

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22542 (4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22910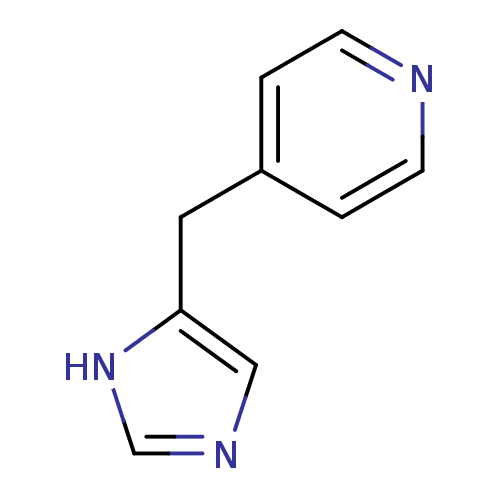 (4-(1H-imidazol-5-ylmethyl)pyridine | Immethridine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50326285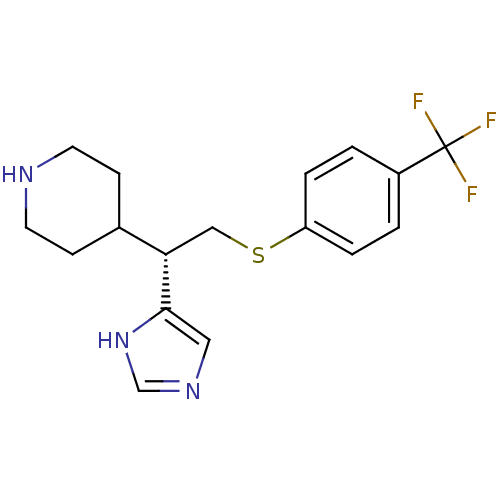 ((S)-4-(1-(1H-Imidazol-4-yl)-2-(4-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50326287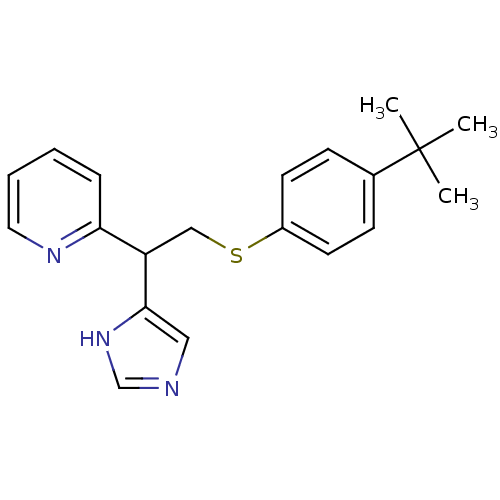 (2-(2-(4-tert-Butylphenylthio)-1-(1H-imidazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50326289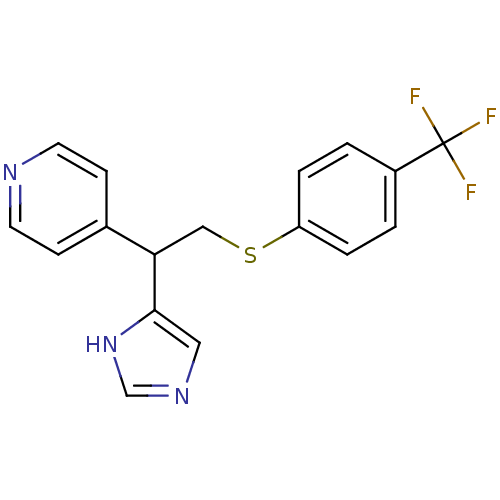 (4-(1-(1H-Imidazole-4-yl)-2-(4-trifluoromeethylphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Binding affinity to human histamine H4 receptor | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50326286 ((R)-4-(1-(1H-Imidazol-4-yl)-2-(4-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50326286 ((R)-4-(1-(1H-Imidazol-4-yl)-2-(4-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Binding affinity to human histamine H1 receptor | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50326288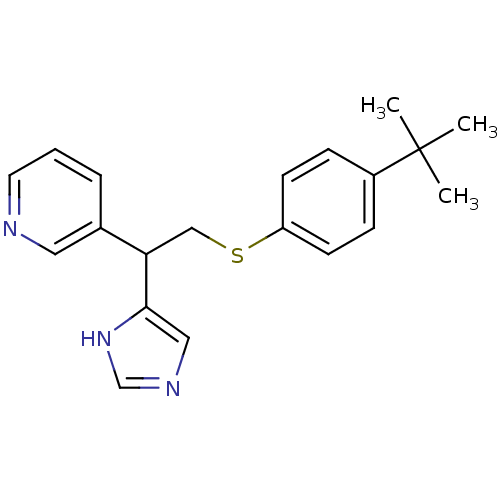 (3-(2-(4-tert-Butylphenylthio)-1-(1H-imidazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50326293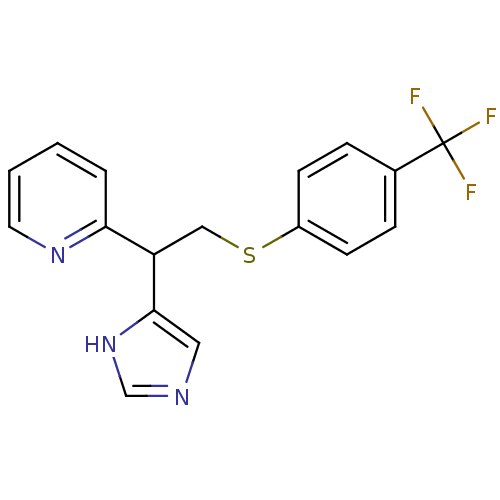 (2-(1-(1H-Imidazole-4-yl)-2-(4-trifluoromeethylphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50326297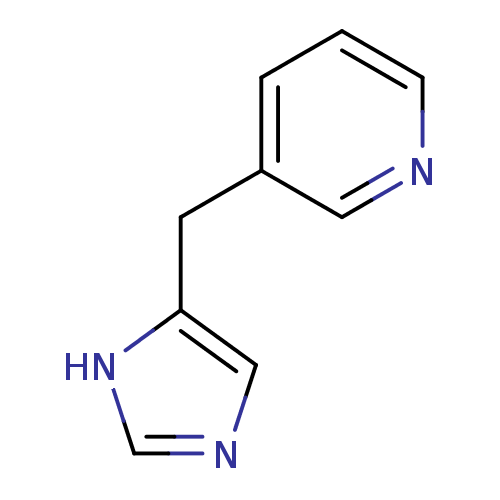 (3-((1H-imidazol-4-yl)methyl)pyridine | 3-(3H-Imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50326294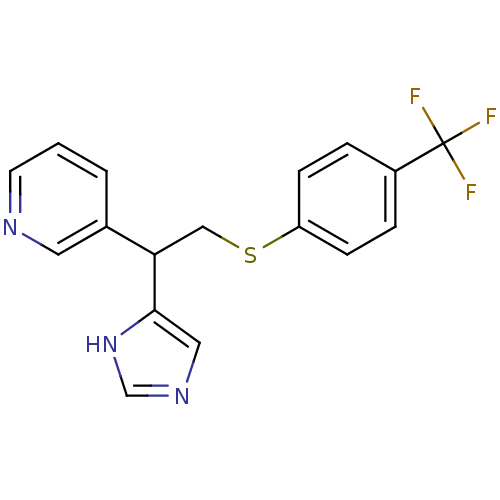 (3-(1-(1H-Imidazole-4-yl)-2-(4-trifluoromeethylphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50326295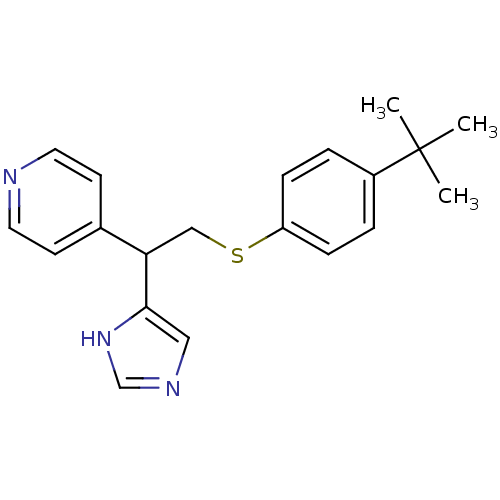 (4-(2-(4-tert-Butylphenylthio)-1-(1H-imidazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50326286 ((R)-4-(1-(1H-Imidazol-4-yl)-2-(4-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50326289 (4-(1-(1H-Imidazole-4-yl)-2-(4-trifluoromeethylphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50326285 ((S)-4-(1-(1H-Imidazol-4-yl)-2-(4-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Binding affinity to human histamine H1 receptor | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50326286 ((R)-4-(1-(1H-Imidazol-4-yl)-2-(4-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Binding affinity to human histamine H4 receptor | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50326288 (3-(2-(4-tert-Butylphenylthio)-1-(1H-imidazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Binding affinity to human histamine H4 receptor | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50326292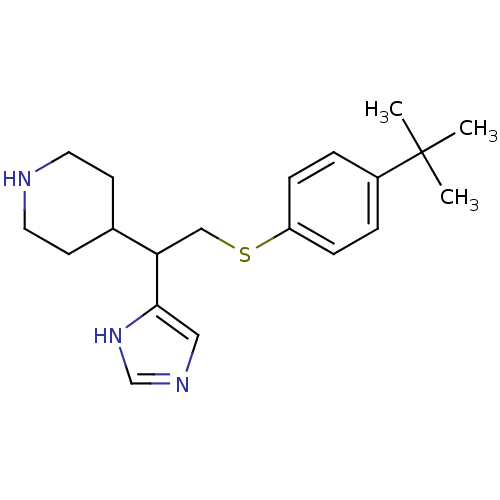 (4-(2-(4-tert-Butylphenylthio)-1-(1H-imidazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50326289 (4-(1-(1H-Imidazole-4-yl)-2-(4-trifluoromeethylphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Binding affinity to human histamine H1 receptor | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50326287 (2-(2-(4-tert-Butylphenylthio)-1-(1H-imidazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Binding affinity to human histamine H4 receptor | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50326291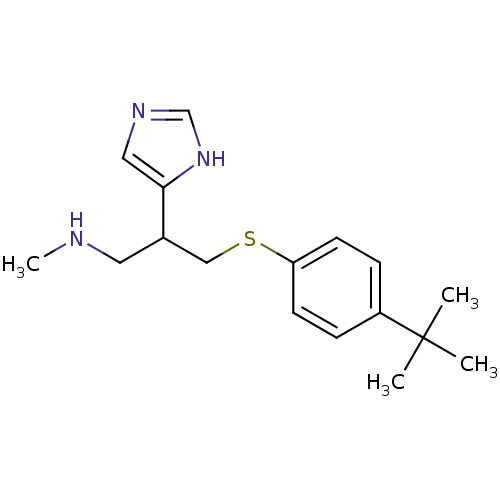 (3-(4-tert-Butylphenylthio)-2-(1H-imidazol-4-yl)-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50326290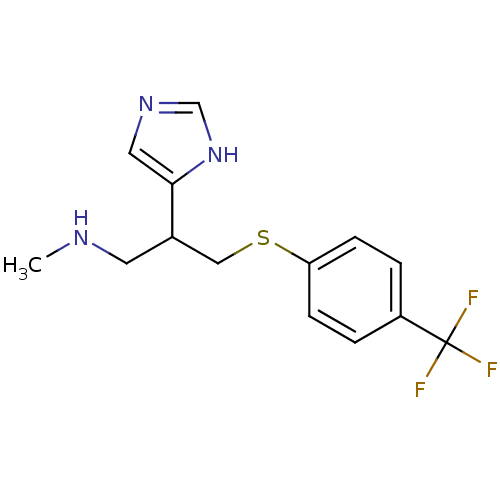 (2-(1H-Imidazol-4-yl)-N-methyl-3-(4-(trifluoromethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50326285 ((S)-4-(1-(1H-Imidazol-4-yl)-2-(4-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Binding affinity to human histamine H4 receptor | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50326296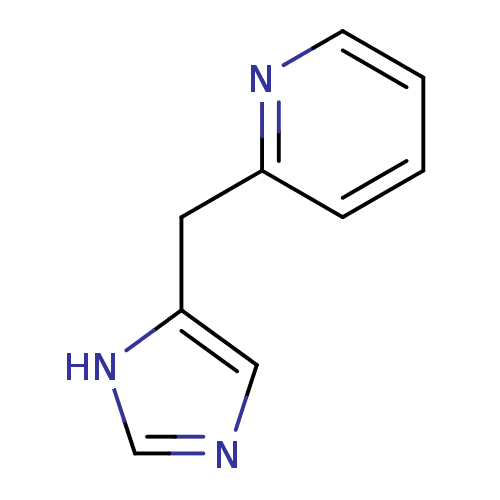 (2-((1H-imidazol-4-yl)methyl)pyridine | 2-(3H-Imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50326288 (3-(2-(4-tert-Butylphenylthio)-1-(1H-imidazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Binding affinity to human histamine H2 receptor | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50326287 (2-(2-(4-tert-Butylphenylthio)-1-(1H-imidazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Binding affinity to human histamine H2 receptor | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50326287 (2-(2-(4-tert-Butylphenylthio)-1-(1H-imidazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Binding affinity to human histamine H1 receptor | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50326288 (3-(2-(4-tert-Butylphenylthio)-1-(1H-imidazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Binding affinity to human histamine H1 receptor | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50326289 (4-(1-(1H-Imidazole-4-yl)-2-(4-trifluoromeethylphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Binding affinity to human histamine H2 receptor | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50326286 ((R)-4-(1-(1H-Imidazol-4-yl)-2-(4-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Binding affinity to human histamine H2 receptor | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50326285 ((S)-4-(1-(1H-Imidazol-4-yl)-2-(4-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Binding affinity to human histamine H2 receptor | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50015318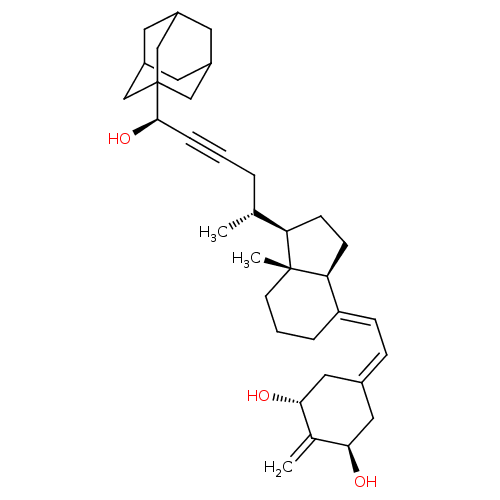 (CHEMBL3263871) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University Curated by ChEMBL | Assay Description Displacement of [3H]-1,25(OH)2D3 from GST-fused human VDR-LBD expressed in Escherichia coli BL21 | J Med Chem 57: 4073-87 (2014) Article DOI: 10.1021/jm401989c BindingDB Entry DOI: 10.7270/Q2ZS2Z2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50015316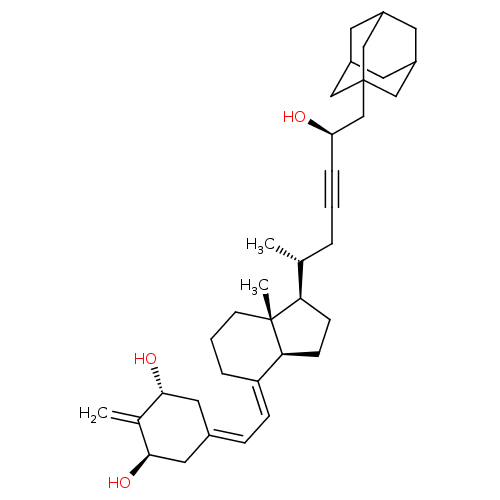 (CHEMBL3263873) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University Curated by ChEMBL | Assay Description Displacement of [3H]-1,25(OH)2D3 from GST-fused human VDR-LBD expressed in Escherichia coli BL21 | J Med Chem 57: 4073-87 (2014) Article DOI: 10.1021/jm401989c BindingDB Entry DOI: 10.7270/Q2ZS2Z2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50015315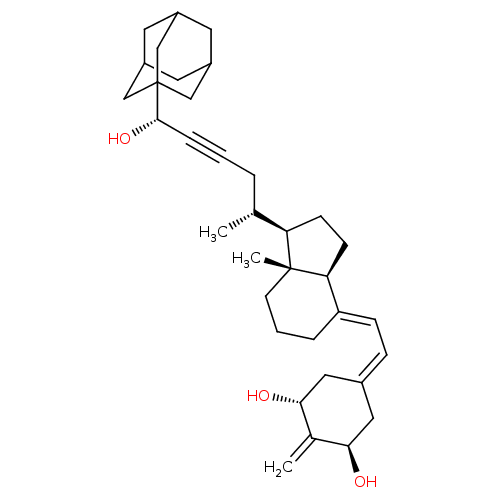 (CHEMBL3263870) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University Curated by ChEMBL | Assay Description Displacement of [3H]-1,25(OH)2D3 from GST-fused human VDR-LBD expressed in Escherichia coli BL21 | J Med Chem 57: 4073-87 (2014) Article DOI: 10.1021/jm401989c BindingDB Entry DOI: 10.7270/Q2ZS2Z2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50015317 (CHEMBL3263872) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University Curated by ChEMBL | Assay Description Displacement of [3H]-1,25(OH)2D3 from GST-fused human VDR-LBD expressed in Escherichia coli BL21 | J Med Chem 57: 4073-87 (2014) Article DOI: 10.1021/jm401989c BindingDB Entry DOI: 10.7270/Q2ZS2Z2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50326289 (4-(1-(1H-Imidazole-4-yl)-2-(4-trifluoromeethylphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50326287 (2-(2-(4-tert-Butylphenylthio)-1-(1H-imidazol-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50326289 (4-(1-(1H-Imidazole-4-yl)-2-(4-trifluoromeethylphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP1A2 | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50326287 (2-(2-(4-tert-Butylphenylthio)-1-(1H-imidazol-4-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50326292 (4-(2-(4-tert-Butylphenylthio)-1-(1H-imidazol-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase IMP-1 (Pseudomonas aeruginosa) | BDBM50493102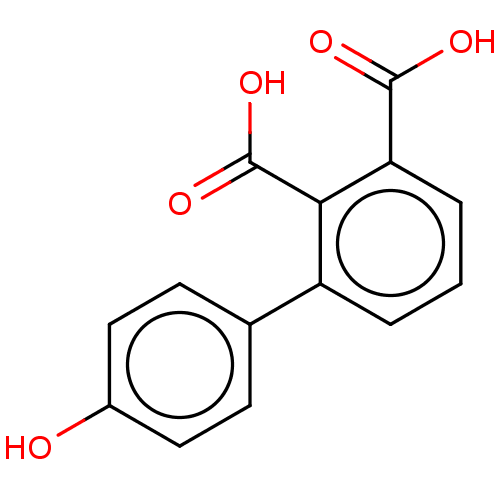 (CHEMBL566579) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Pharma, Co., Ltd Curated by ChEMBL | Assay Description Inhibition of carbapenems-resistant Pseudomonas aeruginosa MSC15369 metallo-beta-lactamase IMP1 expressed in Escherichia coli DH5[alpha] using nitroc... | Bioorg Med Chem 21: 5841-50 (2013) Article DOI: 10.1016/j.bmc.2013.07.006 BindingDB Entry DOI: 10.7270/Q24X5BQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase IMP-1 (Pseudomonas aeruginosa) | BDBM50493088 (CHEMBL2420935) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Pharma, Co., Ltd Curated by ChEMBL | Assay Description Inhibition of carbapenems-resistant Pseudomonas aeruginosa MSC15369 metallo-beta-lactamase IMP1 expressed in Escherichia coli DH5[alpha] using nitroc... | Bioorg Med Chem 21: 5841-50 (2013) Article DOI: 10.1016/j.bmc.2013.07.006 BindingDB Entry DOI: 10.7270/Q24X5BQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase IMP-1 (Pseudomonas aeruginosa) | BDBM50493103 (CHEMBL2420934) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Pharma, Co., Ltd Curated by ChEMBL | Assay Description Inhibition of carbapenems-resistant Pseudomonas aeruginosa MSC15369 metallo-beta-lactamase IMP1 expressed in Escherichia coli DH5[alpha] using nitroc... | Bioorg Med Chem 21: 5841-50 (2013) Article DOI: 10.1016/j.bmc.2013.07.006 BindingDB Entry DOI: 10.7270/Q24X5BQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase IMP-1 (Pseudomonas aeruginosa) | BDBM50493084 (CHEMBL2420944) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Pharma, Co., Ltd Curated by ChEMBL | Assay Description Inhibition of carbapenems-resistant Pseudomonas aeruginosa MSC15369 metallo-beta-lactamase IMP1 expressed in Escherichia coli DH5[alpha] using nitroc... | Bioorg Med Chem 21: 5841-50 (2013) Article DOI: 10.1016/j.bmc.2013.07.006 BindingDB Entry DOI: 10.7270/Q24X5BQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase IMP-1 (Pseudomonas aeruginosa) | BDBM50493083 (CHEMBL2420948) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Pharma, Co., Ltd Curated by ChEMBL | Assay Description Inhibition of carbapenems-resistant Pseudomonas aeruginosa MSC15369 metallo-beta-lactamase IMP1 expressed in Escherichia coli DH5[alpha] using nitroc... | Bioorg Med Chem 21: 5841-50 (2013) Article DOI: 10.1016/j.bmc.2013.07.006 BindingDB Entry DOI: 10.7270/Q24X5BQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 114 total ) | Next | Last >> |