

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50346950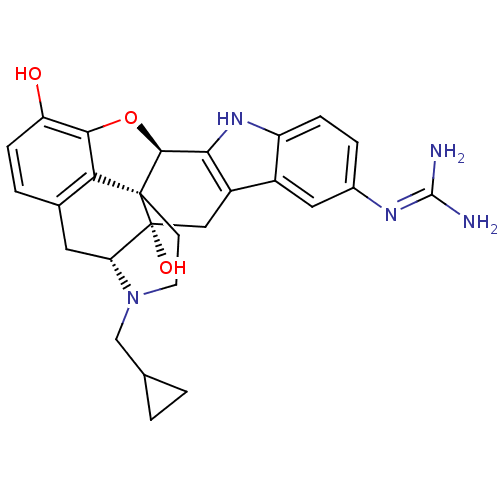 (CHEMBL485832) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Mutant factor of the compound against mu-[K303E] with Opioid receptor kappa 1 for binding affinity | J Med Chem 41: 4911-4 (1999) Article DOI: 10.1021/jm9805182 BindingDB Entry DOI: 10.7270/Q2K9387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50346950 (CHEMBL485832) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Effect on binding affinity of mutational exchange of Glu297 in the Opioid receptor kappa 1 in transiently expressed rat cos-7 cells activity expresse... | J Med Chem 41: 4911-4 (1999) Article DOI: 10.1021/jm9805182 BindingDB Entry DOI: 10.7270/Q2K9387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Effect on binding affinity of mutational exchange of Glu297 in the Opioid receptor kappa 1 in transiently expressed rat cos-7 cells activity expresse... | J Med Chem 41: 4911-4 (1999) Article DOI: 10.1021/jm9805182 BindingDB Entry DOI: 10.7270/Q2K9387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50087456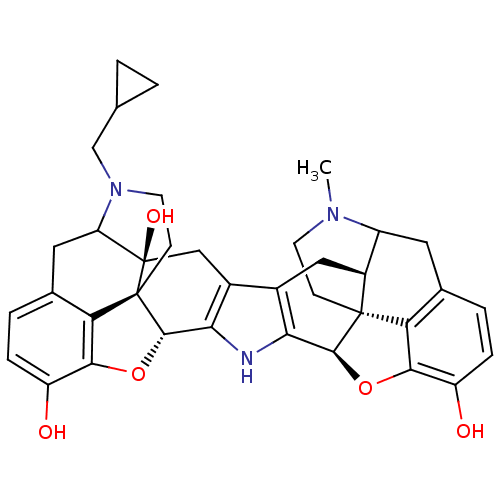 (17-Methyl-17'-cyclopropylmethyl-3'-hydroxy-6,6',7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against K303E Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50068379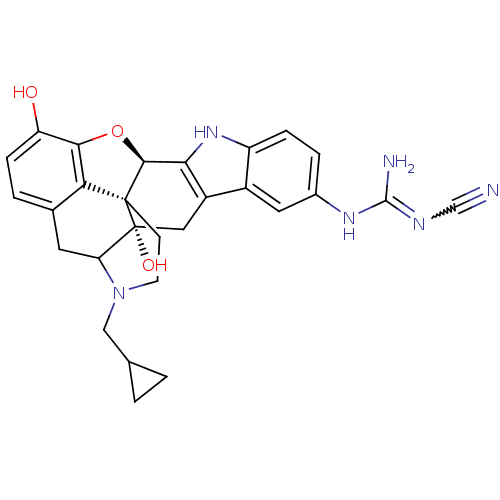 (5'-[N-(N'-Cyano)guanidinyl]-17-cyclopropylmethyl-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Effect on binding affinity of mutational exchange of Glu297 in the Opioid receptor kappa 1 in transiently expressed rat cos-7 cells activity expresse... | J Med Chem 41: 4911-4 (1999) Article DOI: 10.1021/jm9805182 BindingDB Entry DOI: 10.7270/Q2K9387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50001714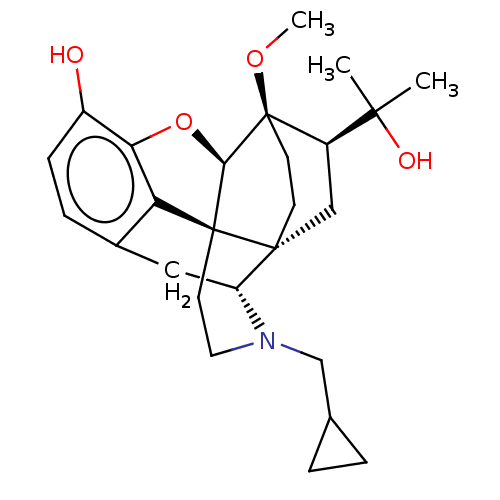 (2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50001714 (2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50068379 (5'-[N-(N'-Cyano)guanidinyl]-17-cyclopropylmethyl-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Mutant factor against mu-[K303E] with Opioid receptor kappa 1 for binding affinity | J Med Chem 41: 4911-4 (1999) Article DOI: 10.1021/jm9805182 BindingDB Entry DOI: 10.7270/Q2K9387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087456 (17-Methyl-17'-cyclopropylmethyl-3'-hydroxy-6,6',7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50001714 (2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against K303E Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50001714 (2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against E297K mutant Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50001714 (2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against E297A mutant Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087460 (17-cyclopropylmethyl-17'-cyclopropylmethyl-3'-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087458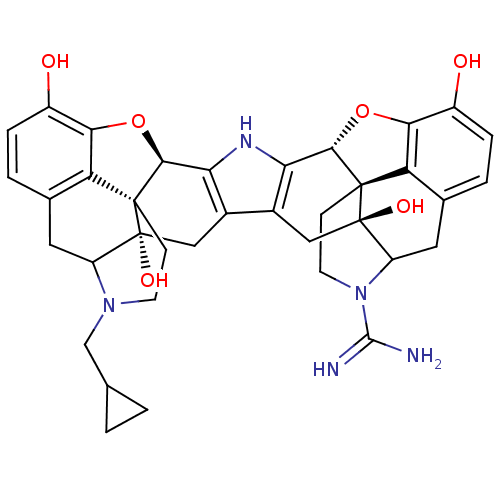 (17-Cyclopropylmethyl-17'-guanidinyl-6,6',7,7'-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor kappa 1r in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50366689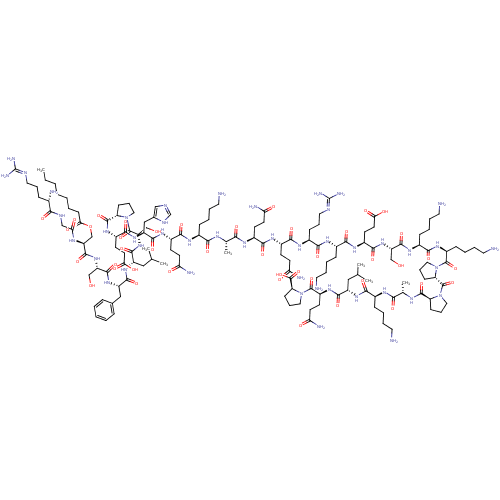 (GHRELIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Leipzig Curated by ChEMBL | Assay Description Displacement of [125I]-His-ghrelin from human ghrelin receptor expressed in COS7 cells incubated for 75 mins by scintillation counting based assay | J Med Chem 55: 7437-49 (2012) Article DOI: 10.1021/jm300414b BindingDB Entry DOI: 10.7270/Q26M37Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Mutant factor against mu-[K303E] with Opioid receptor kappa 1 for binding affinity | J Med Chem 41: 4911-4 (1999) Article DOI: 10.1021/jm9805182 BindingDB Entry DOI: 10.7270/Q2K9387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against K303E Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50068379 (5'-[N-(N'-Cyano)guanidinyl]-17-cyclopropylmethyl-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Mutant factor of the compound against kappa[E297K] with Opioid receptor kappa 1 for binding affinity. | J Med Chem 41: 4911-4 (1999) Article DOI: 10.1021/jm9805182 BindingDB Entry DOI: 10.7270/Q2K9387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50087460 (17-cyclopropylmethyl-17'-cyclopropylmethyl-3'-hydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against K303E Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50068379 (5'-[N-(N'-Cyano)guanidinyl]-17-cyclopropylmethyl-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Effect on binding affinity of mutational exchange of Lys303 in the Opioid receptor mu 1 in transiently expressed rat cos-7 cells activity expressed a... | J Med Chem 41: 4911-4 (1999) Article DOI: 10.1021/jm9805182 BindingDB Entry DOI: 10.7270/Q2K9387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50396915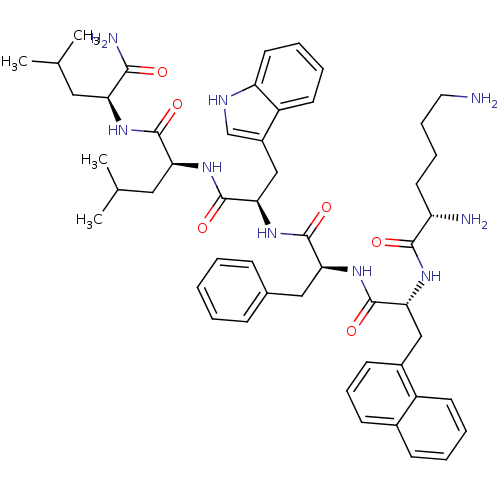 (CHEMBL2170781) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Leipzig Curated by ChEMBL | Assay Description Displacement of [125I]-His-ghrelin from human ghrelin receptor expressed in COS7 cells incubated for 75 mins by scintillation counting based assay | J Med Chem 55: 7437-49 (2012) Article DOI: 10.1021/jm300414b BindingDB Entry DOI: 10.7270/Q26M37Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50346950 (CHEMBL485832) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Effect on binding affinity of mutational exchange of Lys303 in the Opioid receptor mu 1 in transiently expressed rat cos-7 cells activity expressed a... | J Med Chem 41: 4911-4 (1999) Article DOI: 10.1021/jm9805182 BindingDB Entry DOI: 10.7270/Q2K9387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270276 (CHEMBL477121 | N-[1,4,8,11-Tetraazacyclotetradecan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at human wild type CXCR4 expressed in COS7 cells coexpressing G protein Gqi4myr assessed as inhibition of CXCL12-induced phosphat... | J Biol Chem 282: 27354-65 (2007) Article DOI: 10.1074/jbc.M704739200 BindingDB Entry DOI: 10.7270/Q26Q1X1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against E297A mutant Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against E297K mutant Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Mutant factor of the compound against kappa[E297K] with Opioid receptor kappa 1 for binding affinity. | J Med Chem 41: 4911-4 (1999) Article DOI: 10.1021/jm9805182 BindingDB Entry DOI: 10.7270/Q2K9387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50346950 (CHEMBL485832) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Mutant factor of the compound against kappa[E297K] with Opioid receptor kappa 1 for binding affinity. | J Med Chem 41: 4911-4 (1999) Article DOI: 10.1021/jm9805182 BindingDB Entry DOI: 10.7270/Q2K9387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087460 (17-cyclopropylmethyl-17'-cyclopropylmethyl-3'-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against E297A mutant Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50018790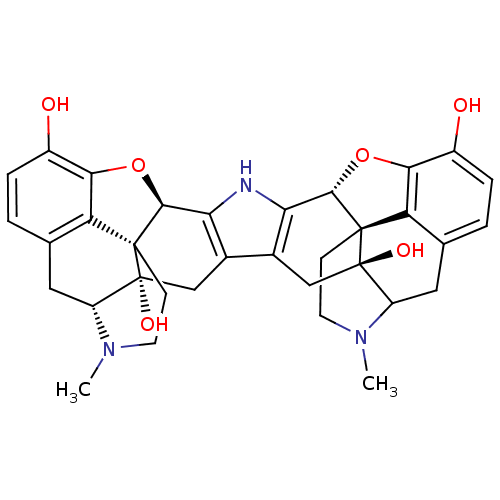 (17,17'-Dimethyl-6,6',7,7'-tetradehydro-4,5:4',5'-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 21.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50396901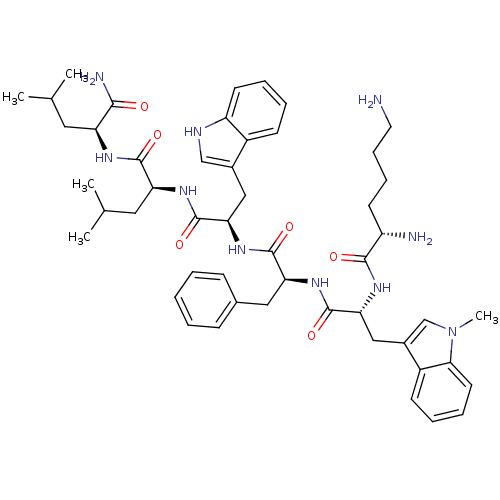 (CHEMBL2170701) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Leipzig Curated by ChEMBL | Assay Description Displacement of [125I]-His-ghrelin from human ghrelin receptor expressed in COS7 cells incubated for 75 mins by scintillation counting based assay | J Med Chem 55: 7437-49 (2012) Article DOI: 10.1021/jm300414b BindingDB Entry DOI: 10.7270/Q26M37Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087459 (17-cyclopropylmethyl-17'-acetyl-3'-hydroxy-6,6',7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 23.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087460 (17-cyclopropylmethyl-17'-cyclopropylmethyl-3'-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against E297K mutant Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50396896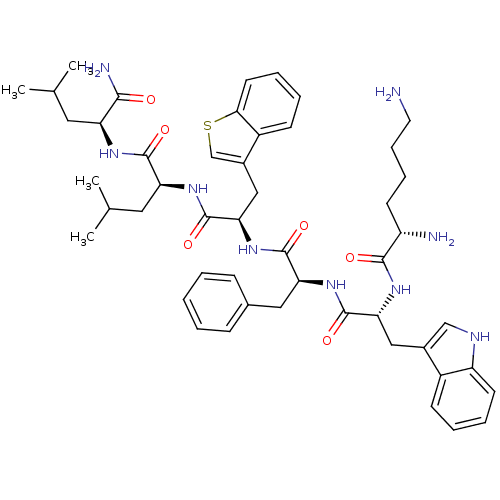 (CHEMBL2170693) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Leipzig Curated by ChEMBL | Assay Description Displacement of [125I]-His-ghrelin from human ghrelin receptor expressed in COS7 cells incubated for 75 mins by scintillation counting based assay | J Med Chem 55: 7437-49 (2012) Article DOI: 10.1021/jm300414b BindingDB Entry DOI: 10.7270/Q26M37Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50396895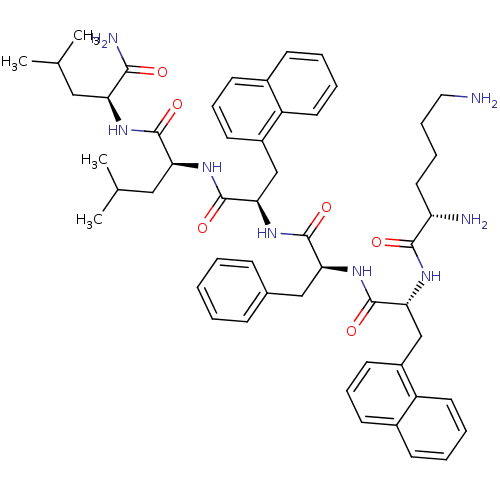 (CHEMBL2170694) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Leipzig Curated by ChEMBL | Assay Description Displacement of [125I]-His-ghrelin from human ghrelin receptor expressed in COS7 cells incubated for 75 mins by scintillation counting based assay | J Med Chem 55: 7437-49 (2012) Article DOI: 10.1021/jm300414b BindingDB Entry DOI: 10.7270/Q26M37Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087457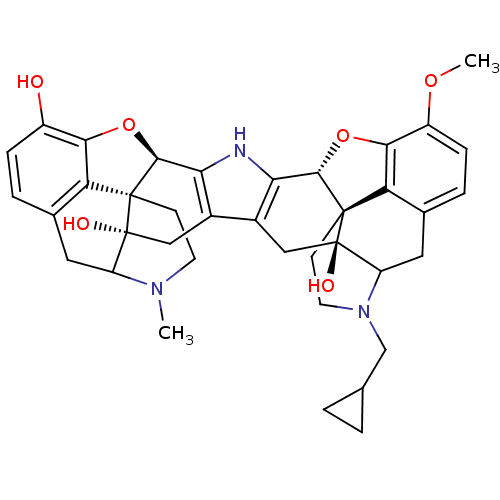 (17-Methyl-17'-cyclopropylmethyl-3'-methoxy-6,6',7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 36.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087456 (17-Methyl-17'-cyclopropylmethyl-3'-hydroxy-6,6',7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 39.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against E297A mutant Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087456 (17-Methyl-17'-cyclopropylmethyl-3'-hydroxy-6,6',7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 39.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against E297K mutant Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50087456 (17-Methyl-17'-cyclopropylmethyl-3'-hydroxy-6,6',7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50018790 (17,17'-Dimethyl-6,6',7,7'-tetradehydro-4,5:4',5'-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 41.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against K303E Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50087459 (17-cyclopropylmethyl-17'-acetyl-3'-hydroxy-6,6',7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against K303E Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50087457 (17-Methyl-17'-cyclopropylmethyl-3'-methoxy-6,6',7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against K303E Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Effect on binding affinity of mutational exchange of Lys303 in the Opioid receptor mu 1 in transiently expressed rat cos-7 cells activity expressed a... | J Med Chem 41: 4911-4 (1999) Article DOI: 10.1021/jm9805182 BindingDB Entry DOI: 10.7270/Q2K9387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50087459 (17-cyclopropylmethyl-17'-acetyl-3'-hydroxy-6,6',7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087459 (17-cyclopropylmethyl-17'-acetyl-3'-hydroxy-6,6',7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against E297A mutant Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087459 (17-cyclopropylmethyl-17'-acetyl-3'-hydroxy-6,6',7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against E297K mutant Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270276 (CHEMBL477121 | N-[1,4,8,11-Tetraazacyclotetradecan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [125I]12G5 antibody from human wild type CXCR4 expressed in COS7 cells | J Biol Chem 282: 27354-65 (2007) Article DOI: 10.1074/jbc.M704739200 BindingDB Entry DOI: 10.7270/Q26Q1X1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50087457 (17-Methyl-17'-cyclopropylmethyl-3'-methoxy-6,6',7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087458 (17-Cyclopropylmethyl-17'-guanidinyl-6,6',7,7'-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against E297K mutant Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 327 total ) | Next | Last >> |