

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50226128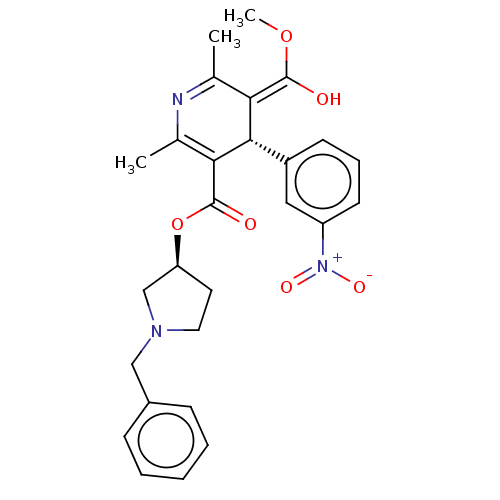 (CHEMBL2093893) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate | J Med Chem 29: 2504-11 (1986) BindingDB Entry DOI: 10.7270/Q2QF8W3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50226129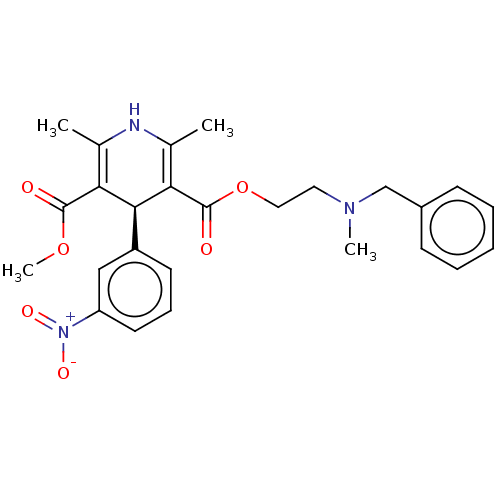 (CHEMBL1314450) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | PubMed | 0.499 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate | J Med Chem 29: 2504-11 (1986) BindingDB Entry DOI: 10.7270/Q2QF8W3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50101815 (CHEBI:7550 | Nicardipine) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate | J Med Chem 29: 2504-11 (1986) BindingDB Entry DOI: 10.7270/Q2QF8W3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50226130 (CHEMBL558616) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate | J Med Chem 29: 2504-11 (1986) BindingDB Entry DOI: 10.7270/Q2QF8W3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50101817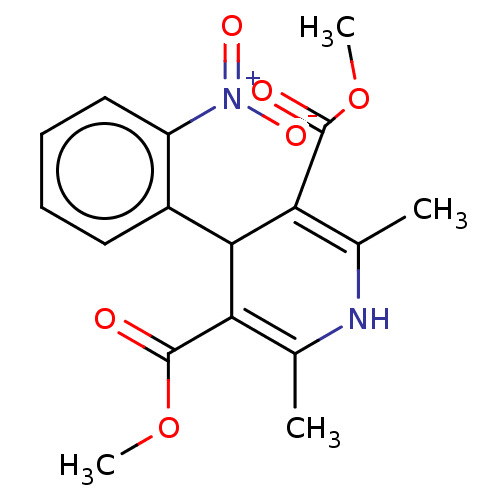 (Adalat | Adalat Cc | Afeditab Cr | BAY-A-1040 | CH...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate | J Med Chem 29: 2504-11 (1986) BindingDB Entry DOI: 10.7270/Q2QF8W3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50226131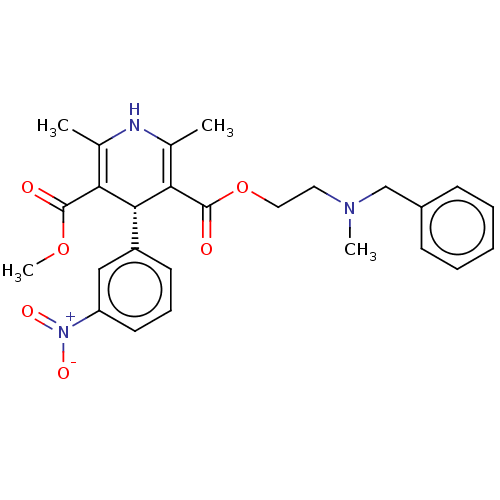 (CHEMBL1598680) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate | J Med Chem 29: 2504-11 (1986) BindingDB Entry DOI: 10.7270/Q2QF8W3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50226127 (CHEMBL542169) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate | J Med Chem 29: 2504-11 (1986) BindingDB Entry DOI: 10.7270/Q2QF8W3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50226126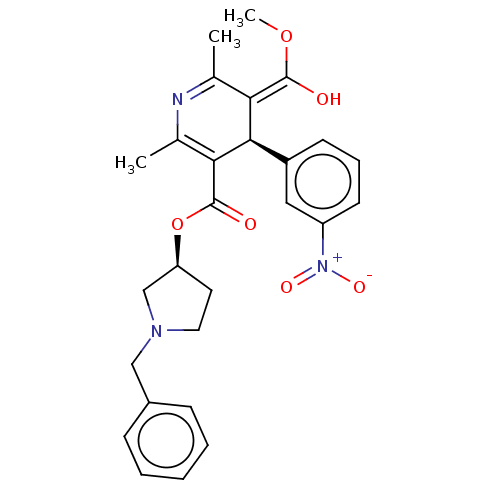 (CHEMBL553553) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate | J Med Chem 29: 2504-11 (1986) BindingDB Entry DOI: 10.7270/Q2QF8W3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine palmitoyltransferase 2 (Homo sapiens (Human)) | BDBM50461646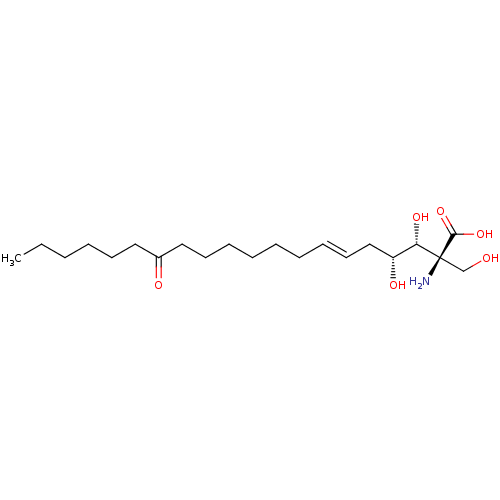 (CHEBI:582124 | Myriocin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... | Bioorg Med Chem 26: 2452-2465 (2018) Article DOI: 10.1016/j.bmc.2018.04.008 BindingDB Entry DOI: 10.7270/Q2RF5XNH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine palmitoyltransferase 2 (Homo sapiens (Human)) | BDBM50461644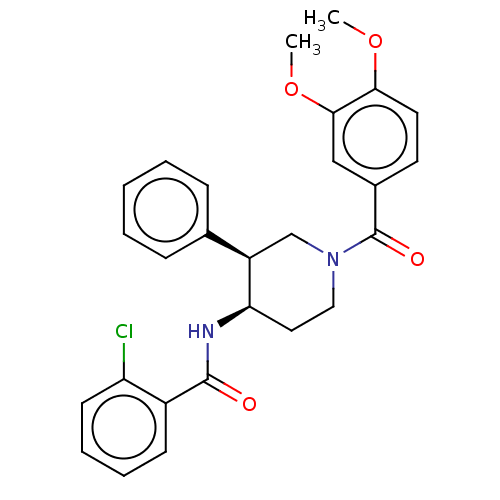 (CHEMBL4228416) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... | Bioorg Med Chem 26: 2452-2465 (2018) Article DOI: 10.1016/j.bmc.2018.04.008 BindingDB Entry DOI: 10.7270/Q2RF5XNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine palmitoyltransferase 2 (Homo sapiens (Human)) | BDBM50461649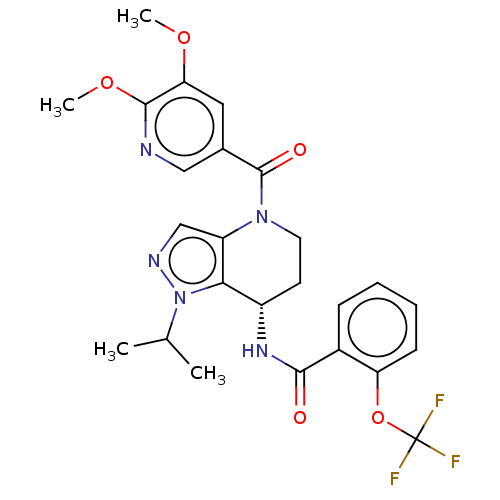 (CHEMBL4225519) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... | Bioorg Med Chem 26: 2452-2465 (2018) Article DOI: 10.1016/j.bmc.2018.04.008 BindingDB Entry DOI: 10.7270/Q2RF5XNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine palmitoyltransferase 2 (Homo sapiens (Human)) | BDBM50461645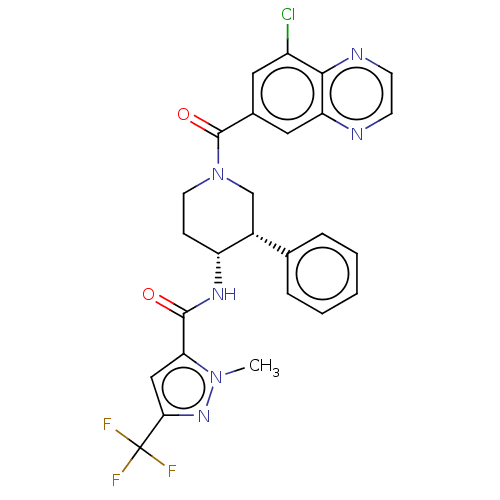 (CHEMBL4228472) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... | Bioorg Med Chem 26: 2452-2465 (2018) Article DOI: 10.1016/j.bmc.2018.04.008 BindingDB Entry DOI: 10.7270/Q2RF5XNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine palmitoyltransferase 2 (Homo sapiens (Human)) | BDBM50461647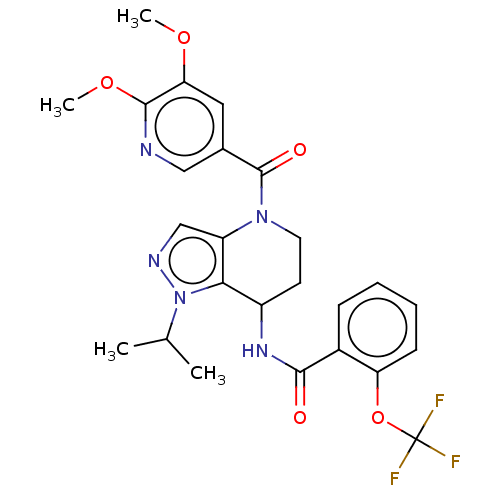 (CHEMBL4225462) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... | Bioorg Med Chem 26: 2452-2465 (2018) Article DOI: 10.1016/j.bmc.2018.04.008 BindingDB Entry DOI: 10.7270/Q2RF5XNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine palmitoyltransferase 2 (Homo sapiens (Human)) | BDBM50461643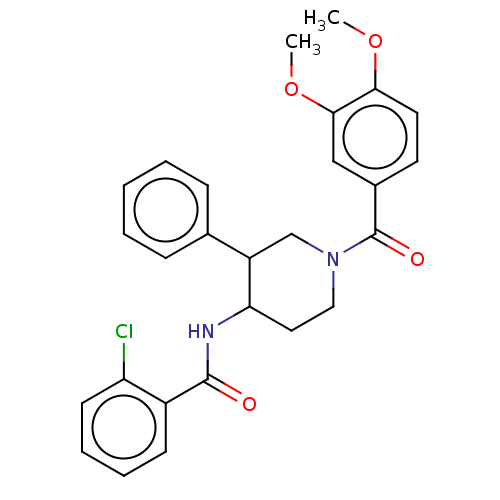 (CHEMBL4225764) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... | Bioorg Med Chem 26: 2452-2465 (2018) Article DOI: 10.1016/j.bmc.2018.04.008 BindingDB Entry DOI: 10.7270/Q2RF5XNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425719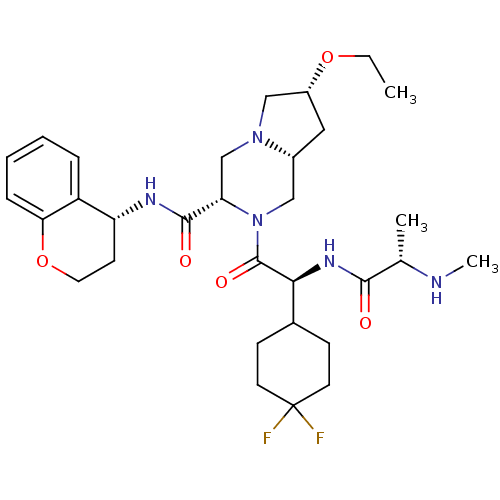 (CHEMBL2316217) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425721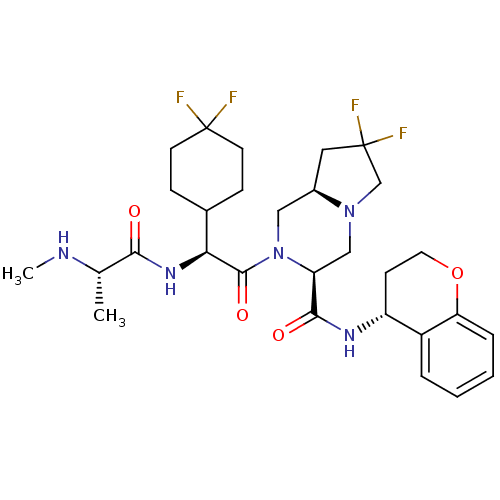 (CHEMBL2311586) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425728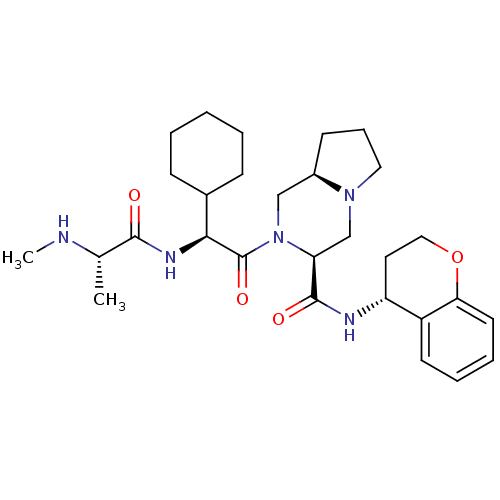 (CHEMBL2365533) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425722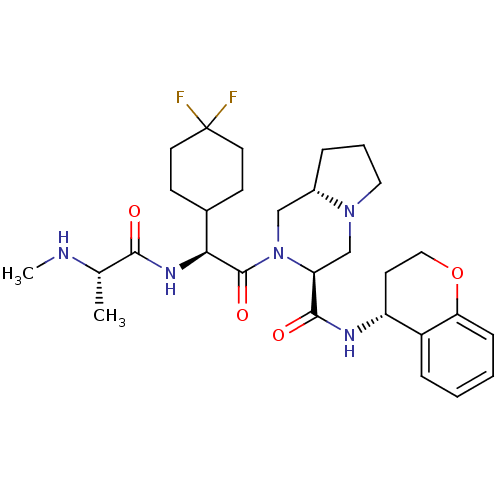 (CHEMBL2316215) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425725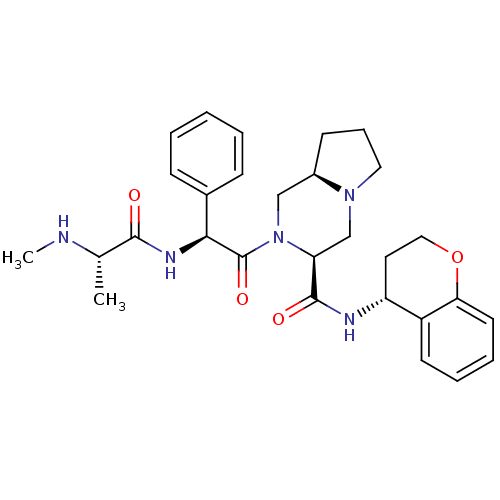 (CHEMBL2316224) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425730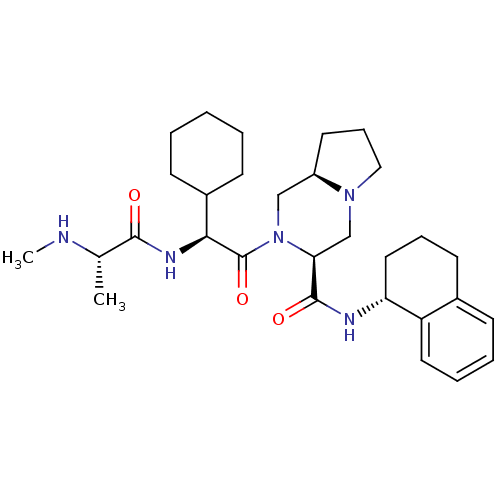 (CHEMBL2316219) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425724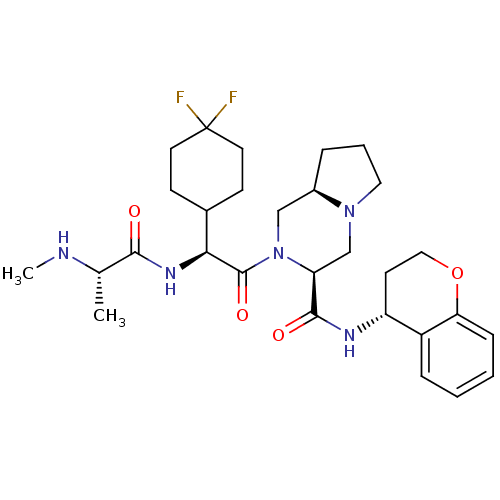 (CHEMBL2316213) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425723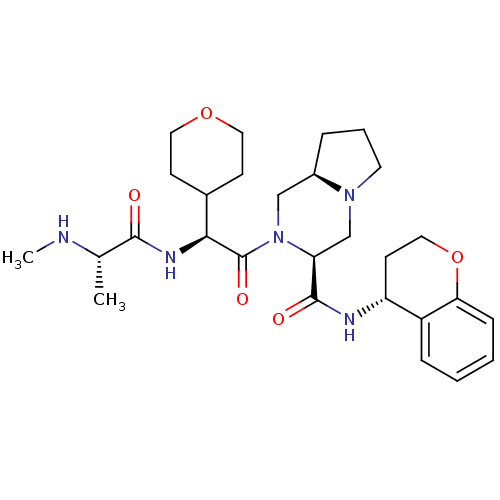 (CHEMBL2316214) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425727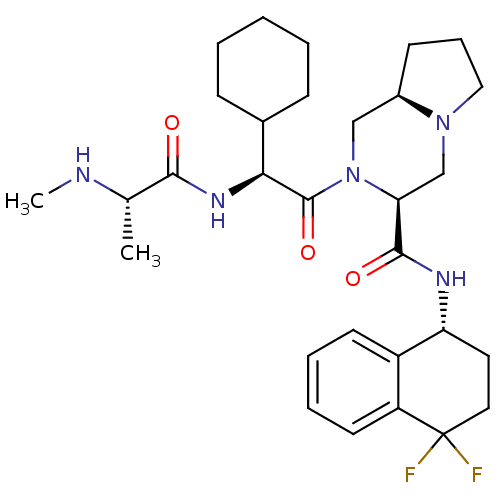 (CHEMBL2316222) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50234480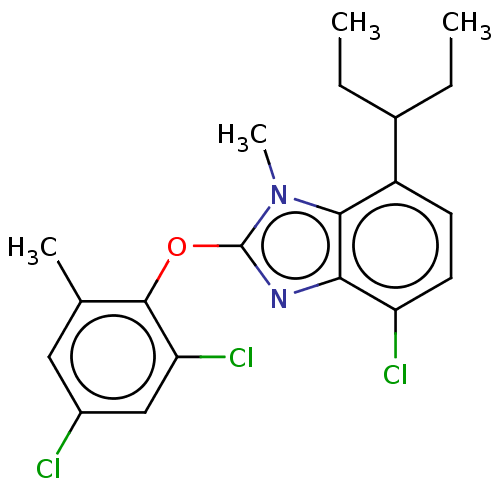 (CHEMBL4069551) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes preincubated for 1 hr followed by [125I]-CRF addition meas... | Bioorg Med Chem 25: 1556-1570 (2017) Article DOI: 10.1016/j.bmc.2016.11.011 BindingDB Entry DOI: 10.7270/Q23R0W4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50460165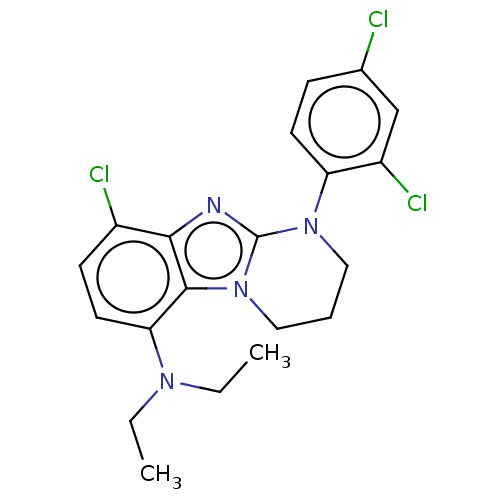 (CHEMBL4225254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human CRF1 receptor expressed in CHO cells assessed as inhibition of human CRF-stimulated cAMP accumulation after 4 hrs by luc... | Bioorg Med Chem 26: 2229-2250 (2018) Article DOI: 10.1016/j.bmc.2018.01.020 BindingDB Entry DOI: 10.7270/Q2474DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425720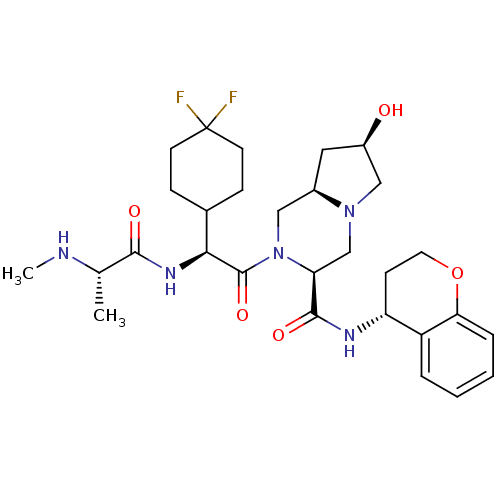 (CHEMBL2316216) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine palmitoyltransferase 2 (Homo sapiens (Human)) | BDBM50461639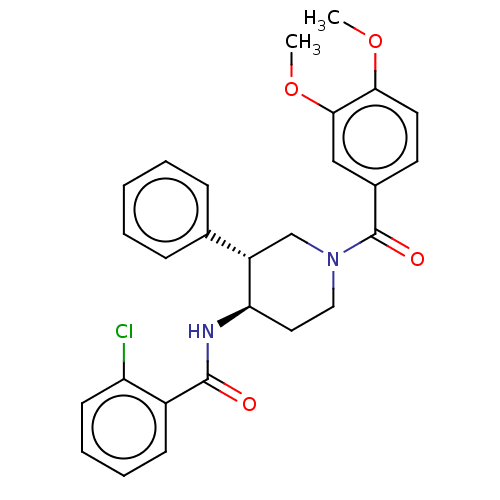 (CHEMBL4229253) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... | Bioorg Med Chem 26: 2452-2465 (2018) Article DOI: 10.1016/j.bmc.2018.04.008 BindingDB Entry DOI: 10.7270/Q2RF5XNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine palmitoyltransferase 2 (Homo sapiens (Human)) | BDBM50461641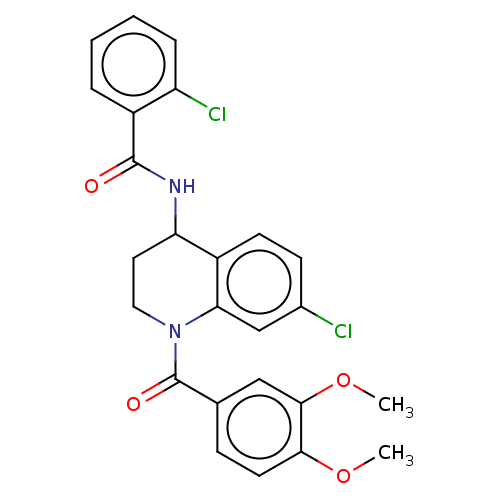 (CHEMBL4225192) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... | Bioorg Med Chem 26: 2452-2465 (2018) Article DOI: 10.1016/j.bmc.2018.04.008 BindingDB Entry DOI: 10.7270/Q2RF5XNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50460165 (CHEMBL4225254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes after 1.5 hrs by liquid scintillation counting method | Bioorg Med Chem 26: 2229-2250 (2018) Article DOI: 10.1016/j.bmc.2018.01.020 BindingDB Entry DOI: 10.7270/Q2474DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50234477 (CHEMBL4087369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes preincubated for 1 hr followed by [125I]-CRF addition meas... | Bioorg Med Chem 25: 1556-1570 (2017) Article DOI: 10.1016/j.bmc.2016.11.011 BindingDB Entry DOI: 10.7270/Q23R0W4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425726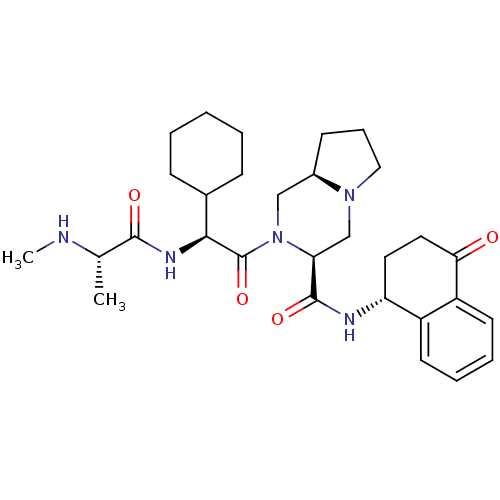 (CHEMBL2316223) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine palmitoyltransferase 2 (Homo sapiens (Human)) | BDBM50461638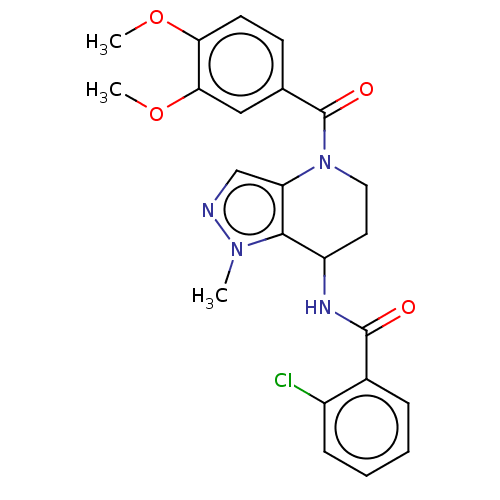 (CHEMBL4226297) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... | Bioorg Med Chem 26: 2452-2465 (2018) Article DOI: 10.1016/j.bmc.2018.04.008 BindingDB Entry DOI: 10.7270/Q2RF5XNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50234487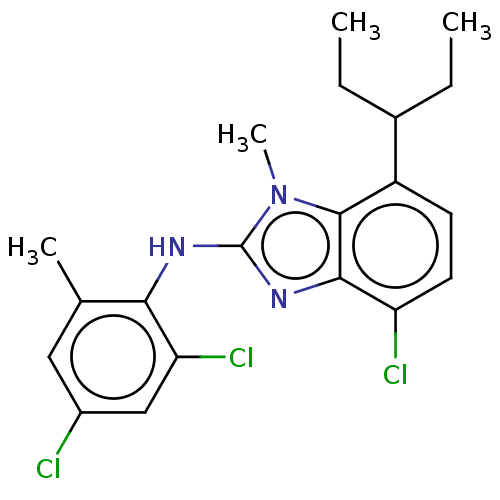 (CHEMBL4070898) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes after 1.5 hrs by liquid scintillation counting method | Bioorg Med Chem 25: 1556-1570 (2017) Article DOI: 10.1016/j.bmc.2016.11.011 BindingDB Entry DOI: 10.7270/Q23R0W4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50234482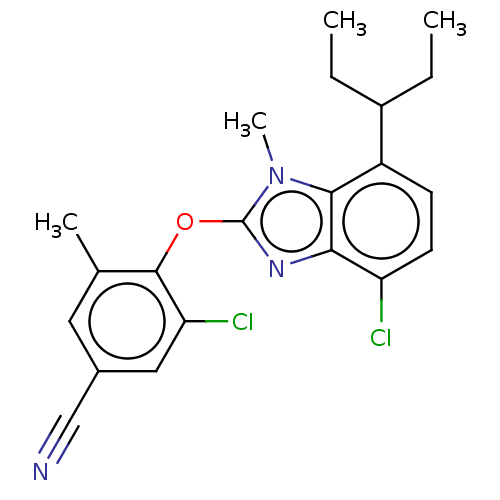 (CHEMBL4096045) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes preincubated for 1 hr followed by [125I]-CRF addition meas... | Bioorg Med Chem 25: 1556-1570 (2017) Article DOI: 10.1016/j.bmc.2016.11.011 BindingDB Entry DOI: 10.7270/Q23R0W4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50161867 (CHEMBL3793277) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes after 1.5 hrs by liquid scintillation counting method | Bioorg Med Chem 26: 2229-2250 (2018) Article DOI: 10.1016/j.bmc.2018.01.020 BindingDB Entry DOI: 10.7270/Q2474DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50161832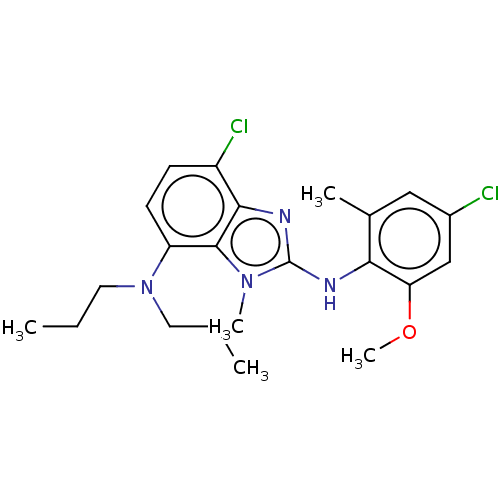 (CHEMBL3792517) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes preincubated for 1 hr followed by [125I]-CRF addition meas... | Bioorg Med Chem 25: 1556-1570 (2017) Article DOI: 10.1016/j.bmc.2016.11.011 BindingDB Entry DOI: 10.7270/Q23R0W4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50460180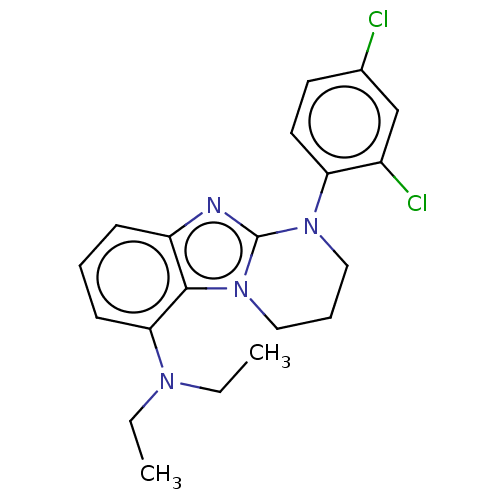 (CHEMBL4224887) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes after 1.5 hrs by liquid scintillation counting method | Bioorg Med Chem 26: 2229-2250 (2018) Article DOI: 10.1016/j.bmc.2018.01.020 BindingDB Entry DOI: 10.7270/Q2474DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50425731 (CHEMBL2316218) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... | J Med Chem 56: 1228-46 (2013) Article DOI: 10.1021/jm301674z BindingDB Entry DOI: 10.7270/Q23N24QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50234478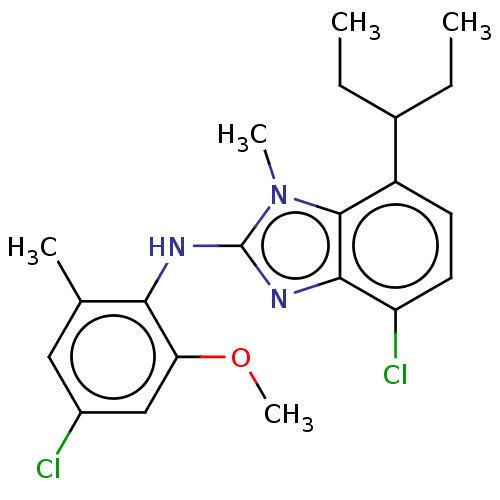 (CHEMBL4101653) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes preincubated for 1 hr followed by [125I]-CRF addition meas... | Bioorg Med Chem 25: 1556-1570 (2017) Article DOI: 10.1016/j.bmc.2016.11.011 BindingDB Entry DOI: 10.7270/Q23R0W4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50116105 (3-(6-(dimethylamino)-4-methylpyridin-3-yl)-2,5-dim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes preincubated for 1 hr followed by [125I]-CRF addition meas... | Bioorg Med Chem 25: 1556-1570 (2017) Article DOI: 10.1016/j.bmc.2016.11.011 BindingDB Entry DOI: 10.7270/Q23R0W4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50460172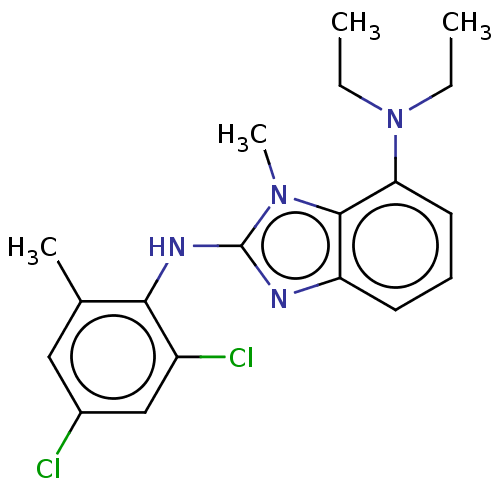 (CHEMBL4225516) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes after 1.5 hrs by liquid scintillation counting method | Bioorg Med Chem 26: 2229-2250 (2018) Article DOI: 10.1016/j.bmc.2018.01.020 BindingDB Entry DOI: 10.7270/Q2474DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50234484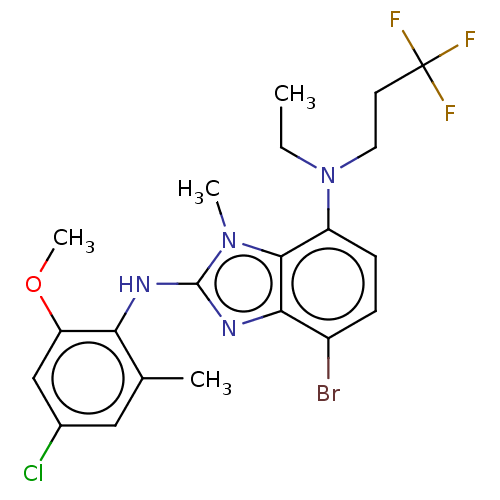 (CHEMBL4070523) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes preincubated for 1 hr followed by [125I]-CRF addition meas... | Bioorg Med Chem 25: 1556-1570 (2017) Article DOI: 10.1016/j.bmc.2016.11.011 BindingDB Entry DOI: 10.7270/Q23R0W4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50460167 (CHEMBL4225316) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes after 1.5 hrs by liquid scintillation counting method | Bioorg Med Chem 26: 2229-2250 (2018) Article DOI: 10.1016/j.bmc.2018.01.020 BindingDB Entry DOI: 10.7270/Q2474DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50234483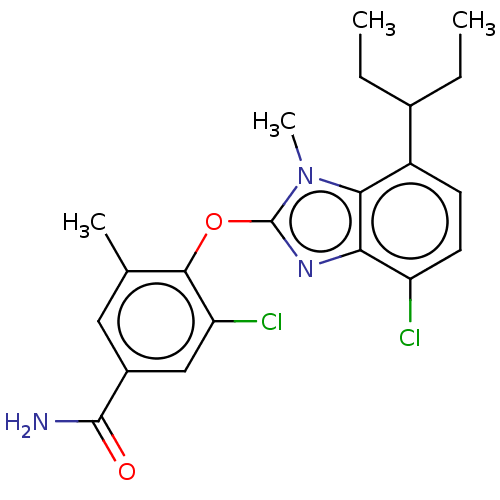 (CHEMBL4068170) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes preincubated for 1 hr followed by [125I]-CRF addition meas... | Bioorg Med Chem 25: 1556-1570 (2017) Article DOI: 10.1016/j.bmc.2016.11.011 BindingDB Entry DOI: 10.7270/Q23R0W4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50234480 (CHEMBL4069551) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes preincubated for 1 hr followed by [125I]-CRF addition meas... | Bioorg Med Chem 25: 1556-1570 (2017) Article DOI: 10.1016/j.bmc.2016.11.011 BindingDB Entry DOI: 10.7270/Q23R0W4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50161867 (CHEMBL3793277) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes preincubated for 1 hr followed by [125I]-CRF addition meas... | Bioorg Med Chem 25: 1556-1570 (2017) Article DOI: 10.1016/j.bmc.2016.11.011 BindingDB Entry DOI: 10.7270/Q23R0W4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50234480 (CHEMBL4069551) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes preincubated for 1 hr followed by compound washout for 2 h... | Bioorg Med Chem 25: 1556-1570 (2017) Article DOI: 10.1016/j.bmc.2016.11.011 BindingDB Entry DOI: 10.7270/Q23R0W4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50460183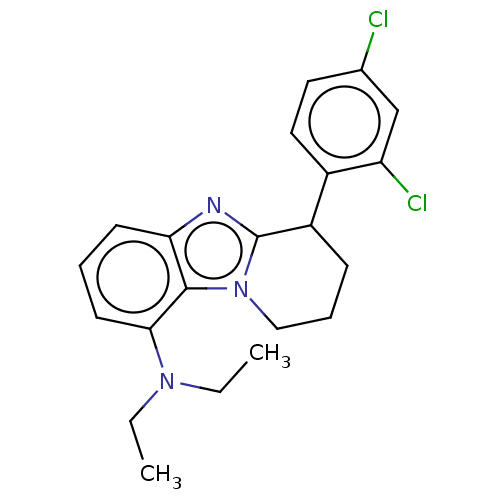 (CHEMBL4227554) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes after 1.5 hrs by liquid scintillation counting method | Bioorg Med Chem 26: 2229-2250 (2018) Article DOI: 10.1016/j.bmc.2018.01.020 BindingDB Entry DOI: 10.7270/Q2474DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50460170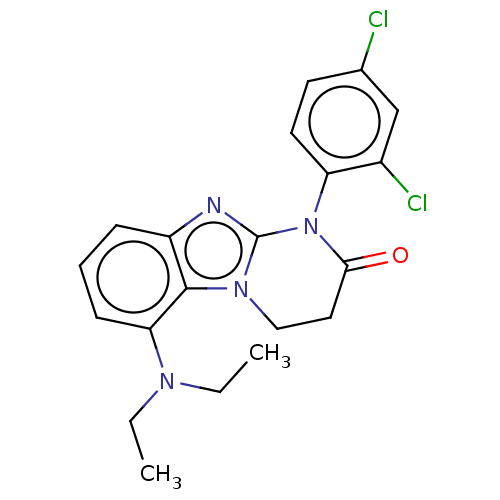 (CHEMBL4227537) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes after 1.5 hrs by liquid scintillation counting method | Bioorg Med Chem 26: 2229-2250 (2018) Article DOI: 10.1016/j.bmc.2018.01.020 BindingDB Entry DOI: 10.7270/Q2474DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50234480 (CHEMBL4069551) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human CRF1 receptor expressed in CHO cells assessed as inhibition of human CRF-stimulated cAMP accumulation | Bioorg Med Chem 25: 1556-1570 (2017) Article DOI: 10.1016/j.bmc.2016.11.011 BindingDB Entry DOI: 10.7270/Q23R0W4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3525 total ) | Next | Last >> |