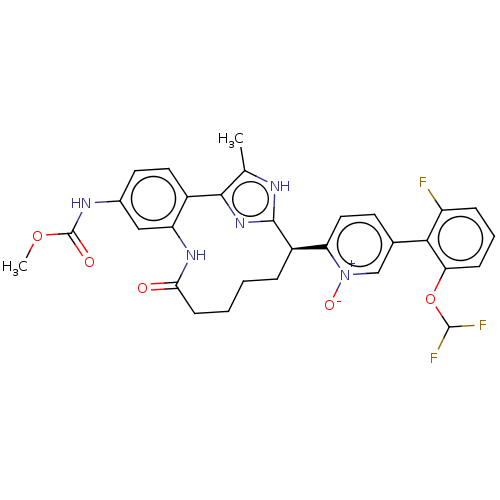 (US10214512, Example 126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357192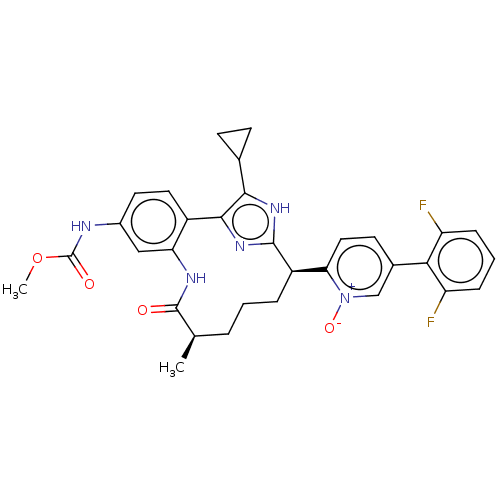 (US10214512, Example 145-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357143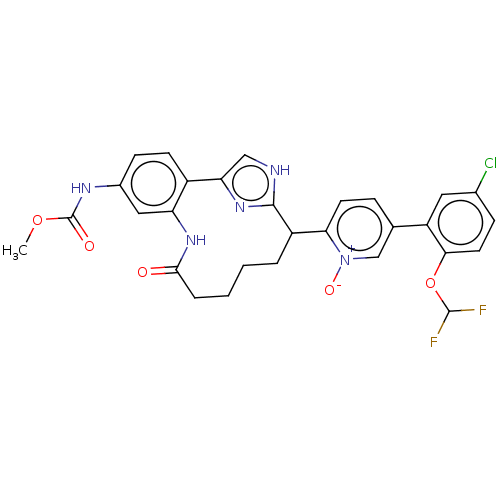 ((Z)-5-(5-Chloro-2-(difluoromethoxy)phenyl)-2-(24-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357110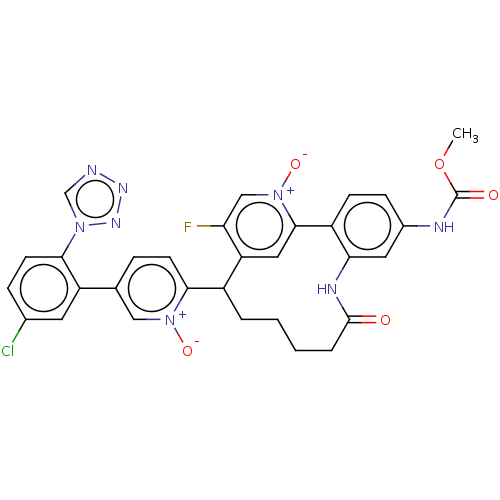 (9-(5-(5-chloro-2-(1h-tetrazol-1-yl)phenyl)-1-oxido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357196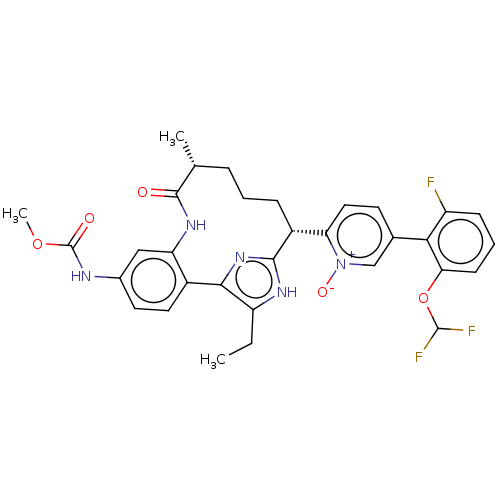 (US10214512, Example 146-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518249 (CHEMBL4588827) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357231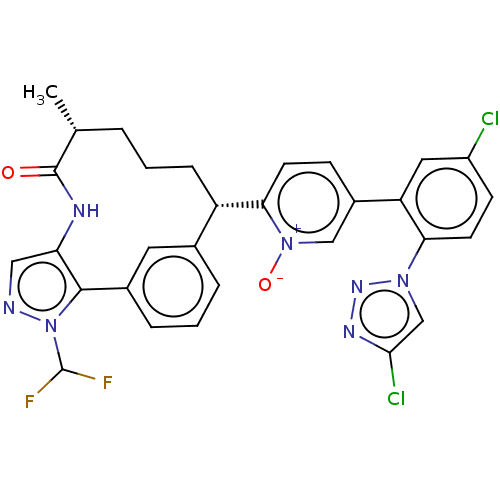 (US10214512, Example 166-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357174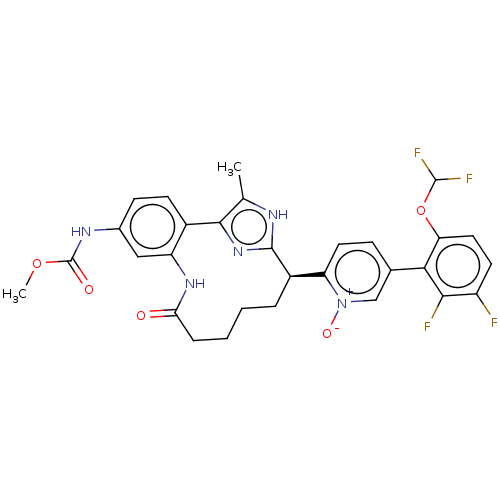 (US10214512, Example 130) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357171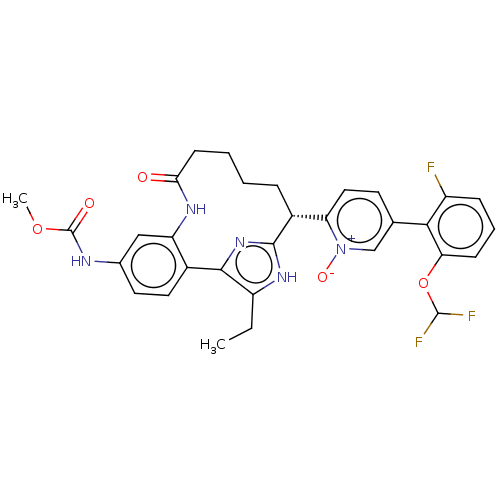 (US10214512, Example 127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50122293 (CHEMBL401000 | CHEMBL63064 | N-(2,5-Dimethoxy-benz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]DAA1106 from Sprague-Dawley rat brain PBR | J Med Chem 49: 2735-42 (2006) Article DOI: 10.1021/jm060006k BindingDB Entry DOI: 10.7270/Q20K29CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50122293 (CHEMBL401000 | CHEMBL63064 | N-(2,5-Dimethoxy-benz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description In vitro inhibition of [11C]2 binding to Peripheral benzodiazepine receptor (PBR) in rat brain | J Med Chem 47: 2228-35 (2004) Article DOI: 10.1021/jm0304919 BindingDB Entry DOI: 10.7270/Q20G3KXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM357210 (US10214512, Example 151-a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Kallikrein can be determined using a relevant purified serine protease, a... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357099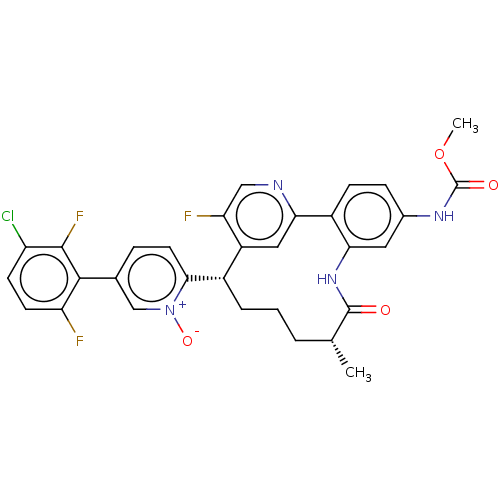 (5-(3-chloro-2,6-difluorophenyl)-2-((5R,9S)-15-fluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357140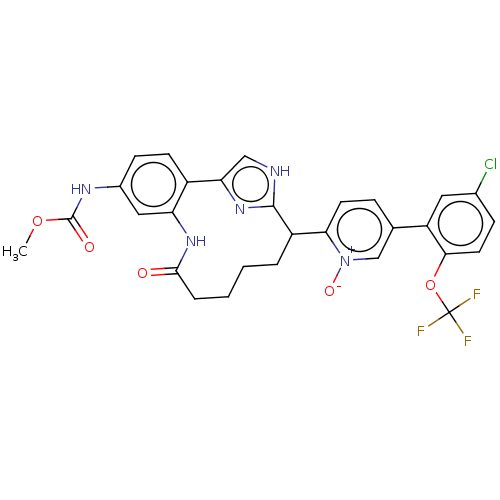 ((Z)-5-(5-chloro-2-(trifluoromethoxy)phenyl)-2-(24-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194658 ((2R)-N-(1-cyclopropylmethyl-4-piperidinylmethyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357178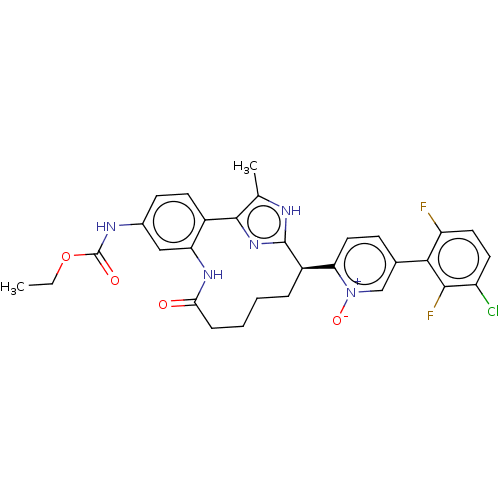 (US10214512, Example 134) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357155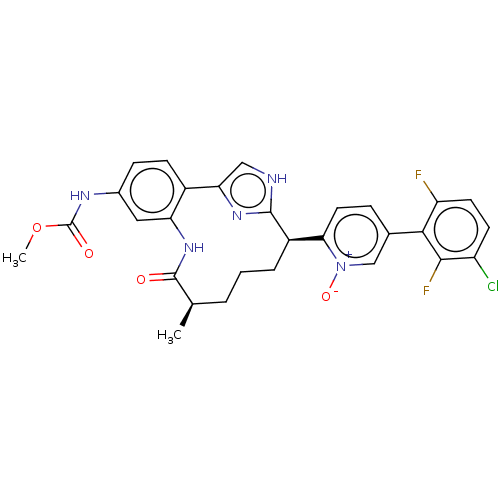 ((Z)-5-(3-chloro-2,6-difluorophenyl)-2-(24-((methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357202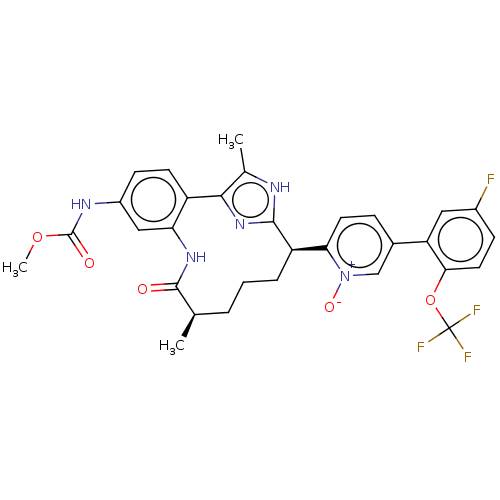 (US10214512, Example 149-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50122295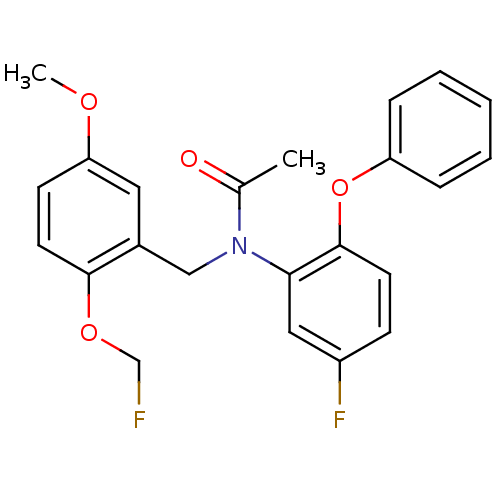 (CHEMBL63065 | N-(2-Fluoromethoxy-5-methoxy-benzyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description In vitro inhibition of [11C]2 binding to Peripheral benzodiazepine receptor (PBR) in rat brain | J Med Chem 47: 2228-35 (2004) Article DOI: 10.1021/jm0304919 BindingDB Entry DOI: 10.7270/Q20G3KXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357022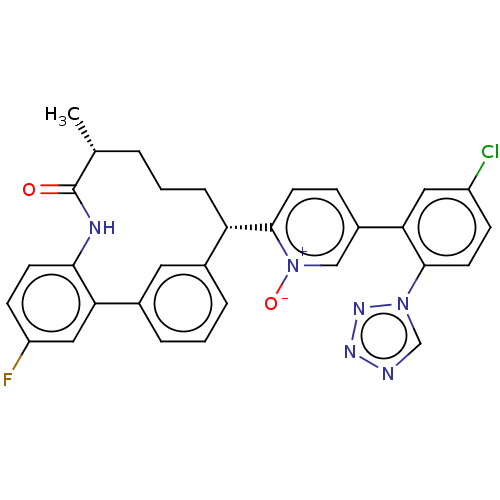 (5-(5-Chloro-2-(1H-tetrazol-1-yl)phenyl)-2-((5R,9S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357149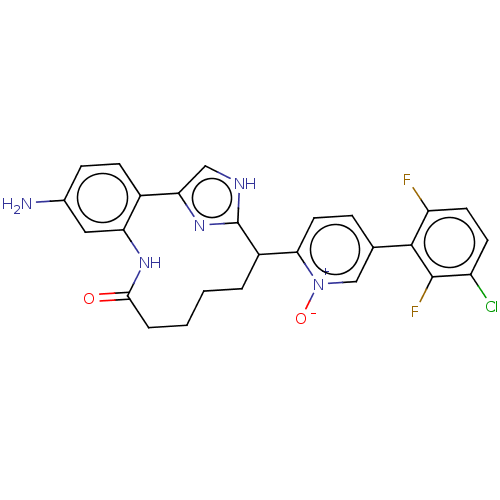 ((Z)-2-(24-Amino-4-OXO-11H-3-aza-1(4,2)-imidazola-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Homo sapiens (Human)) | BDBM50122293 (CHEMBL401000 | CHEMBL63064 | N-(2,5-Dimethoxy-benz...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description In vitro binding affinity for PBR (peripheral benzodiazepine receptor) in monkey brain | Bioorg Med Chem Lett 13: 201-4 (2002) BindingDB Entry DOI: 10.7270/Q2PZ585X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357152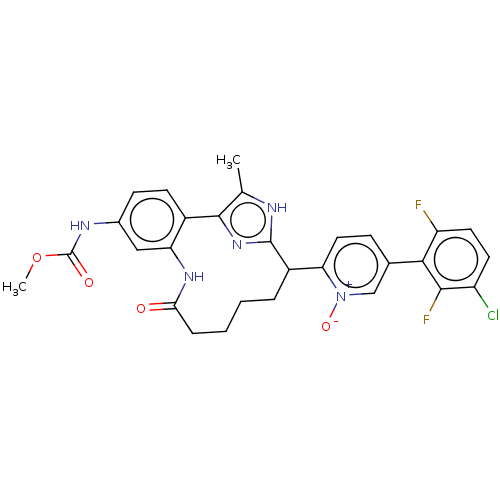 ((Z)-5-(3-chloro-2,6-difluorophenyl)-2-(24-((methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50185957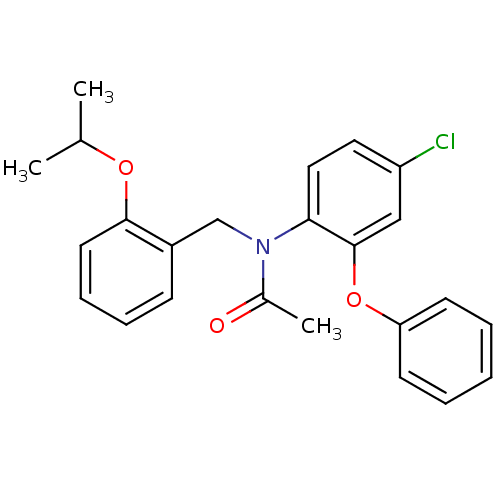 (CHEMBL205767 | N-(2-isopropoxybenzyl)-N-(4-chloro-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C](R)-PK1119 from Sprague-Dawley rat brain PBR | J Med Chem 49: 2735-42 (2006) Article DOI: 10.1021/jm060006k BindingDB Entry DOI: 10.7270/Q20K29CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194659 ((2R)-N-(1-cyclopentylmethyl-4-piperidinylmethyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357061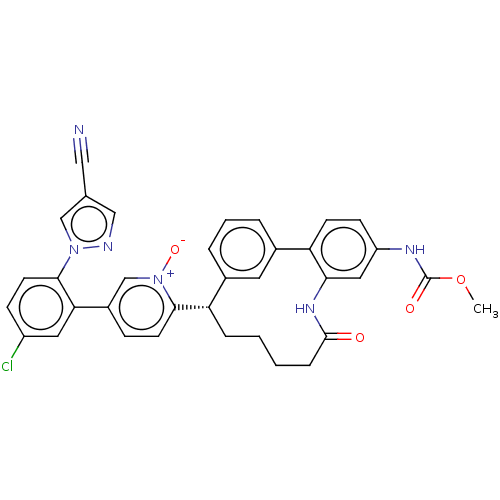 (US10214512, Example 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 16841 total ) | Next | Last >> |