

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM86636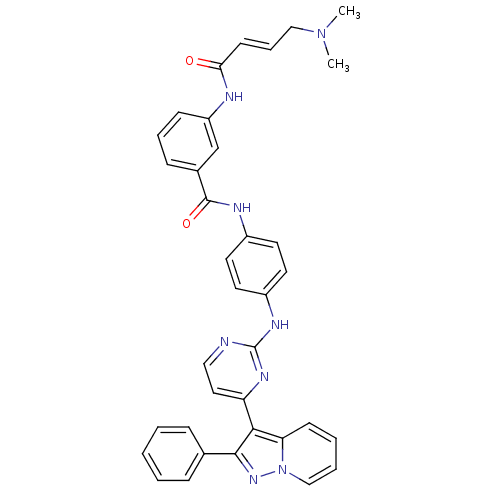 (JNK-IN-11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM86636 (JNK-IN-11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM86635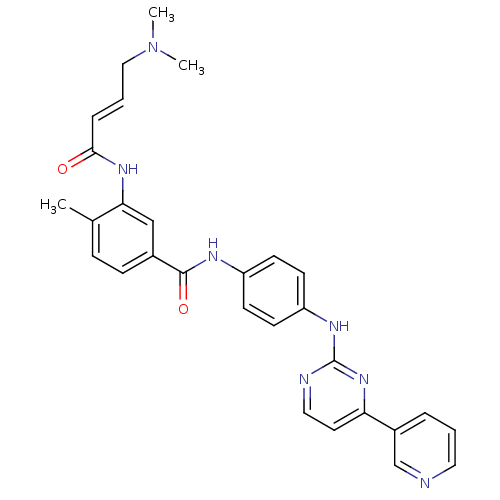 (JNK-IN-10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM86634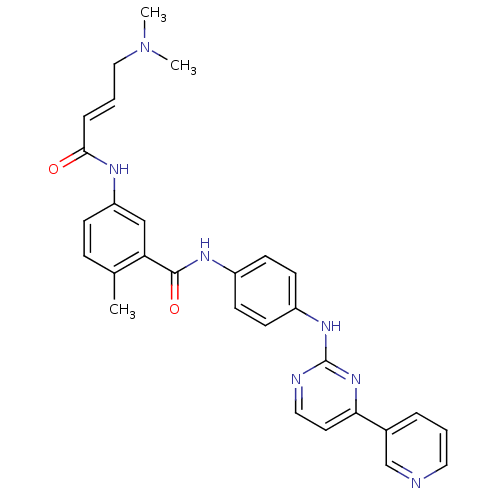 (JNK-IN-9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM86632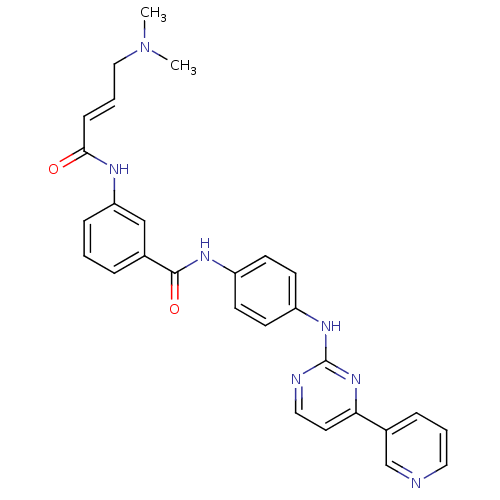 (JNK-IN-7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM86630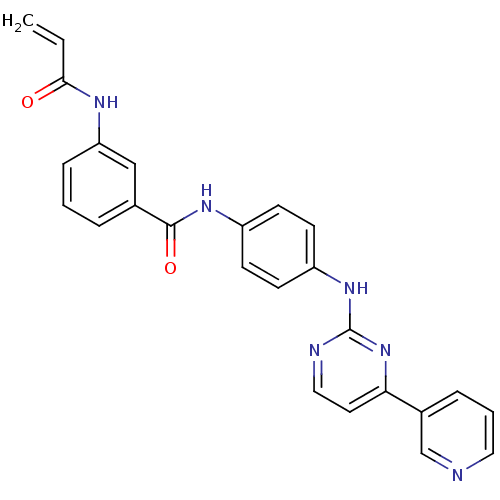 (JNK-IN-5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM86633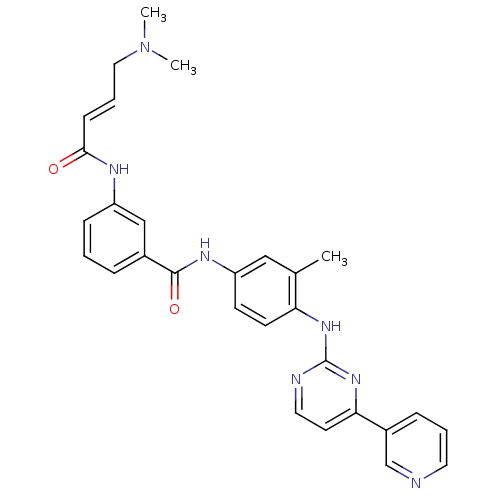 (JNK-IN-8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM86636 (JNK-IN-11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.34 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM86632 (JNK-IN-7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM86630 (JNK-IN-5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM86632 (JNK-IN-7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM86630 (JNK-IN-5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.11 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM86633 (JNK-IN-8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.67 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50335377 (CHEMBL1651534 | N-Pyridazin-3-yl-4-(3-{[5-(trifluo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of His-tagged human FAAH N-terminal transmembrane-deleted truncated form expressed in Escherichia coli preincubated for 60 mins before ole... | ACS Med Chem Lett 2: 91-96 (2011) Article DOI: 10.1021/ml100190t BindingDB Entry DOI: 10.7270/Q25X29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50335377 (CHEMBL1651534 | N-Pyridazin-3-yl-4-(3-{[5-(trifluo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of His-tagged rat FAAH N-terminal transmembrane-deleted truncated form expressed in Escherichia coli preincubated for 60 mins... | ACS Med Chem Lett 2: 91-96 (2011) Article DOI: 10.1021/ml100190t BindingDB Entry DOI: 10.7270/Q25X29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM86637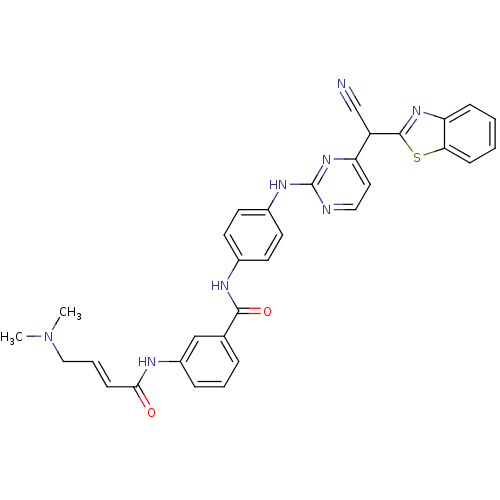 (JNK-IN-12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM86637 (JNK-IN-12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM86637 (JNK-IN-12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26740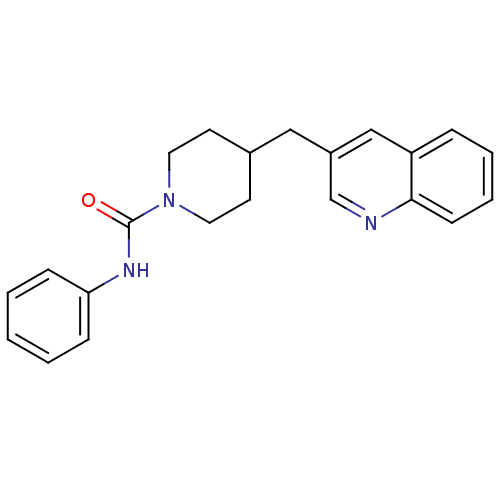 (N-phenyl-4-(quinolin-3-ylmethyl)piperidine-1-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16.2 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description FAAH activity was measured by following the production of ammonia generated from the hydrolysis of oleamide by FAAH. GDH catalyzes the condensation o... | Biochemistry 46: 13019-30 (2007) Article DOI: 10.1021/bi701378g BindingDB Entry DOI: 10.7270/Q2VT1QCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM86633 (JNK-IN-8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26741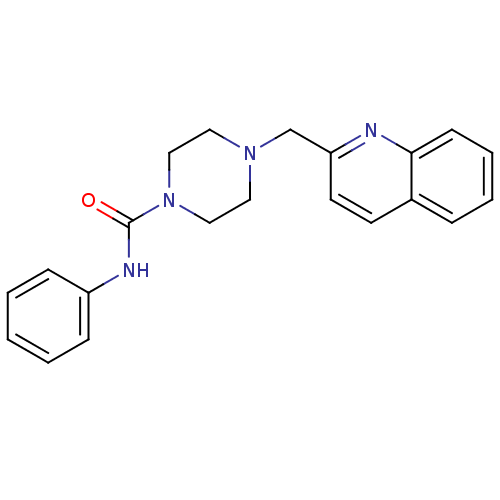 (N-phenyl-4-(quinolin-2-ylmethyl)piperazine-1-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description FAAH activity was measured by following the production of ammonia generated from the hydrolysis of oleamide by FAAH. GDH catalyzes the condensation o... | Biochemistry 46: 13019-30 (2007) Article DOI: 10.1021/bi701378g BindingDB Entry DOI: 10.7270/Q2VT1QCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26740 (N-phenyl-4-(quinolin-3-ylmethyl)piperidine-1-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51.9 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description FAAH activity was measured by following the production of ammonia generated from the hydrolysis of oleamide by FAAH. GDH catalyzes the condensation o... | Biochemistry 46: 13019-30 (2007) Article DOI: 10.1021/bi701378g BindingDB Entry DOI: 10.7270/Q2VT1QCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50169959 (2-(benzo[d]thiazol-2(3H)-ylidene)-2-(2-(2-(pyridin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26739 (3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description FAAH activity was measured by following the production of ammonia generated from the hydrolysis of oleamide by FAAH. GDH catalyzes the condensation o... | Biochemistry 46: 13019-30 (2007) Article DOI: 10.1021/bi701378g BindingDB Entry DOI: 10.7270/Q2VT1QCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM16018 (14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM86631 (JNK-IN-6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50169959 (2-(benzo[d]thiazol-2(3H)-ylidene)-2-(2-(2-(pyridin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM16018 (14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM86638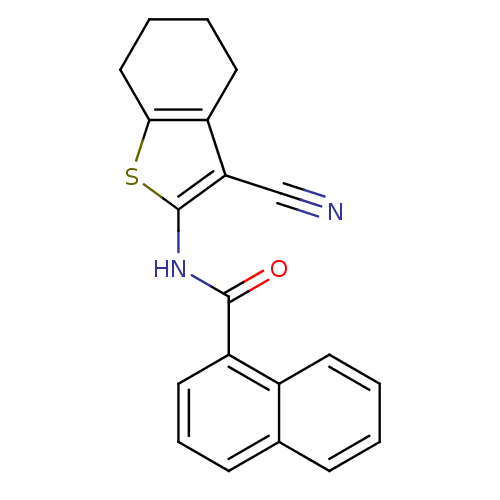 | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23120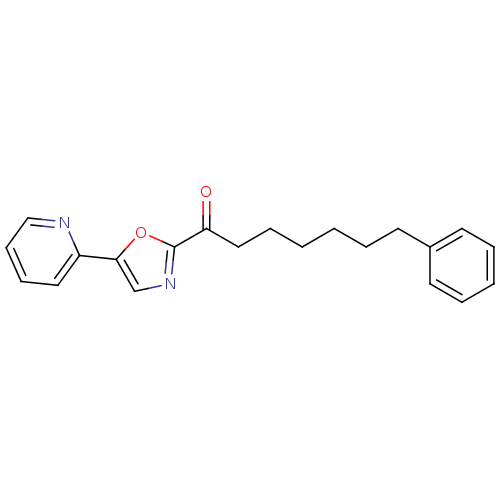 (7-phenyl-1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]hepta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 208 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description FAAH activity was measured by following the production of ammonia generated from the hydrolysis of oleamide by FAAH. GDH catalyzes the condensation o... | Biochemistry 46: 13019-30 (2007) Article DOI: 10.1021/bi701378g BindingDB Entry DOI: 10.7270/Q2VT1QCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50169959 (2-(benzo[d]thiazol-2(3H)-ylidene)-2-(2-(2-(pyridin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23120 (7-phenyl-1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]hepta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description FAAH activity was measured by following the production of ammonia generated from the hydrolysis of oleamide by FAAH. GDH catalyzes the condensation o... | Biochemistry 46: 13019-30 (2007) Article DOI: 10.1021/bi701378g BindingDB Entry DOI: 10.7270/Q2VT1QCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26740 (N-phenyl-4-(quinolin-3-ylmethyl)piperidine-1-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 248 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description FAAH activity was measured by following the production of ammonia generated from the hydrolysis of oleamide by FAAH. GDH catalyzes the condensation o... | Biochemistry 46: 13019-30 (2007) Article DOI: 10.1021/bi701378g BindingDB Entry DOI: 10.7270/Q2VT1QCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23120 (7-phenyl-1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]hepta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 252 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description FAAH activity was measured by following the production of ammonia generated from the hydrolysis of oleamide by FAAH. GDH catalyzes the condensation o... | Biochemistry 46: 13019-30 (2007) Article DOI: 10.1021/bi701378g BindingDB Entry DOI: 10.7270/Q2VT1QCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23120 (7-phenyl-1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]hepta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 297 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description FAAH activity was measured by following the production of ammonia generated from the hydrolysis of oleamide by FAAH. GDH catalyzes the condensation o... | Biochemistry 46: 13019-30 (2007) Article DOI: 10.1021/bi701378g BindingDB Entry DOI: 10.7270/Q2VT1QCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26741 (N-phenyl-4-(quinolin-2-ylmethyl)piperazine-1-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 473 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description FAAH activity was measured by following the production of ammonia generated from the hydrolysis of oleamide by FAAH. GDH catalyzes the condensation o... | Biochemistry 46: 13019-30 (2007) Article DOI: 10.1021/bi701378g BindingDB Entry DOI: 10.7270/Q2VT1QCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26739 (3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 572 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description FAAH activity was measured by following the production of ammonia generated from the hydrolysis of oleamide by FAAH. GDH catalyzes the condensation o... | Biochemistry 46: 13019-30 (2007) Article DOI: 10.1021/bi701378g BindingDB Entry DOI: 10.7270/Q2VT1QCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26740 (N-phenyl-4-(quinolin-3-ylmethyl)piperidine-1-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 595 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description FAAH activity was measured by following the production of ammonia generated from the hydrolysis of oleamide by FAAH. GDH catalyzes the condensation o... | Biochemistry 46: 13019-30 (2007) Article DOI: 10.1021/bi701378g BindingDB Entry DOI: 10.7270/Q2VT1QCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26741 (N-phenyl-4-(quinolin-2-ylmethyl)piperazine-1-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 687 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description FAAH activity was measured by following the production of ammonia generated from the hydrolysis of oleamide by FAAH. GDH catalyzes the condensation o... | Biochemistry 46: 13019-30 (2007) Article DOI: 10.1021/bi701378g BindingDB Entry DOI: 10.7270/Q2VT1QCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM86627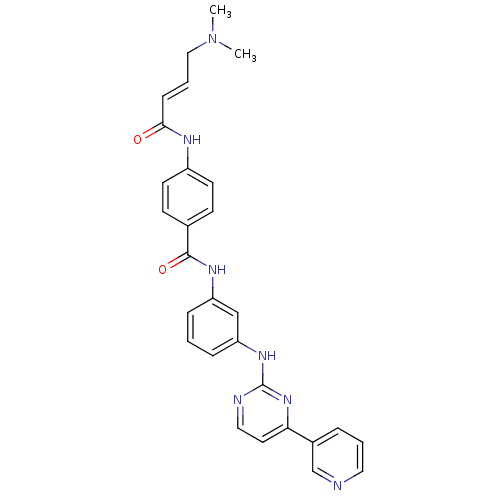 (JNK-IN-2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 709 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50335380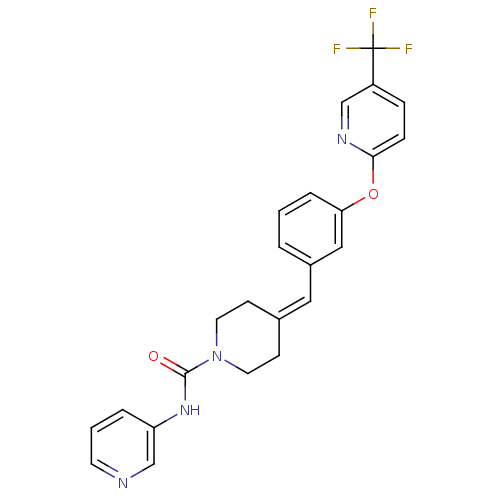 (CHEMBL1651525 | N-Pyridin-3-yl-4-(3-{[5-(trifluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 uisng testosterone substrate | ACS Med Chem Lett 2: 91-96 (2011) Article DOI: 10.1021/ml100190t BindingDB Entry DOI: 10.7270/Q25X29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM86627 (JNK-IN-2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 809 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26739 (3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 856 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description FAAH activity was measured by following the production of ammonia generated from the hydrolysis of oleamide by FAAH. GDH catalyzes the condensation o... | Biochemistry 46: 13019-30 (2007) Article DOI: 10.1021/bi701378g BindingDB Entry DOI: 10.7270/Q2VT1QCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26739 (3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 986 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description FAAH activity was measured by following the production of ammonia generated from the hydrolysis of oleamide by FAAH. GDH catalyzes the condensation o... | Biochemistry 46: 13019-30 (2007) Article DOI: 10.1021/bi701378g BindingDB Entry DOI: 10.7270/Q2VT1QCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26741 (N-phenyl-4-(quinolin-2-ylmethyl)piperazine-1-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 991 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description FAAH activity was measured by following the production of ammonia generated from the hydrolysis of oleamide by FAAH. GDH catalyzes the condensation o... | Biochemistry 46: 13019-30 (2007) Article DOI: 10.1021/bi701378g BindingDB Entry DOI: 10.7270/Q2VT1QCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM86627 (JNK-IN-2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50335380 (CHEMBL1651525 | N-Pyridin-3-yl-4-(3-{[5-(trifluoro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | ACS Med Chem Lett 2: 91-96 (2011) Article DOI: 10.1021/ml100190t BindingDB Entry DOI: 10.7270/Q25X29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50335379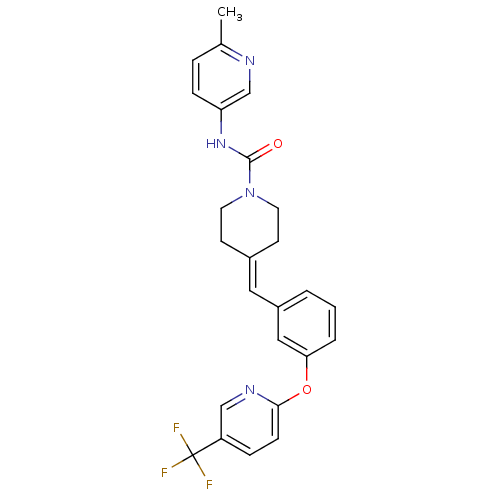 (CHEMBL1651532 | N-(6-Methylpyridin-3-yl)-4-(3-{[5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | ACS Med Chem Lett 2: 91-96 (2011) Article DOI: 10.1021/ml100190t BindingDB Entry DOI: 10.7270/Q25X29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM86629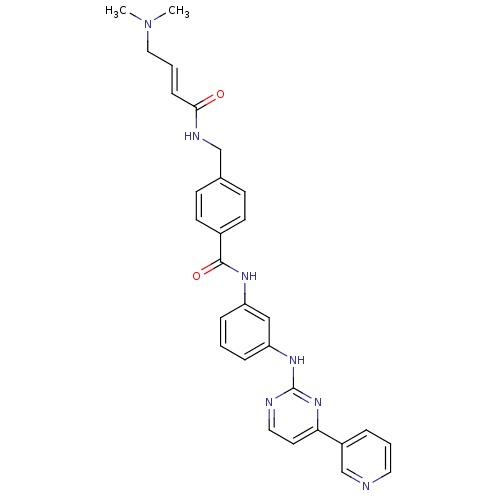 (JNK-IN-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. | Chem Biol 19: 140-54 (2012) Article DOI: 10.1016/j.chembiol.2011.11.010 BindingDB Entry DOI: 10.7270/Q2K35S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50335379 (CHEMBL1651532 | N-(6-Methylpyridin-3-yl)-4-(3-{[5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 uisng testosterone substrate | ACS Med Chem Lett 2: 91-96 (2011) Article DOI: 10.1021/ml100190t BindingDB Entry DOI: 10.7270/Q25X29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 67 total ) | Next | Last >> |