

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459581 (CHEMBL4218481) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459578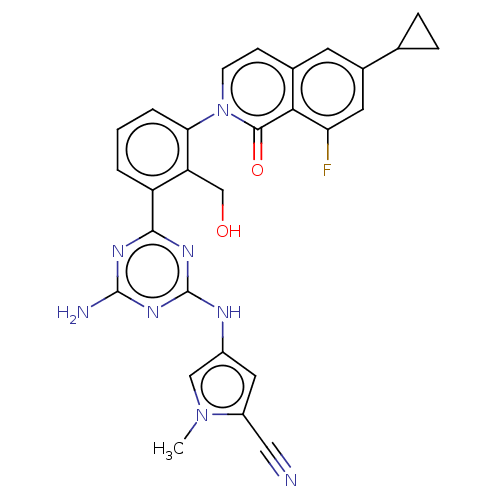 (CHEMBL4204675) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459553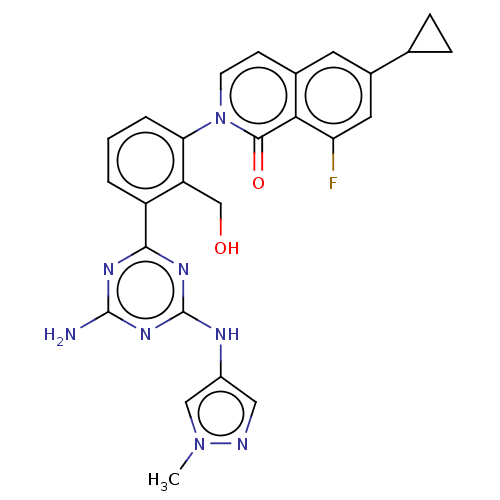 (CHEMBL4209441) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459577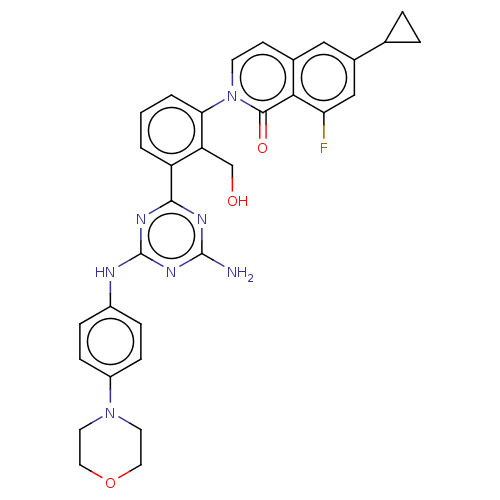 (CHEMBL4217816) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272596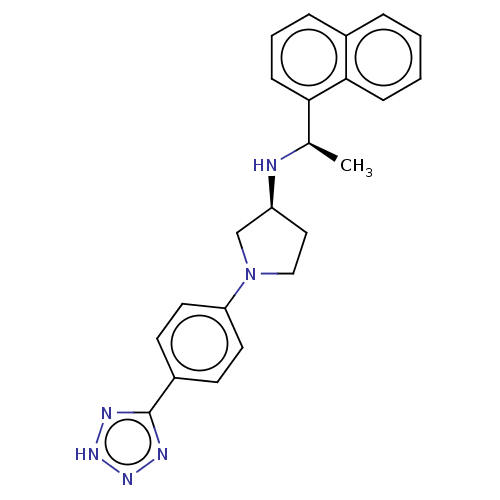 (CHEMBL4126450) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459580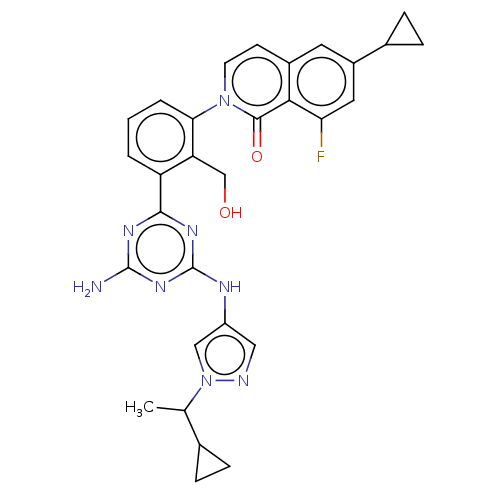 (CHEMBL4211893) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459560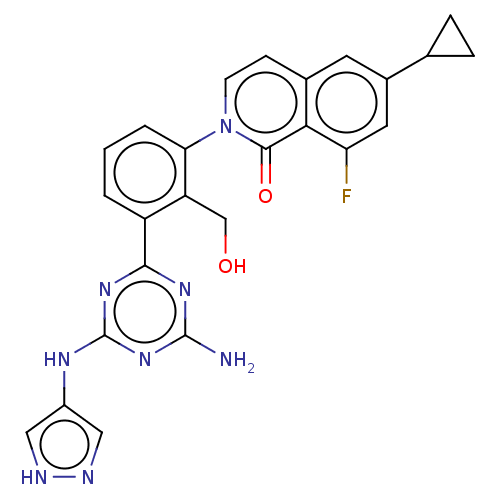 (CHEMBL4214343) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459573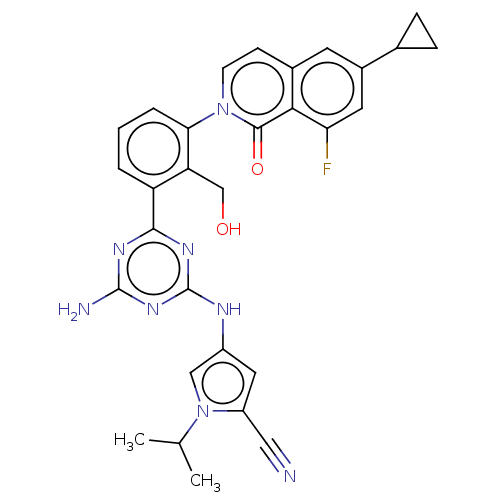 (CHEMBL4208062) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated activated human recombinant BTK using FITC-labeled Srctide peptide substrate by mobility shift ... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459579 (CHEMBL4212436) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459558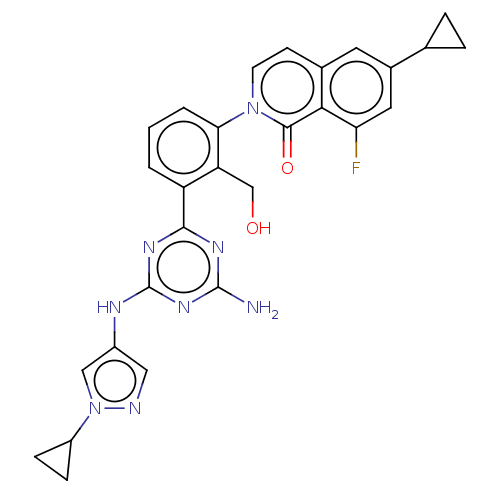 (CHEMBL4216862) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459572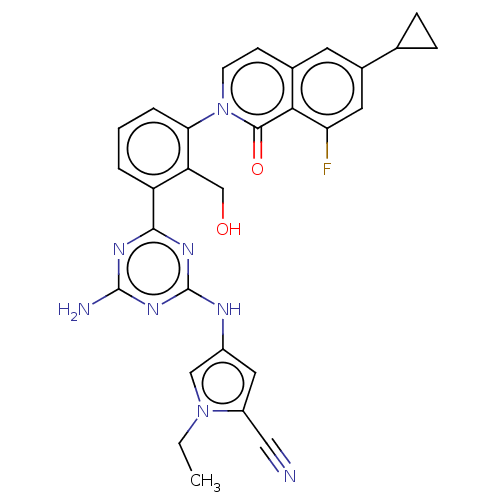 (CHEMBL4216339) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459571 (CHEMBL4212860) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459554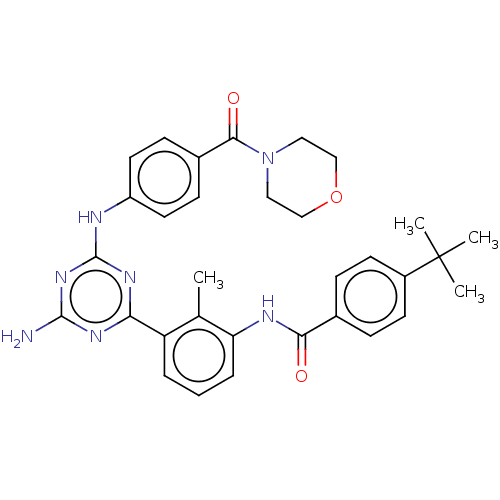 (CHEMBL4209148) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459565 (CHEMBL4217003) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459568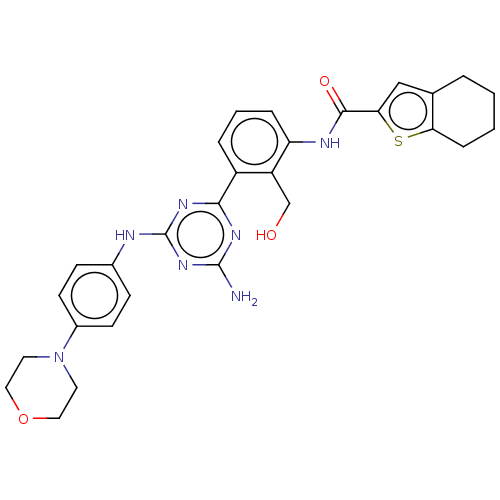 (CHEMBL4213416) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459559 (CHEMBL4203192) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272602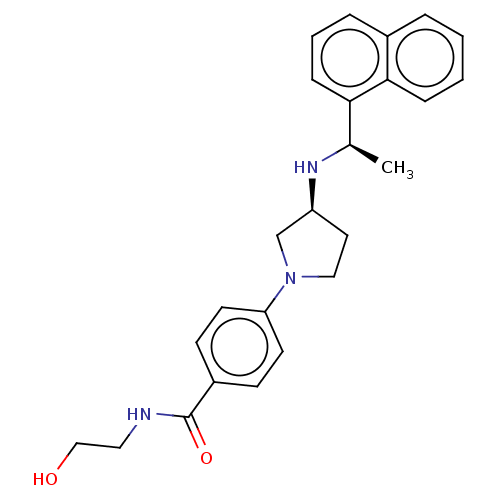 (CHEMBL4126877) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223914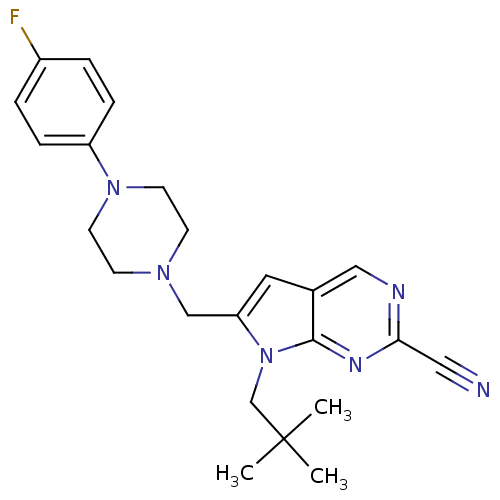 (6-((4-(4-fluorophenyl)piperazin-1-yl)methyl)-7-neo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223939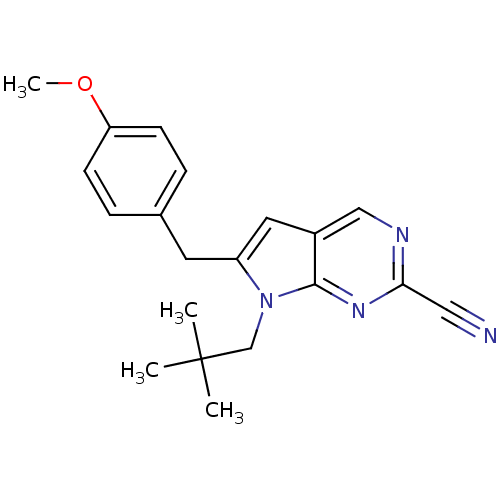 (6-(4-methoxybenzyl)-7-neopentyl-7H-pyrrolo[2,3-d]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223921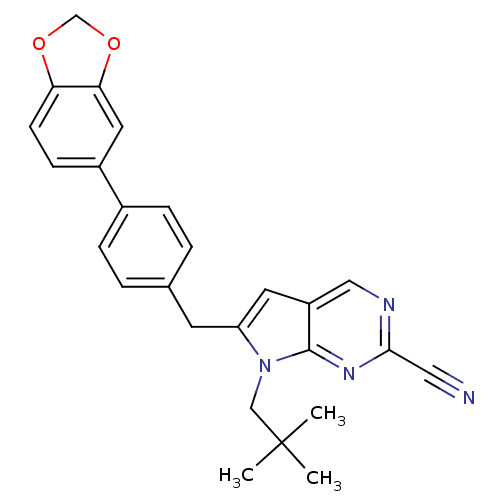 (6-(4-(benzo[d][1,3]dioxol-5-yl)benzyl)-7-neopentyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223935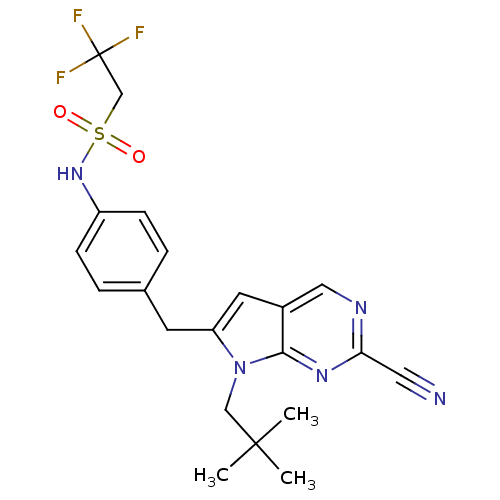 (CHEMBL399842 | N-(4-((2-cyano-7-neopentyl-7H-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223915 (6-benzyl-7-neopentyl-7H-pyrrolo[2,3-d]pyrimidine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272605 (CHEMBL4128542) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223925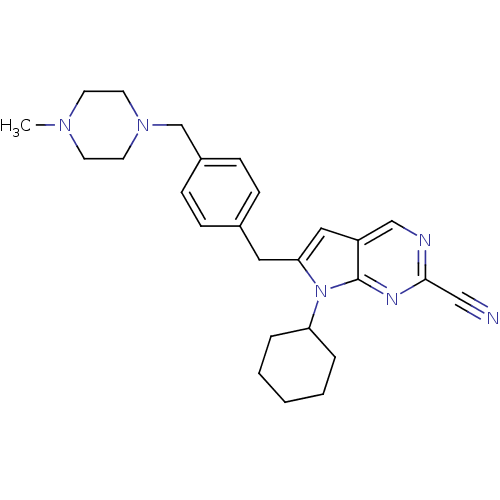 (6-(4-((4-methylpiperazin-1-yl)methyl)benzyl)-7-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459555 (CHEMBL4205586) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223919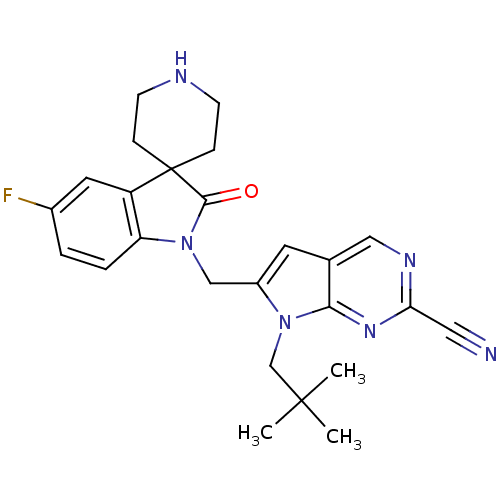 (7-(2,2-dimethylpropyl)-6-[(5-fluoro-2-oxospiro[ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272606 (CHEMBL4125917) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459575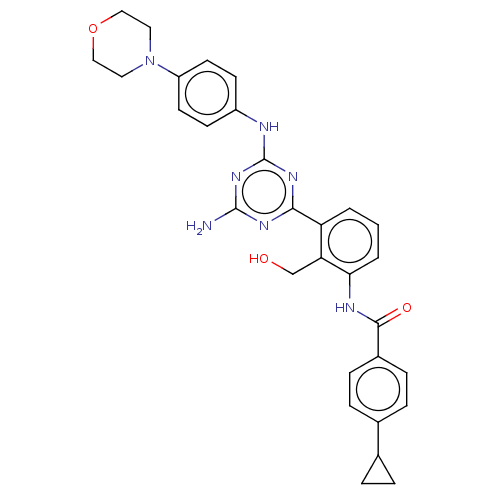 (CHEMBL4204802) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459570 (CHEMBL4217295) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459580 (CHEMBL4211893) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated activated human recombinant BTK using FITC-labeled Srctide peptide substrate by mobility shift ... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223910 (6-(4-chlorobenzyl)-7-cyclohexyl-7H-pyrrolo[2,3-d]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50452721 (CHEMBL4203409) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459556 (CHEMBL4205287) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223911 (7-neopentyl-6-((pyridin-4-yloxy)methyl)-7H-pyrrolo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50376511 (CHEMBL261511) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K by fluorescence assay | Bioorg Med Chem Lett 18: 2599-603 (2008) Article DOI: 10.1016/j.bmcl.2008.03.036 BindingDB Entry DOI: 10.7270/Q2HT2Q6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223920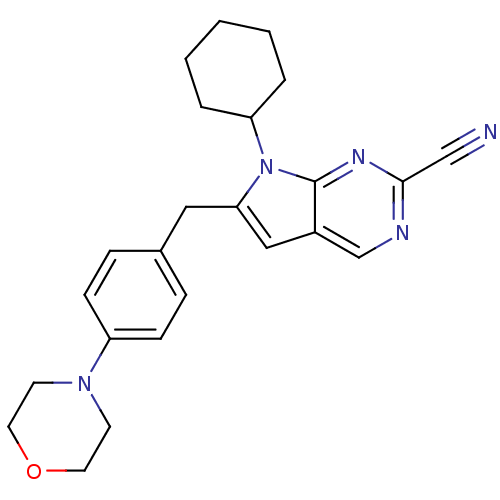 (6-(4-morpholinobenzyl)-7-cyclohexyl-7H-pyrrolo[2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50376500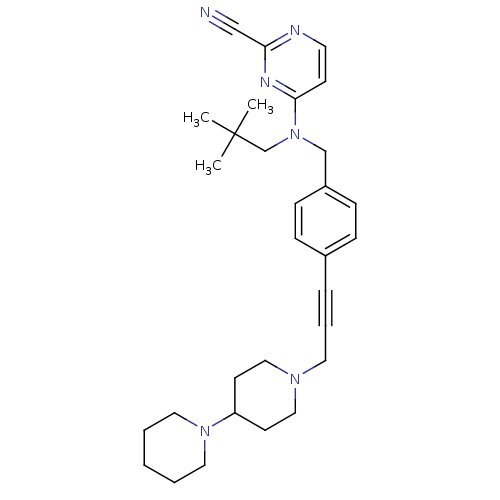 (CHEMBL261700) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K by fluorescence assay | Bioorg Med Chem Lett 18: 2599-603 (2008) Article DOI: 10.1016/j.bmcl.2008.03.036 BindingDB Entry DOI: 10.7270/Q2HT2Q6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50376507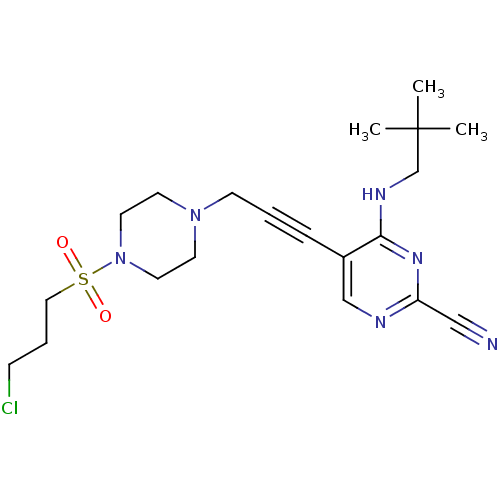 (CHEMBL429147) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K by fluorescence assay | Bioorg Med Chem Lett 18: 2599-603 (2008) Article DOI: 10.1016/j.bmcl.2008.03.036 BindingDB Entry DOI: 10.7270/Q2HT2Q6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459552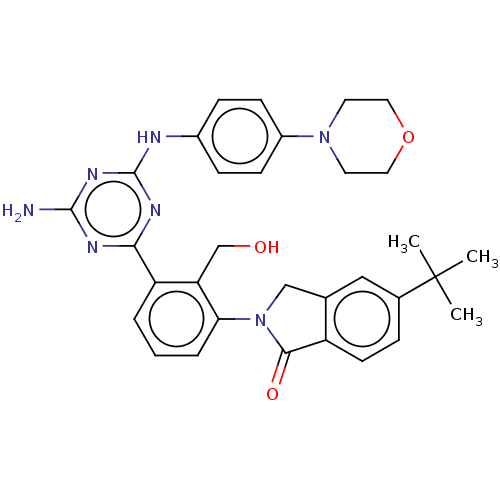 (CHEMBL4208482) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50376503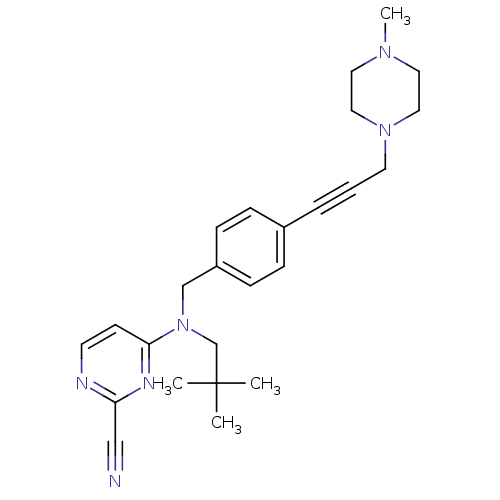 (CHEMBL261516) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K by fluorescence assay | Bioorg Med Chem Lett 18: 2599-603 (2008) Article DOI: 10.1016/j.bmcl.2008.03.036 BindingDB Entry DOI: 10.7270/Q2HT2Q6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223940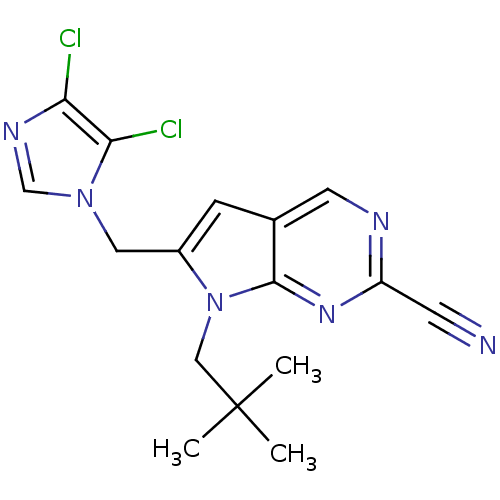 (6-((4,5-dichloro-1H-imidazol-1-yl)methyl)-7-neopen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223936 (6-((5,5-dimethyl-2,4-dioxooxazolidin-3-yl)methyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459567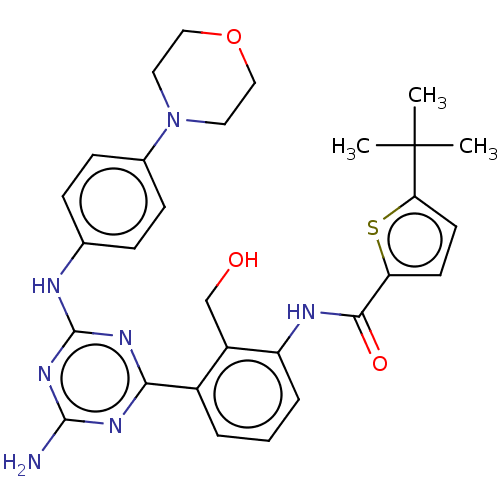 (CHEMBL4215004) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459553 (CHEMBL4209441) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated activated human recombinant BTK using FITC-labeled Srctide peptide substrate by mobility shift ... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459569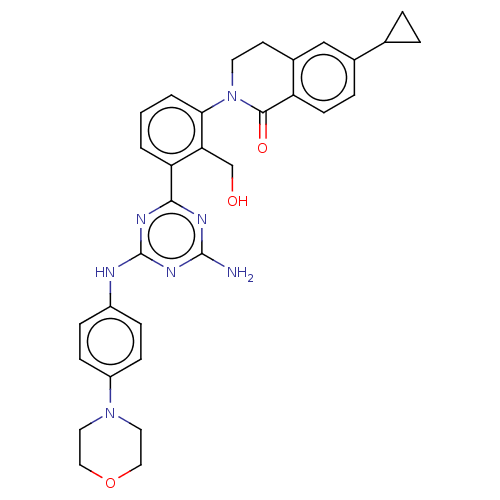 (CHEMBL4204140) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459557 (CHEMBL4209020) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Rattus norvegicus) | BDBM50272607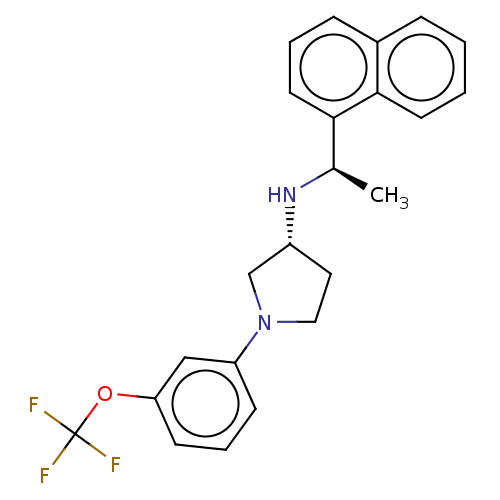 (CHEMBL4126057) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA | Bioorg Med Chem Lett 28: 2055-2060 (2018) Article DOI: 10.1016/j.bmcl.2018.04.055 BindingDB Entry DOI: 10.7270/Q2DF6TP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50459576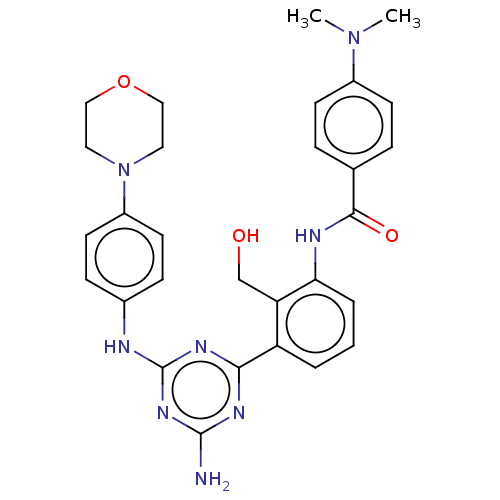 (CHEMBL4209666) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... | J Med Chem 61: 8917-8933 (2018) Article DOI: 10.1021/acs.jmedchem.8b01147 BindingDB Entry DOI: 10.7270/Q2ZS305M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 257 total ) | Next | Last >> |