

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343590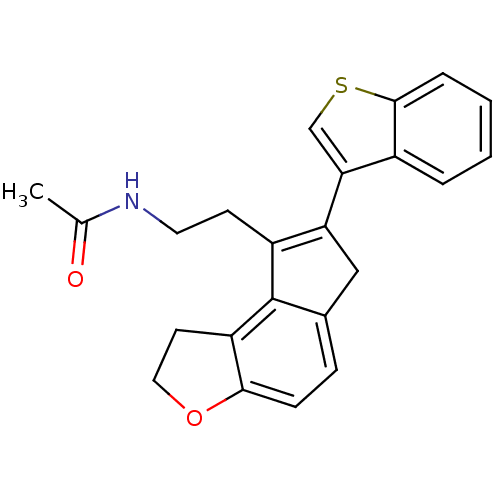 (CHEMBL1774531 | N-{2-[7-(1-Benzothien-3-yl)-1,6-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343599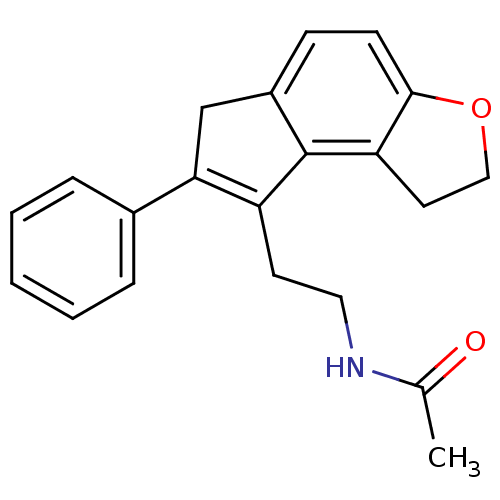 (CHEMBL1774522 | N-[2-(7-Phenyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT2 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343598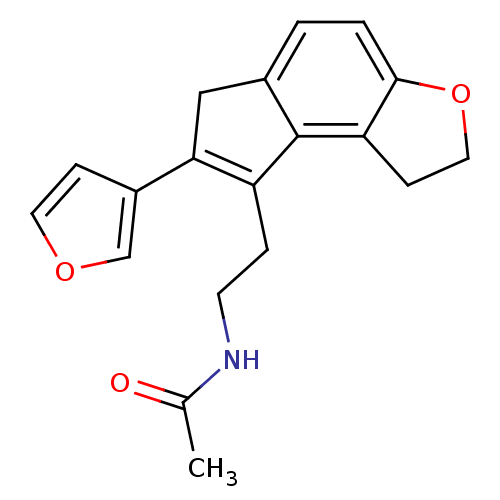 (CHEMBL1774523 | N-{2-[7-(3-Furyl)-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343599 (CHEMBL1774522 | N-[2-(7-Phenyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343601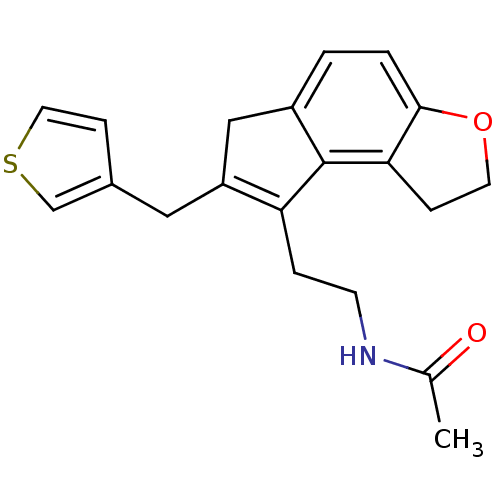 (CHEMBL1774520 | N-{2-[7-(3-Thienylmethyl)-1,6-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343599 (CHEMBL1774522 | N-[2-(7-Phenyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343599 (CHEMBL1774522 | N-[2-(7-Phenyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343603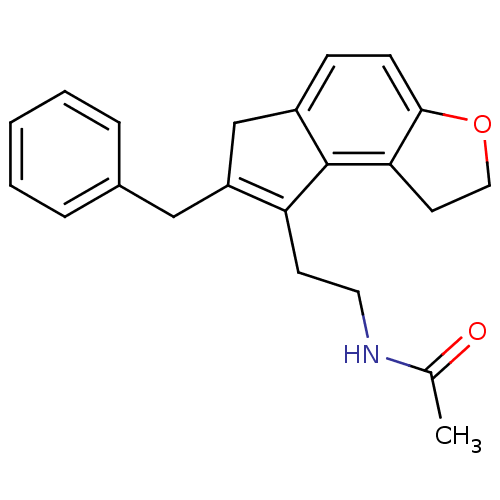 (CHEMBL1774518 | N-[2-(7-Benzyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343600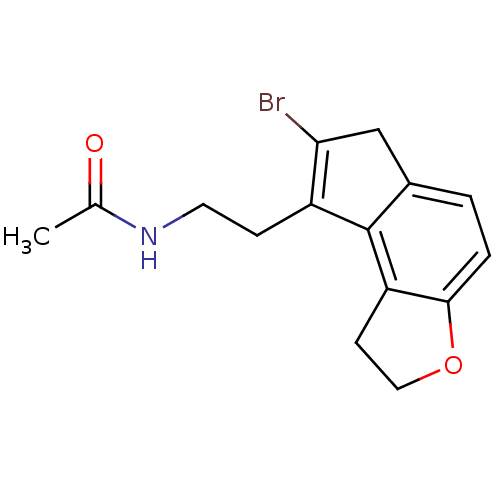 (CHEMBL1774521 | N-[2-(7-Bromo-1,6-dihydro-2H-inden...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343595 (CHEMBL1774526 | N-{2-[7-(3-Methoxyphenyl)-1,6-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343598 (CHEMBL1774523 | N-{2-[7-(3-Furyl)-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343605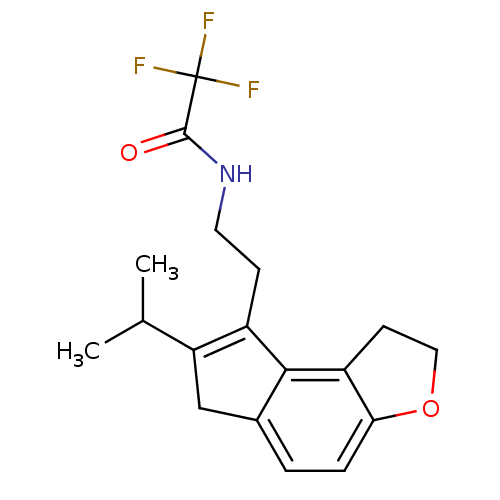 (2,2,2-Trifluoro-N-[2-(7-isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343607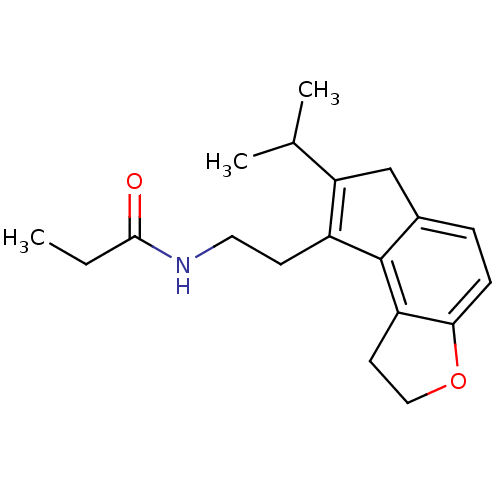 (CHEMBL1774514 | N-[2-(7-Isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343592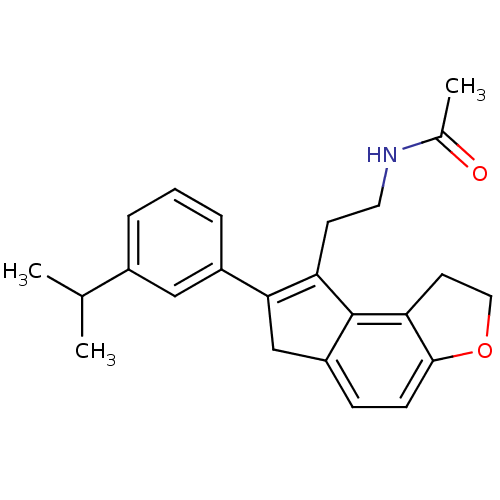 (CHEMBL1774529 | N-{2-[7-(3-Isopropylphenyl)-1,6-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343602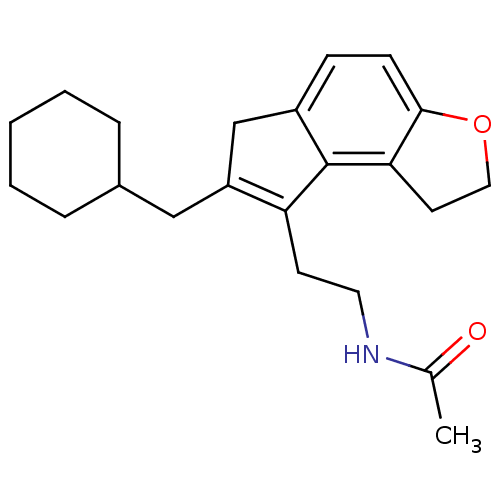 (CHEMBL1774519 | N-{2-[7-(Cyclohexylmethyl)-1,6-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343607 (CHEMBL1774514 | N-[2-(7-Isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343600 (CHEMBL1774521 | N-[2-(7-Bromo-1,6-dihydro-2H-inden...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50118470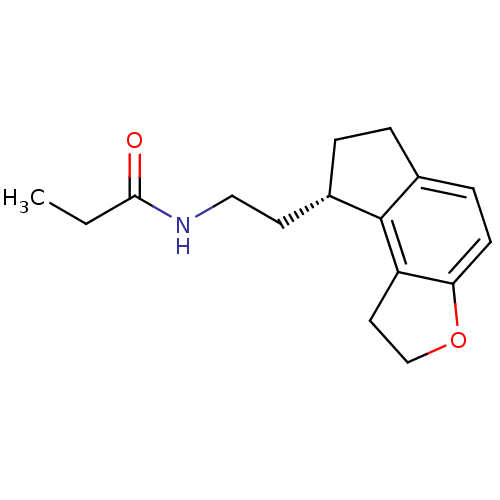 (CHEMBL1218 | N-[2-(1,6,7,8-Tetrahydro-2H-3-oxa-as-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human MT1 expressed in CHO cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343608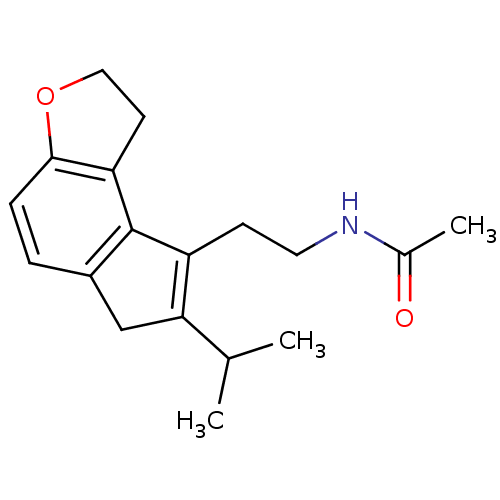 (CHEMBL1774513 | N-[2-(7-Isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343596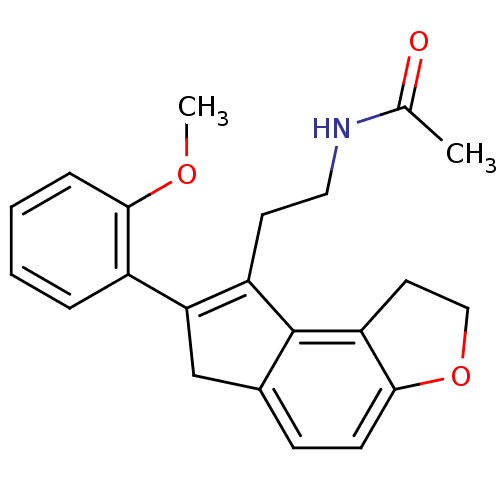 (CHEMBL1774525 | N-{2-[7-(2-Methoxyphenyl)-1,6-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343609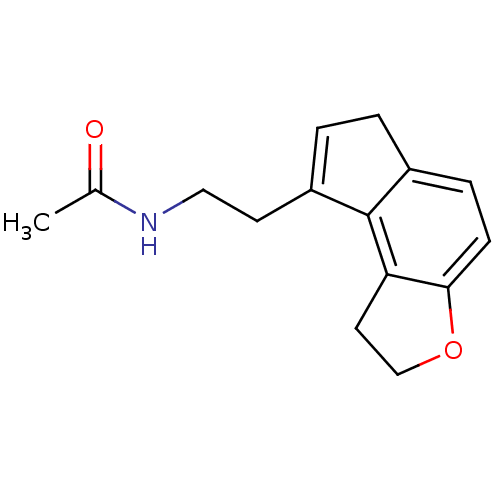 (CHEMBL1774512 | N-[2-(1,6-Dihydro-2H-indeno[5,4-b]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343608 (CHEMBL1774513 | N-[2-(7-Isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50347590 (CHEMBL1802028) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50575683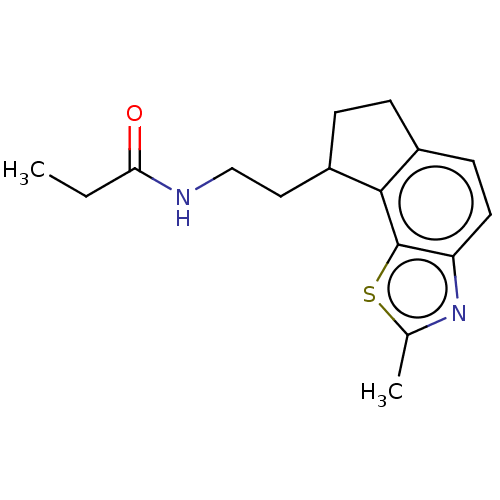 (CHEMBL4868554) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[125l]-lodomelatonin from human MT1 expressed in CHO cell membrane incubated for 150 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM103443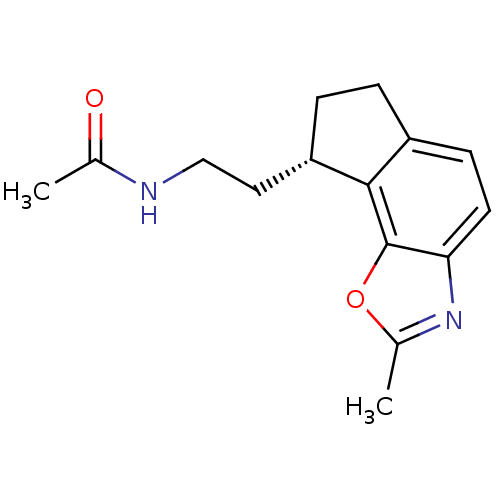 (US8552037, 90) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[125l]-lodomelatonin from human MT1 expressed in CHO cell membrane incubated for 150 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343605 (2,2,2-Trifluoro-N-[2-(7-isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343597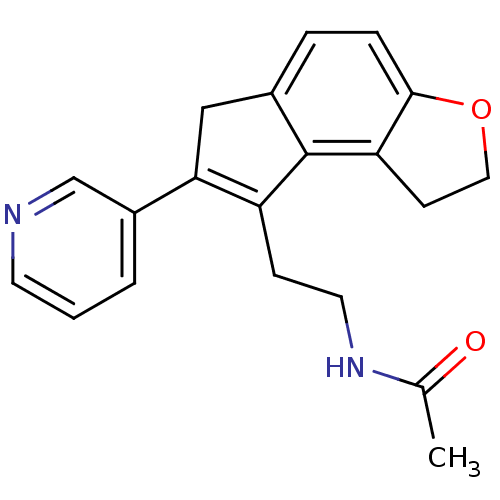 (CHEMBL1774524 | N-[2-(7-Pyridin-3-yl-1,6-dihydro-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343606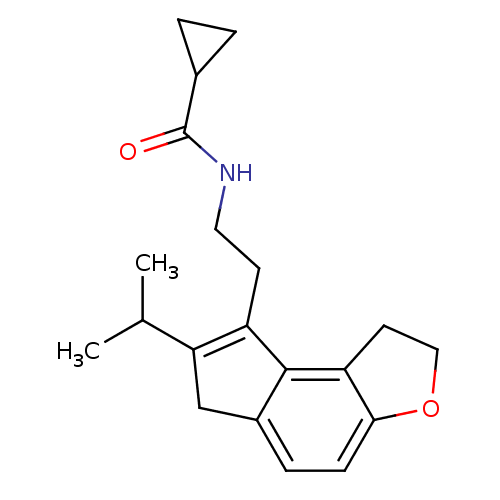 (CHEMBL1774515 | N-[2-(7-Isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM103449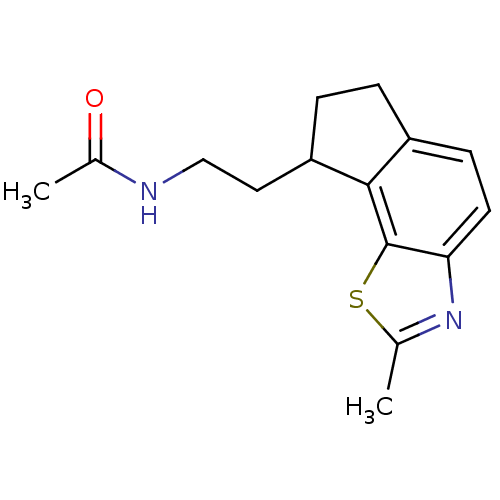 (US8552037, 97) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[125l]-lodomelatonin from human MT1 expressed in CHO cell membrane incubated for 150 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343594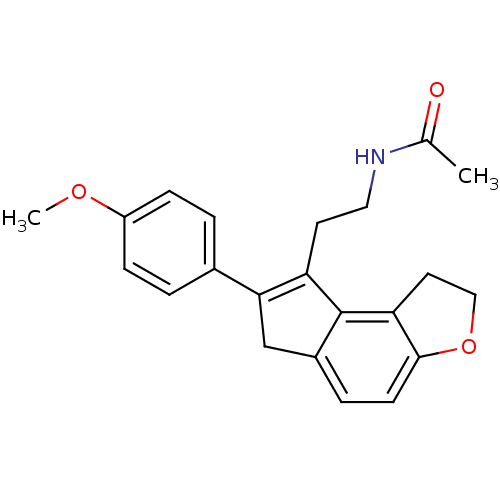 (CHEMBL1774527 | N-{2-[7-(4-Methoxyphenyl)-1,6-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50347593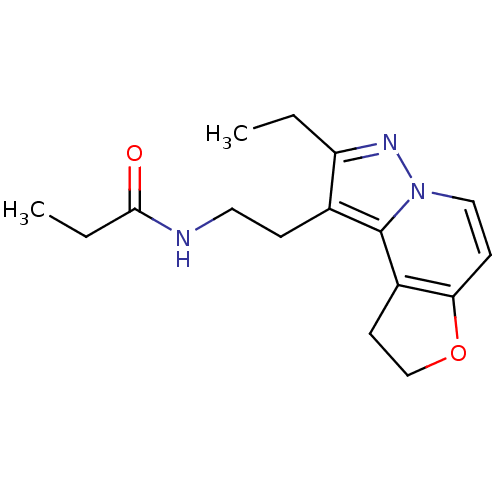 (CHEMBL1802026) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50130611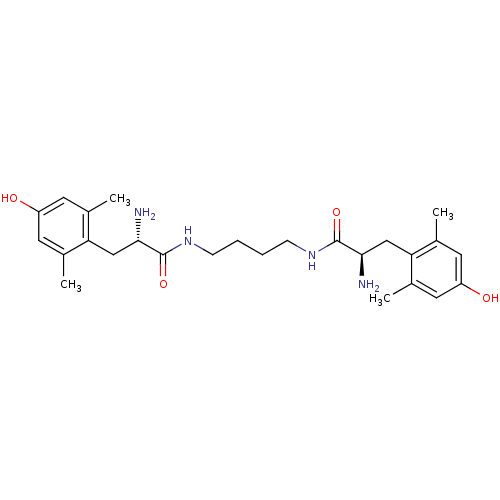 (2-Amino-N-{4-[2-amino-3-(4-hydroxy-2,6-dimethyl-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor mu 1 using [3H]-DAGO in rat brain P2 synaptosomal preparation | J Med Chem 46: 3201-9 (2003) Article DOI: 10.1021/jm020459z BindingDB Entry DOI: 10.7270/Q28C9VMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50221787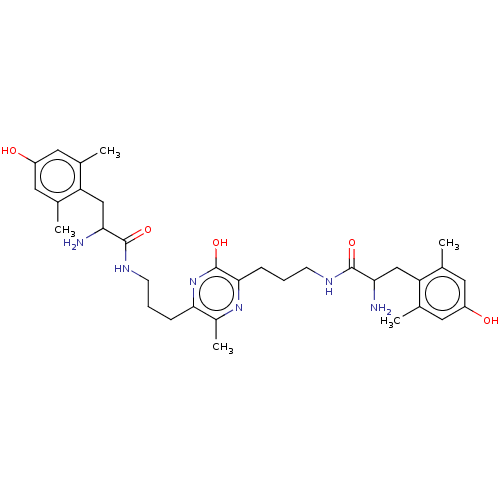 (CHEMBL3216418) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for Opioid receptor mu 1 agonism in isolated tissues from guinea pig ileum | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50347589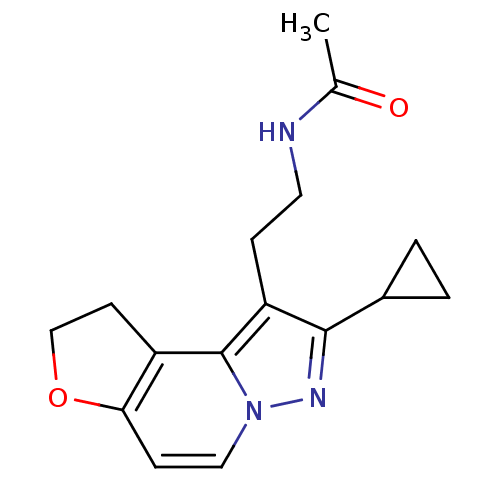 (CHEMBL1802027) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343609 (CHEMBL1774512 | N-[2-(1,6-Dihydro-2H-indeno[5,4-b]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50130617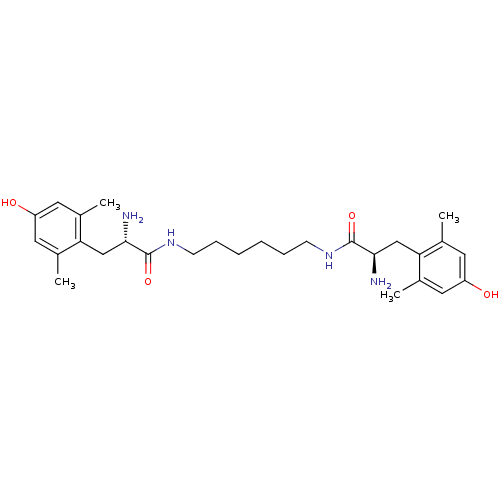 (2-Amino-N-{6-[2-amino-3-(4-hydroxy-2,6-dimethyl-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor mu 1 using [3H]-DAGO in rat brain P2 synaptosomal preparation | J Med Chem 46: 3201-9 (2003) Article DOI: 10.1021/jm020459z BindingDB Entry DOI: 10.7270/Q28C9VMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343595 (CHEMBL1774526 | N-{2-[7-(3-Methoxyphenyl)-1,6-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50575683 (CHEMBL4868554) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[125l]-lodomelatonin from human MT2 expressed in CHO cell membrane incubated for 150 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (RAT) | BDBM82421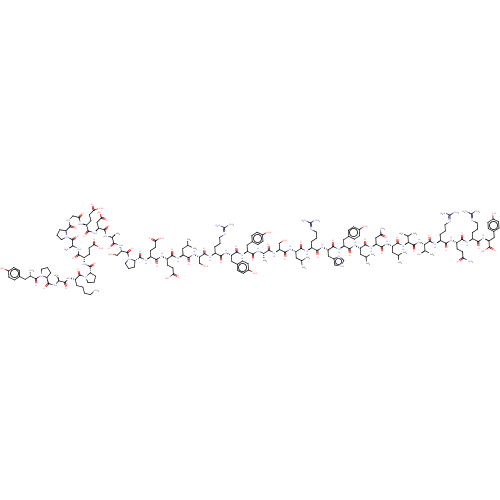 (CAS_81858-94-8 | PYY, porcine) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U410 Curated by PDSP Ki Database | Mol Pharmacol 60: 124-34 (2001) Article DOI: 10.1124/mol.60.1.124 BindingDB Entry DOI: 10.7270/Q2C827VN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50347588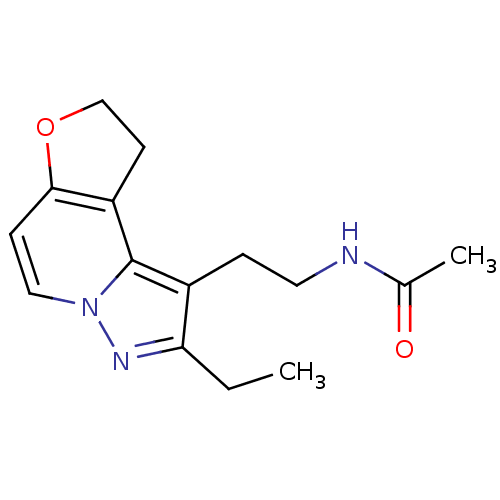 (CHEMBL1802025) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM103442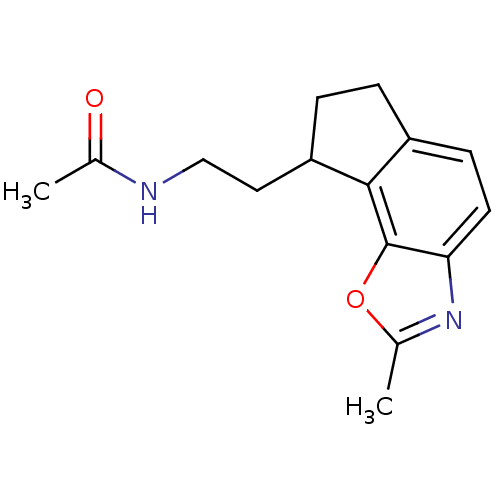 (US8552037, 89) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[125l]-lodomelatonin from human MT1 expressed in CHO cell membrane incubated for 150 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM103445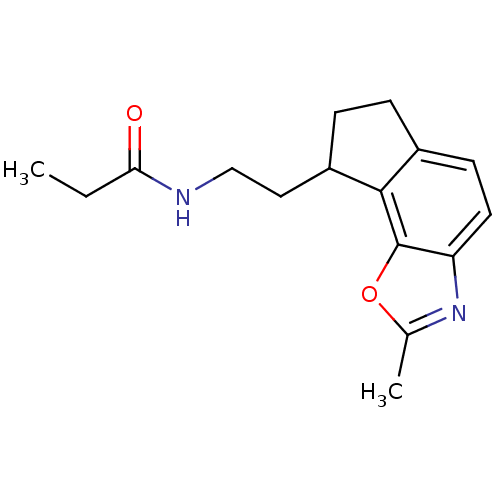 (US8552037, 92) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[125l]-lodomelatonin from human MT1 expressed in CHO cell membrane incubated for 150 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343597 (CHEMBL1774524 | N-[2-(7-Pyridin-3-yl-1,6-dihydro-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (RAT) | BDBM82420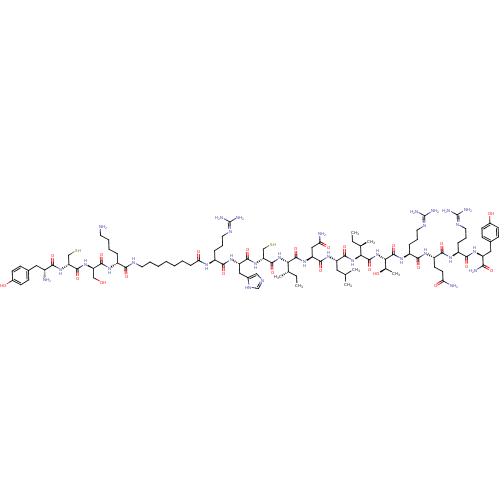 (NPY, C2 | NPY, C2, porcine | PYY 3-36, porcine) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U410 Curated by PDSP Ki Database | Mol Pharmacol 60: 124-34 (2001) Article DOI: 10.1124/mol.60.1.124 BindingDB Entry DOI: 10.7270/Q2C827VN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM103443 (US8552037, 90) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[125l]-lodomelatonin from human MT2 expressed in CHO cell membrane incubated for 150 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM103441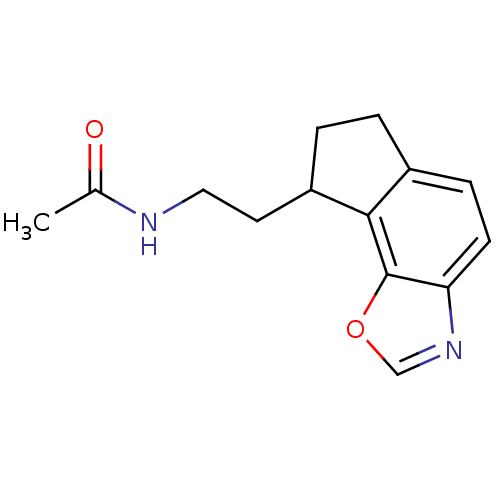 (US8552037, 87) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[125l]-lodomelatonin from human MT2 expressed in CHO cell membrane incubated for 150 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50109635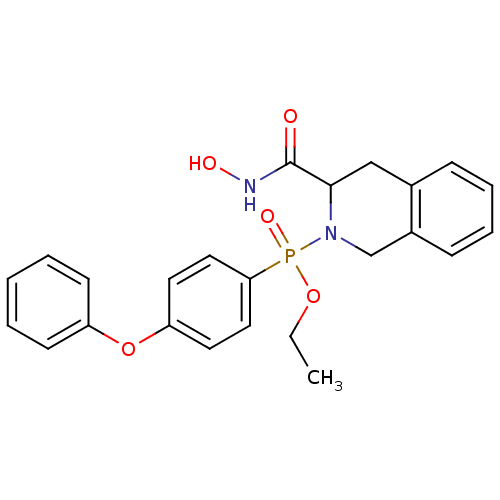 ((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K. Curated by ChEMBL | Assay Description Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). | J Med Chem 45: 919-29 (2002) BindingDB Entry DOI: 10.7270/Q2XK8G9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50347585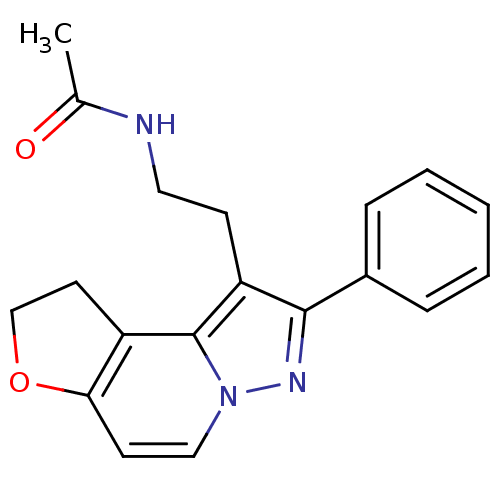 (CHEMBL1802022) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50347587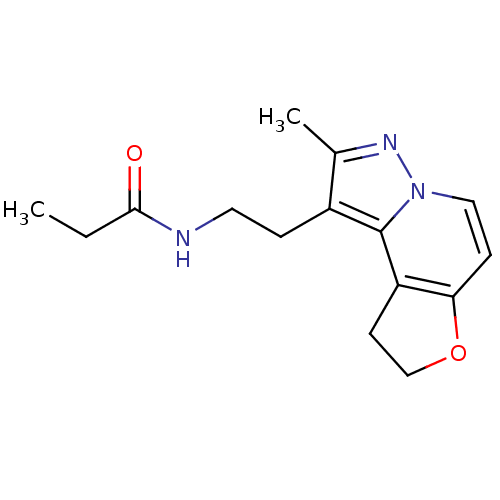 (CHEMBL1802024) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343604 (CHEMBL1774517 | N-Ethyl-N'-[2-(7-isopropyl-1,6-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7557 total ) | Next | Last >> |