

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylistamine from human histamine H3 receptor by cell-based assay | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylistamine from rat histamine H3 receptor by cell-based assay | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50262939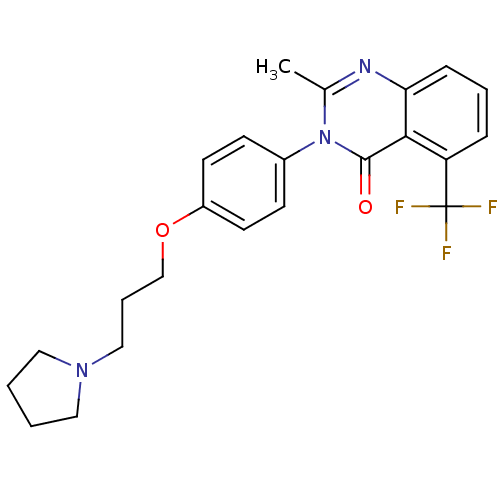 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rhesus monkey histamine H3 receptor expressed in HEK293T cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylistamine from mouse histamine H3 receptor by cell-based assay | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262939 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50262939 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat histamine H3 receptor expressed in HEK293 cells coexpressed with CRE-beta-lactamase | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50067491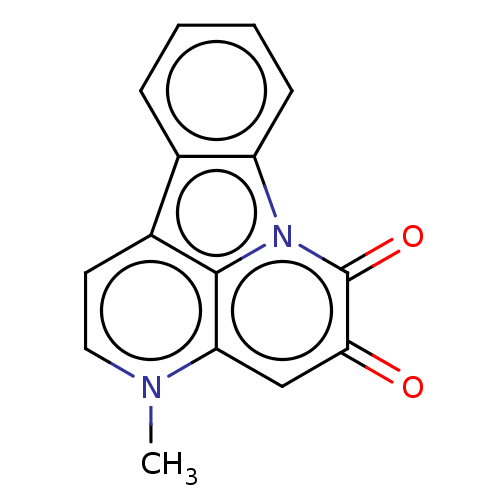 (CHEMBL3400663) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Competitive inhibition of PTP1B (unknown origin) assessed as hydrolysis of para-nitrophenylphosphate by Lineweaver-Burk plot | Bioorg Med Chem Lett 25: 1979-81 (2015) Article DOI: 10.1016/j.bmcl.2015.03.014 BindingDB Entry DOI: 10.7270/Q23N252T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50067500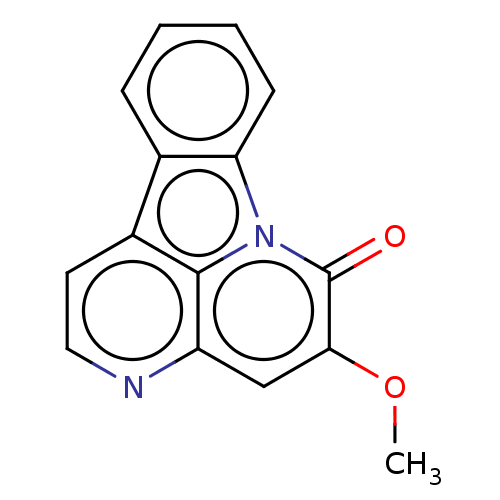 (5-Methoxycanthinone) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Non-competitive inhibition of PTP1B (unknown origin) assessed as hydrolysis of para-nitrophenylphosphate by Lineweaver-Burk plot | Bioorg Med Chem Lett 25: 1979-81 (2015) Article DOI: 10.1016/j.bmcl.2015.03.014 BindingDB Entry DOI: 10.7270/Q23N252T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50067496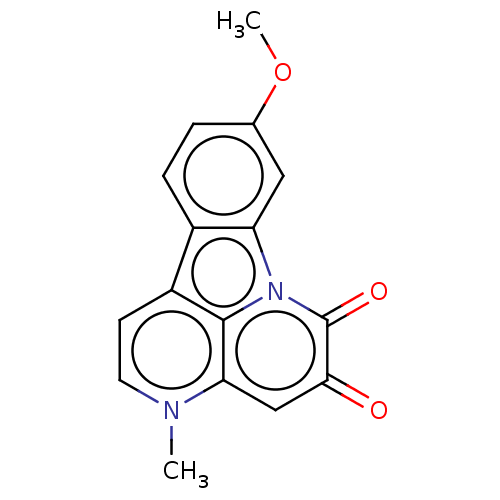 (CHEMBL3400666) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Non-competitive inhibition of PTP1B (unknown origin) assessed as hydrolysis of para-nitrophenylphosphate by Lineweaver-Burk plot | Bioorg Med Chem Lett 25: 1979-81 (2015) Article DOI: 10.1016/j.bmcl.2015.03.014 BindingDB Entry DOI: 10.7270/Q23N252T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50327036 ((1R,3S,4S,5S)-3-[(E)-3-(3,4-Dihydroxy-phenyl)-acry...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Non-competitive inhibition of PTP1B (unknown origin) using p-NPP as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 28: 1194-1197 (2018) Article DOI: 10.1016/j.bmcl.2018.02.052 BindingDB Entry DOI: 10.7270/Q20Z75V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50067501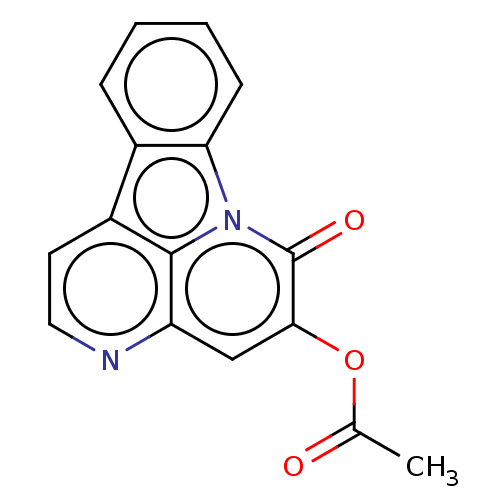 (CHEMBL3400667) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Non-competitive inhibition of PTP1B (unknown origin) assessed as hydrolysis of para-nitrophenylphosphate by Lineweaver-Burk plot | Bioorg Med Chem Lett 25: 1979-81 (2015) Article DOI: 10.1016/j.bmcl.2015.03.014 BindingDB Entry DOI: 10.7270/Q23N252T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50067492 (CHEMBL3400664) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Non-competitive inhibition of PTP1B (unknown origin) assessed as hydrolysis of para-nitrophenylphosphate by Lineweaver-Burk plot | Bioorg Med Chem Lett 25: 1979-81 (2015) Article DOI: 10.1016/j.bmcl.2015.03.014 BindingDB Entry DOI: 10.7270/Q23N252T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50067493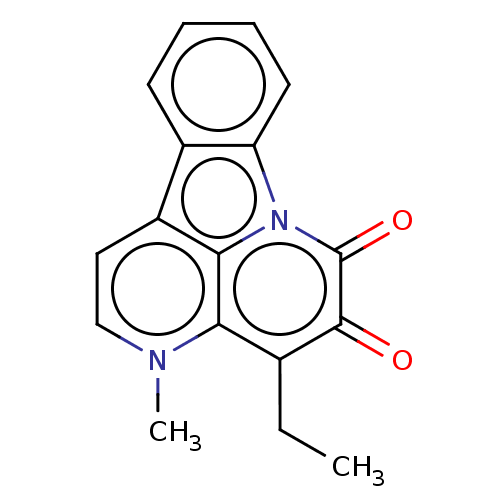 (CHEMBL3400665) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Non-competitive inhibition of PTP1B (unknown origin) assessed as hydrolysis of para-nitrophenylphosphate by Lineweaver-Burk plot | Bioorg Med Chem Lett 25: 1979-81 (2015) Article DOI: 10.1016/j.bmcl.2015.03.014 BindingDB Entry DOI: 10.7270/Q23N252T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50395732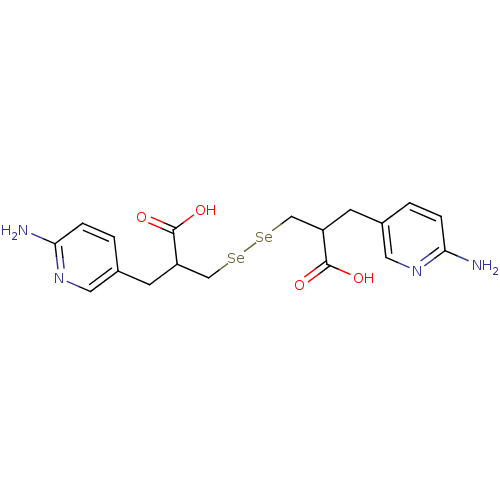 (CHEMBL2164462) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human activated TAFI using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by spectrophot... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50395730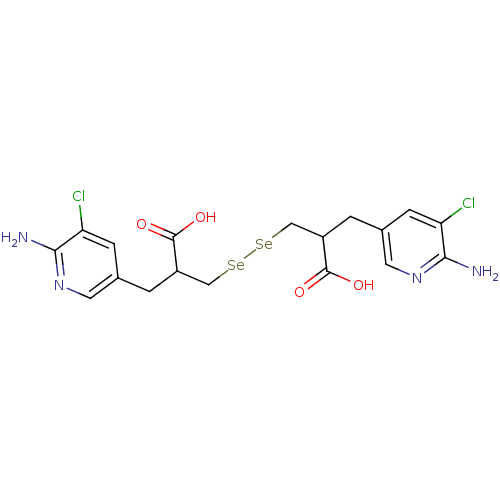 (CHEMBL2164450) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human activated TAFI using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by spectrophot... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50512479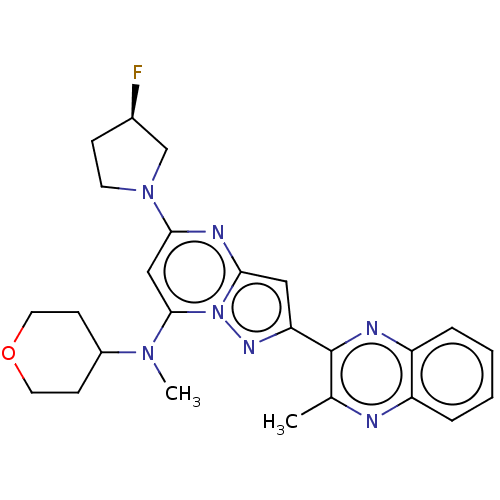 (CHEMBL4456440) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human N-terminal FLAG-tagged PDE10A (1 to 90 residues) expressed in using African green monkey COS7 cells using [3H]cAMP or [3H]cGMP as... | Bioorg Med Chem 27: 3440-3450 (2019) Article DOI: 10.1016/j.bmc.2019.06.021 BindingDB Entry DOI: 10.7270/Q2SB492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM144390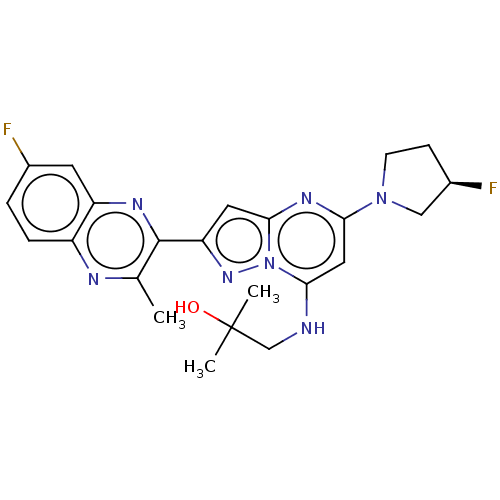 (US8969376, 1.006) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human N-terminal FLAG-tagged PDE10A (1 to 90 residues) expressed in using African green monkey COS7 cells using [3H]cAMP or [3H]cGMP as... | Bioorg Med Chem 27: 3440-3450 (2019) Article DOI: 10.1016/j.bmc.2019.06.021 BindingDB Entry DOI: 10.7270/Q2SB492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296178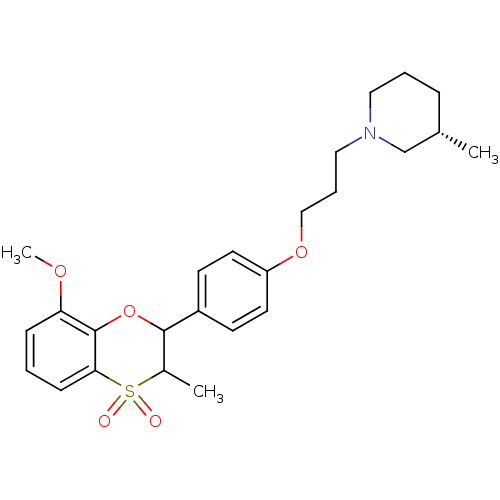 ((+/-)-(S)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296179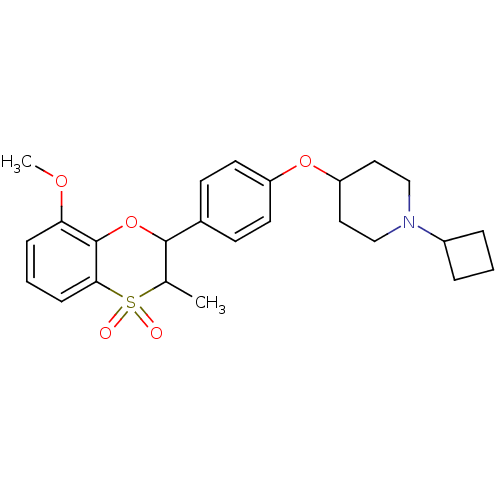 ((+/-)-1-Cyclobutyl-4-[4-(8-methoxy-3-methyl-4,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.177 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MMP-9 by fluorometric assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Sus scrofa) | BDBM50395732 (CHEMBL2164462) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic carboxypeptidase B using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins b... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296176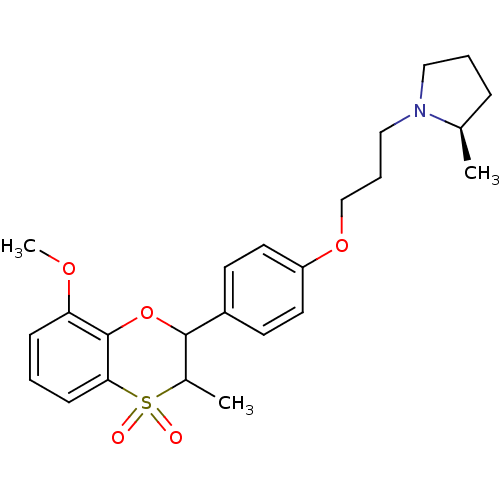 ((+/-)-(R)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296174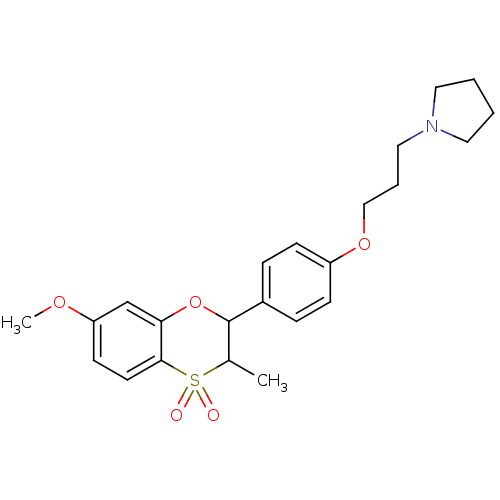 ((+/-)-1-{3-[4-(7-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296175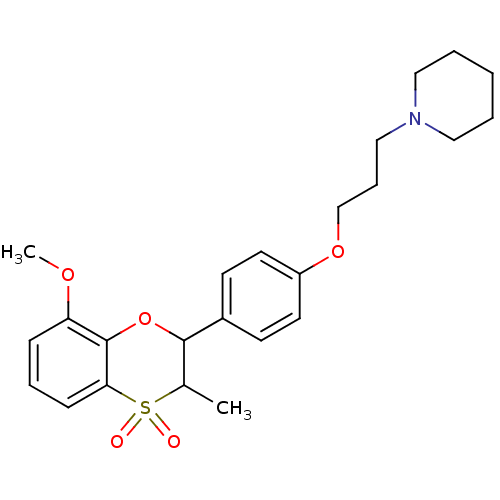 ((+/-)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296173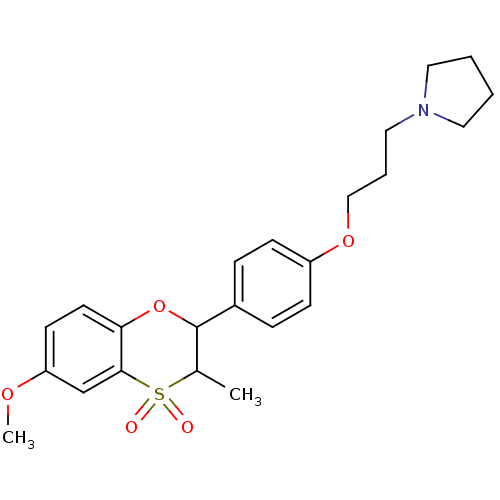 ((+/-)-1-{3-[4-(6-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.422 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MMP-2 by fluorometric assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296172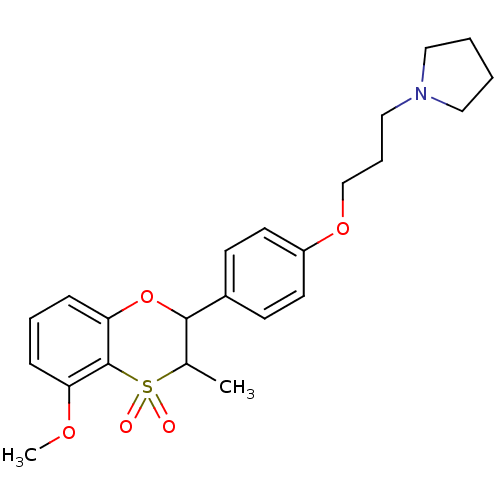 ((+/-)-1-{3-[4-(5-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296188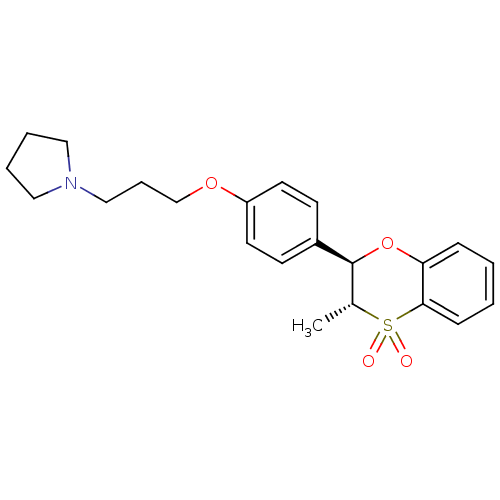 (CHEMBL558248 | trans-1-{3-[4-(3-Methyl-4,4-dioxo-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296181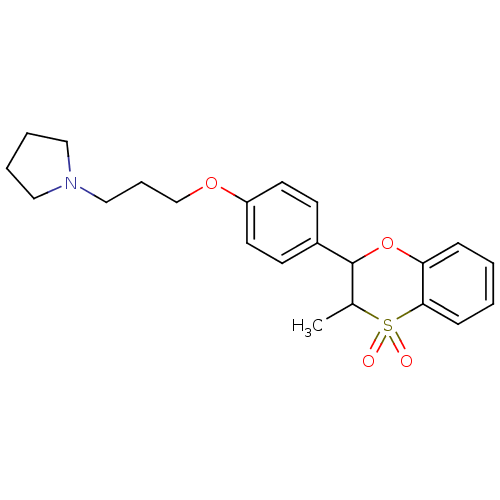 ((+/-)-1-{3-[4-(3-Methyl-4,4-dioxo-3,4-dihydro-2H-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296180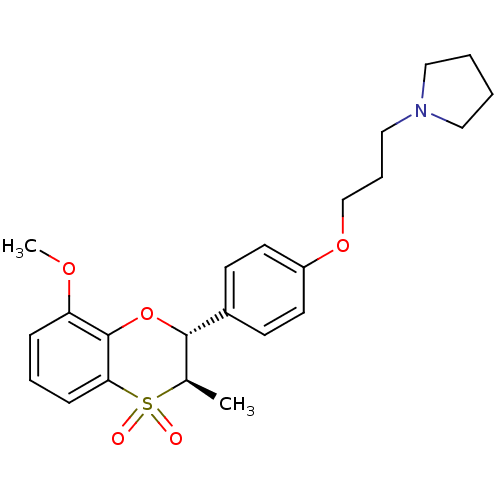 (1-{3-[4-((2R,3R)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.607 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262794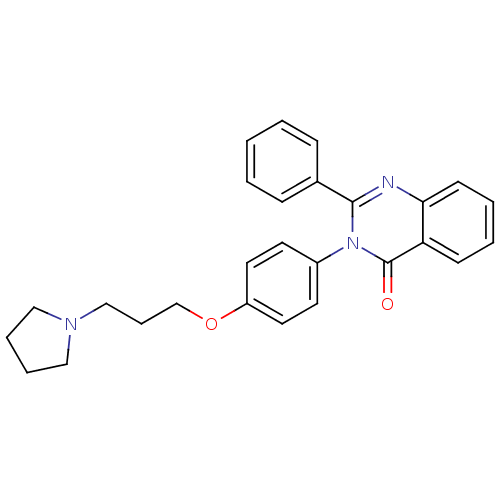 (2-Phenyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM222178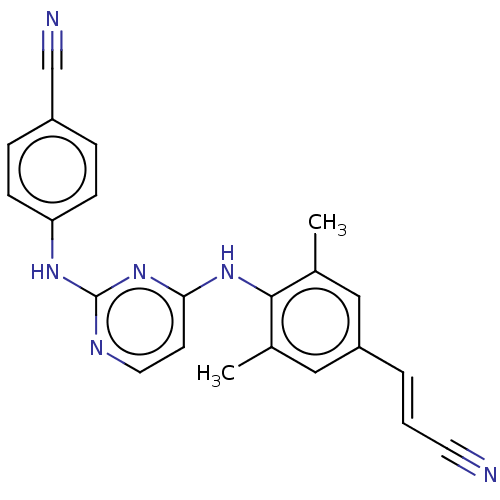 (Rilpivirine) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase p66/p51 Y181C mutant expressed in Escherichia coli BL21 (DE3) pLysS cells preincubated followed ... | Bioorg Med Chem Lett 29: 2182-2188 (2019) Article DOI: 10.1016/j.bmcl.2019.06.047 BindingDB Entry DOI: 10.7270/Q2BR8WKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262792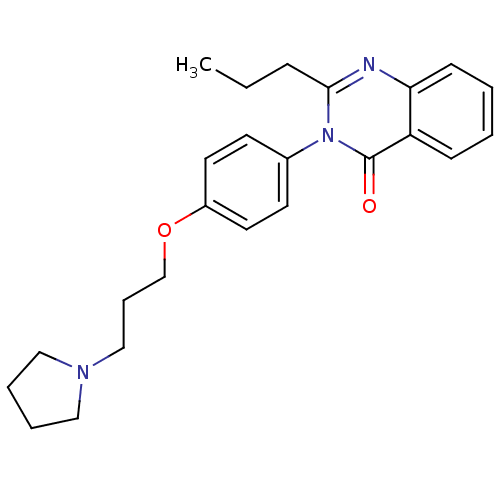 (2-Propyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262747 (2-Methyl-3-[4-({3-[(3S)-3-methyl-1-piperidinyl]pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262793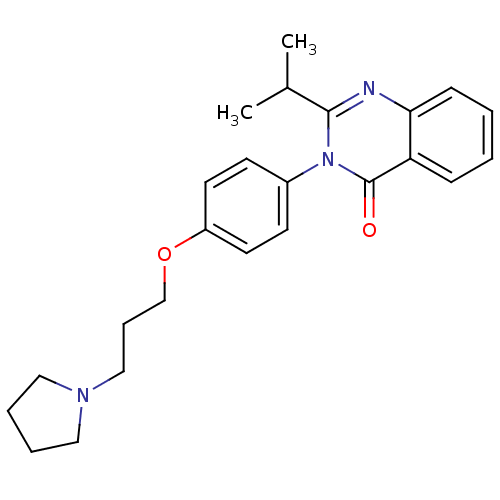 (2-Isopropyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296177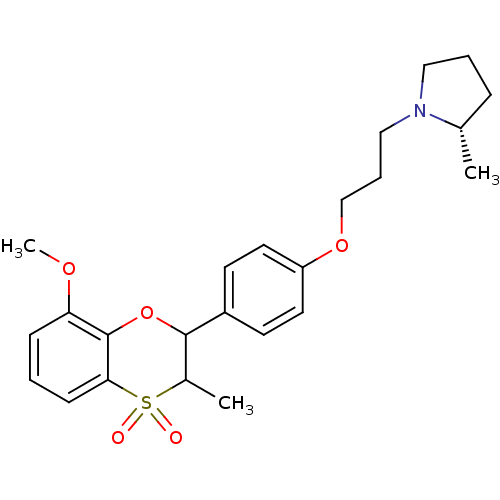 ((+/-)-(S)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296189 (1-{3-[4-(3-Methyl-4,4-dioxo-4H-4lambda*6*-benzo[1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262937 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262795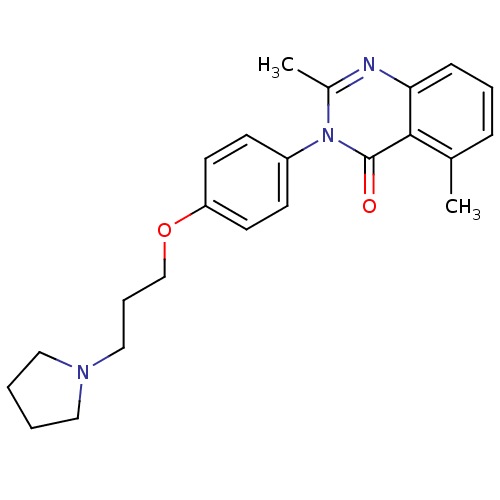 (2,5-Dimethyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262838 (6-Methoxy-2-methyl-3-(4-{[3-(1-pyrrolidinyl)propyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM252685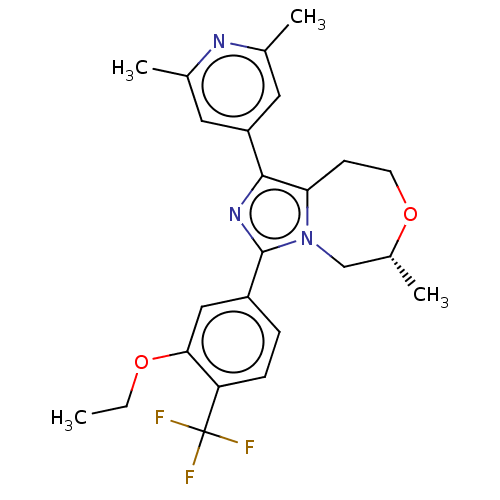 (US9458176, 82) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | 25 |
Eisai R&D Management Co., Ltd. US Patent | Assay Description The frozen cell membrane fraction prepared as described above was thawed before use, and the resultant was diluted with a buffer for a binding assay ... | US Patent US9458176 (2016) BindingDB Entry DOI: 10.7270/Q2PK0F3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Sus scrofa) | BDBM50395735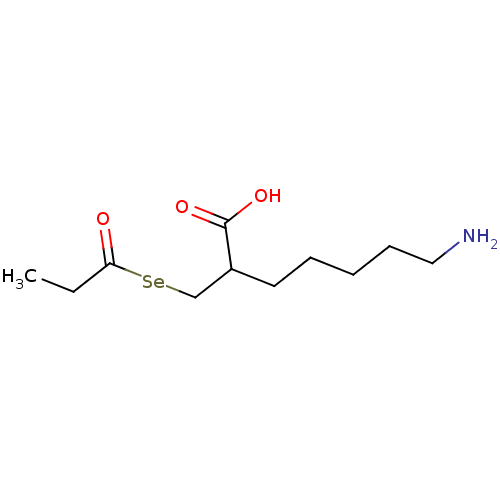 (CHEMBL2164459) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic carboxypeptidase B using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins b... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50395735 (CHEMBL2164459) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human activated TAFI using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by spectrophot... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Sus scrofa) | BDBM50395737 (CHEMBL2164457) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic carboxypeptidase B using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins b... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262835 (2,7-Dimethyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262837 (5-Methoxy-2-methyl-3-(4-{[3-(1-pyrrolidinyl)propyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262936 (5-Chloro-2-methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262887 (8-Fluoro-2-methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 883 total ) | Next | Last >> |