

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380754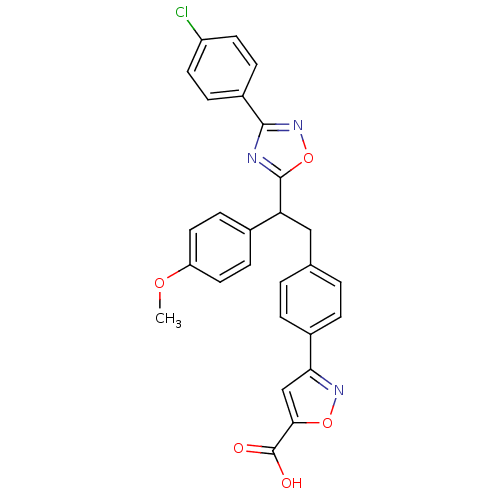 (CHEMBL2017853) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380753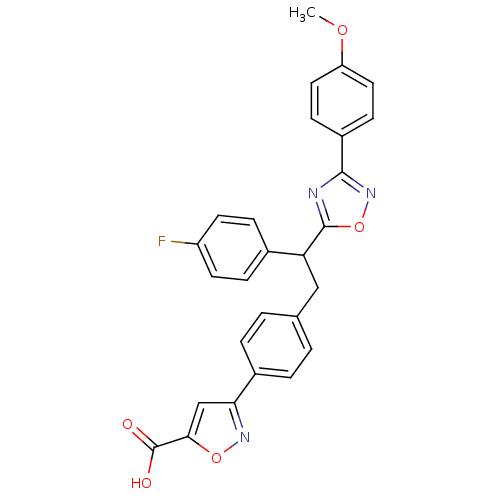 (CHEMBL2017852) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380756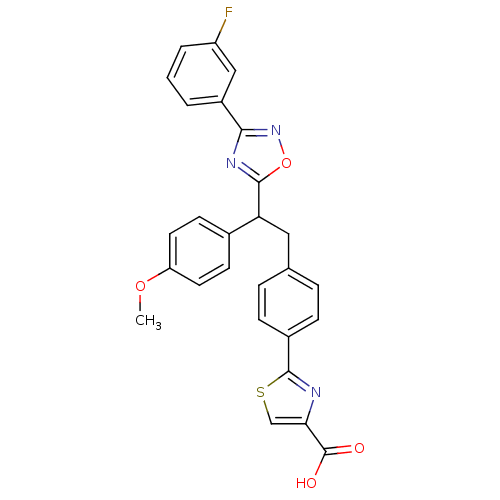 (CHEMBL2017855) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380755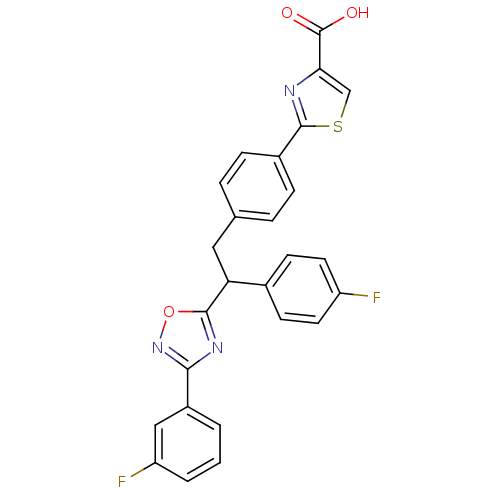 (CHEMBL2017854) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380751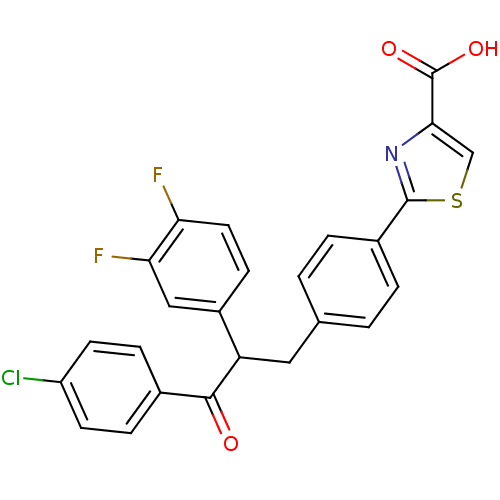 (CHEMBL2017850) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380749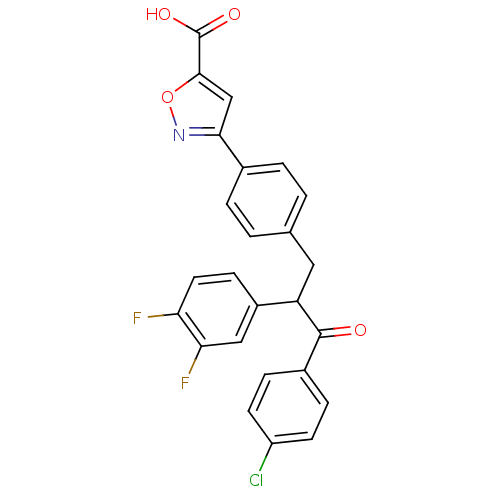 (CHEMBL2017848) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380738 (CHEMBL2017836) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380741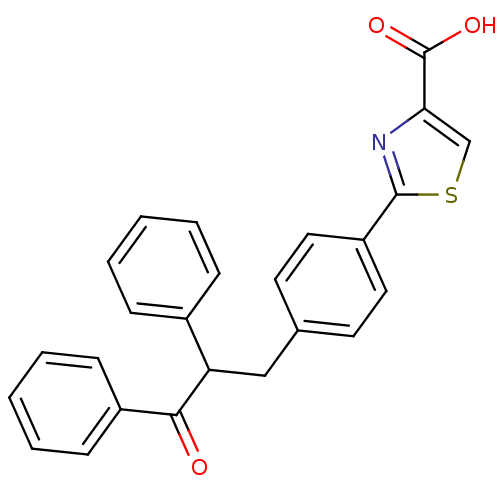 (CHEMBL2017840) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380748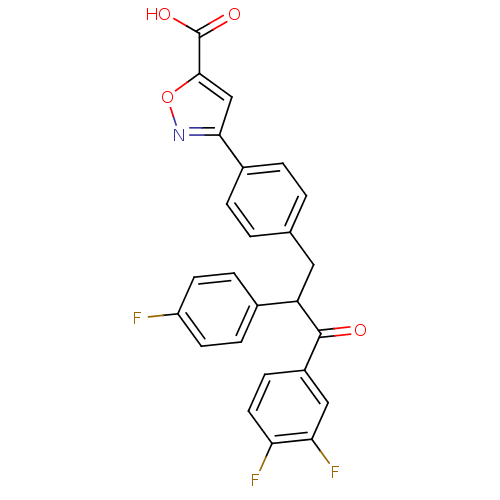 (CHEMBL2017847) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380747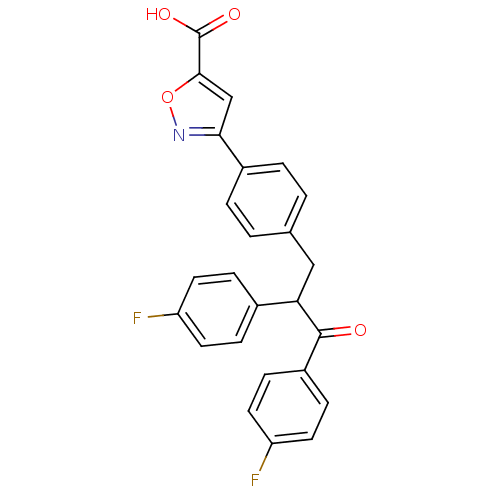 (CHEMBL2017846) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380750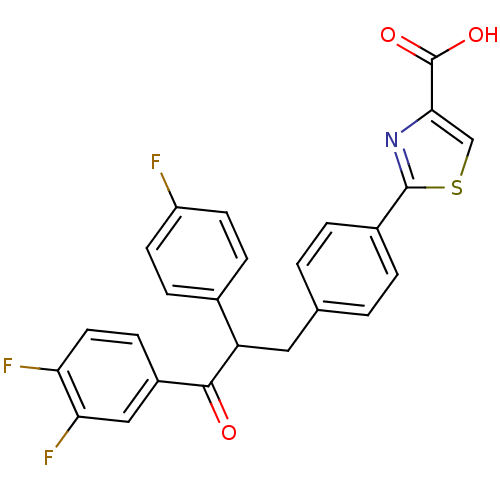 (CHEMBL2017849) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380746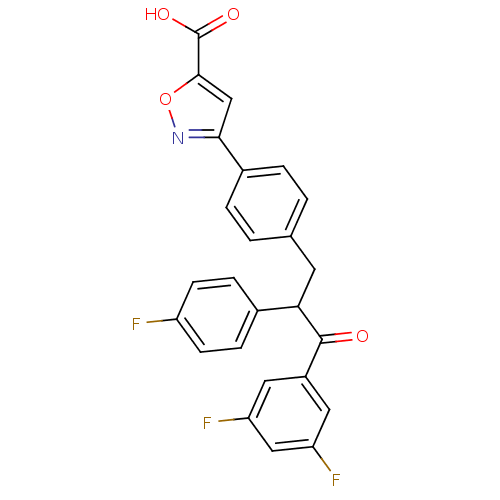 (CHEMBL2017845) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380742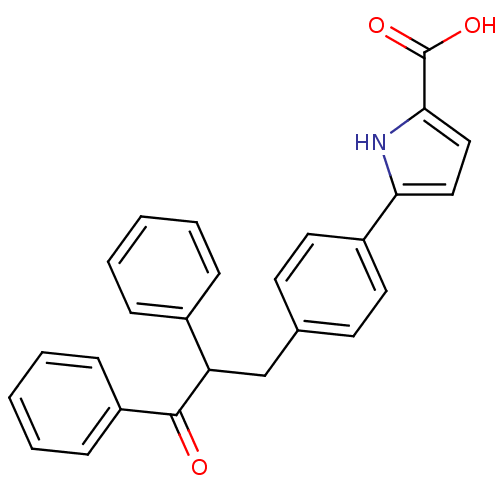 (CHEMBL2017841) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380752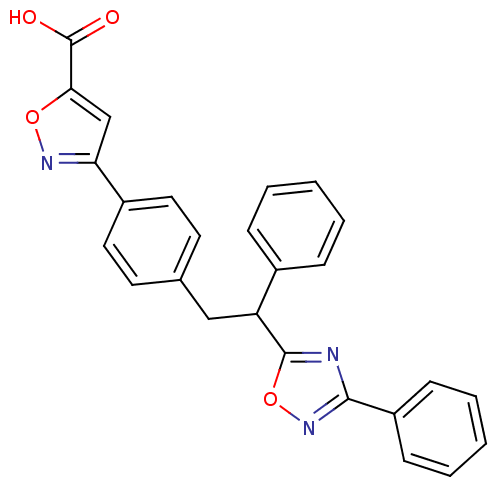 (CHEMBL2017851) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380744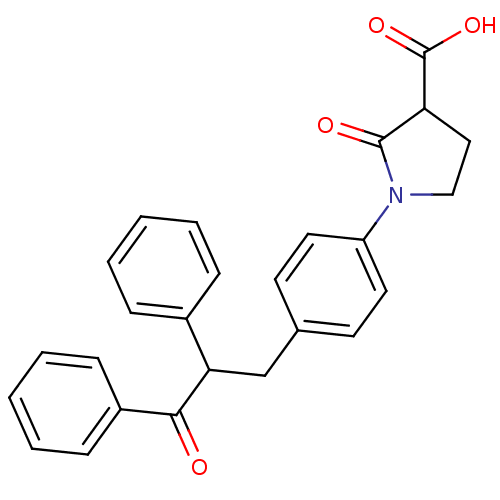 (CHEMBL2017843) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM13990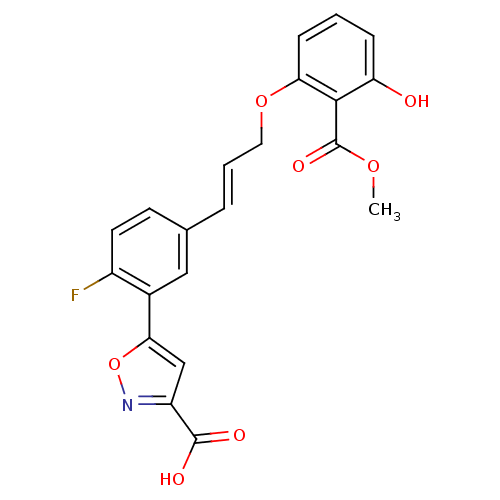 (5-{2-fluoro-5-[(1E)-3-[3-hydroxy-2-(methoxycarbony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380743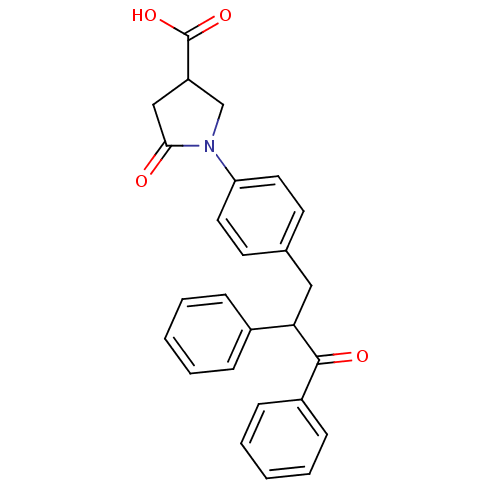 (CHEMBL2017842) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380740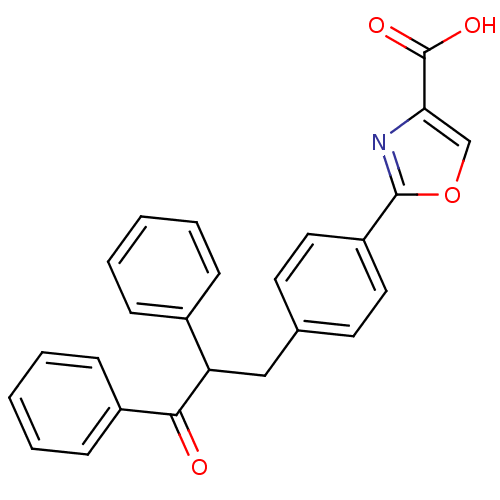 (CHEMBL2017839) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380737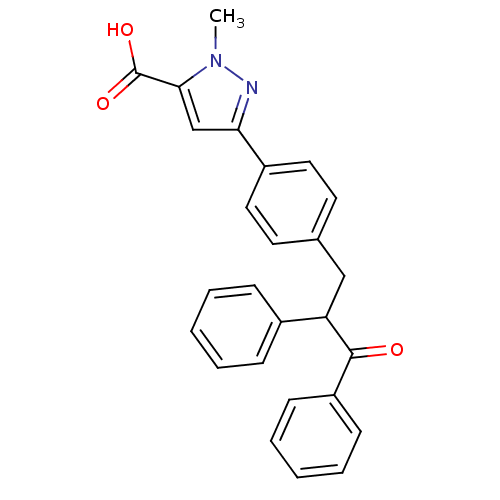 (CHEMBL2017838) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380739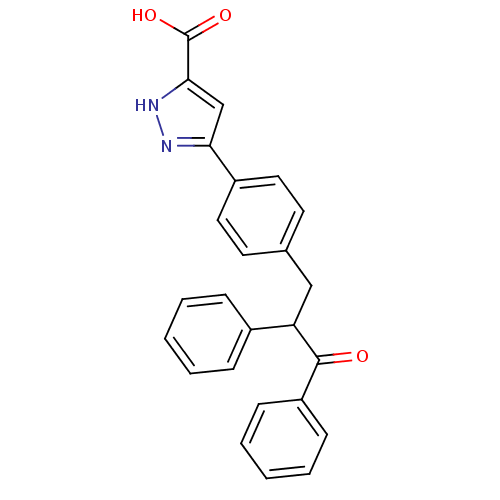 (CHEMBL2017837) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50380745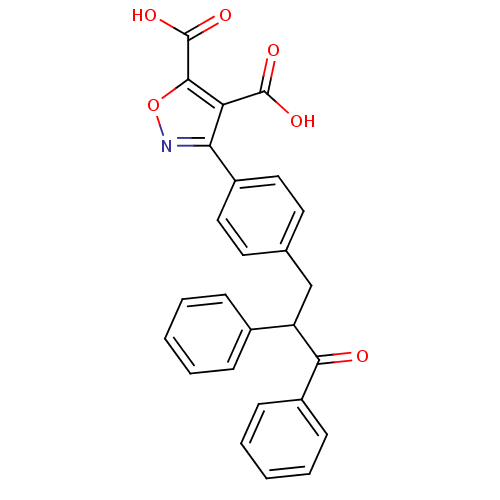 (CHEMBL2017844) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13990 (5-{2-fluoro-5-[(1E)-3-[3-hydroxy-2-(methoxycarbony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of TCPTP | Bioorg Med Chem Lett 22: 2843-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.070 BindingDB Entry DOI: 10.7270/Q2FB53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143373 (US9682953, 20.A-10 | US9682953, 20.A-9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143358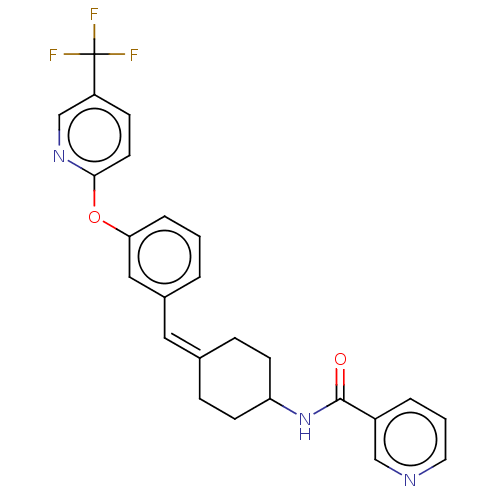 (US9682953, 20.A-2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143449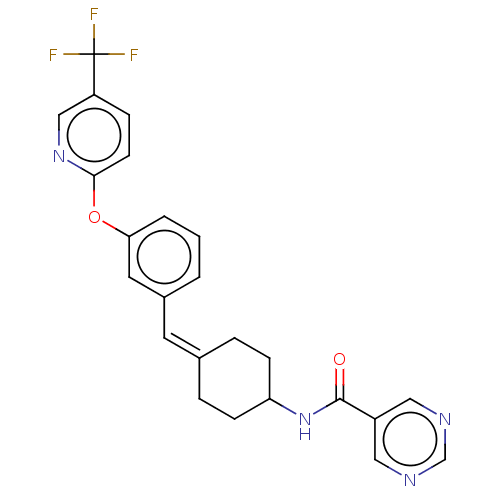 (US9682953, 20.A-19 | US9682953, 20.A-20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143291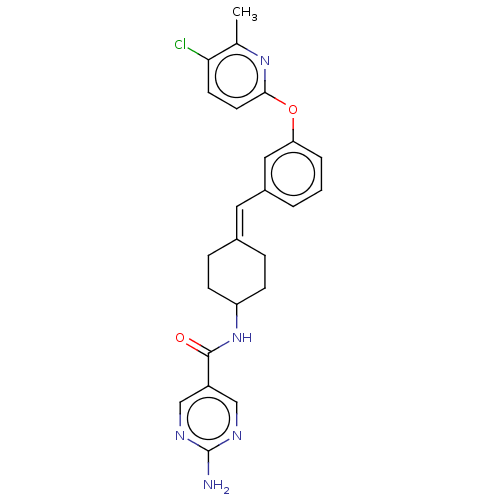 (US9682953, 2.E-27) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143361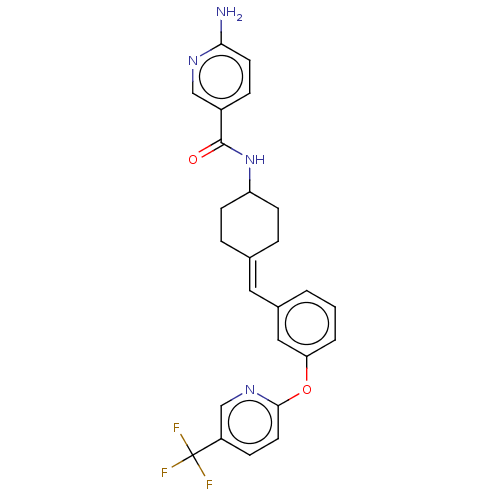 (US9682953, 20.A-4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143366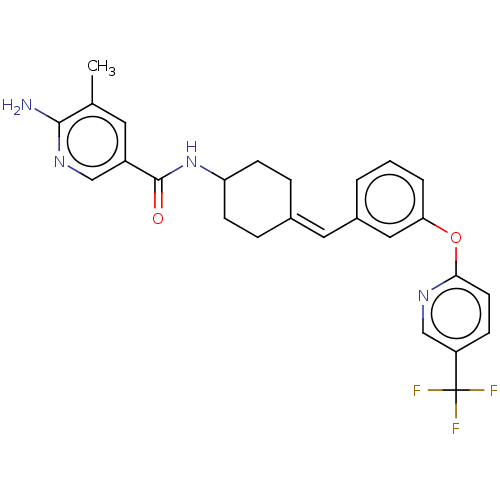 (US9682953, 20.A-6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143368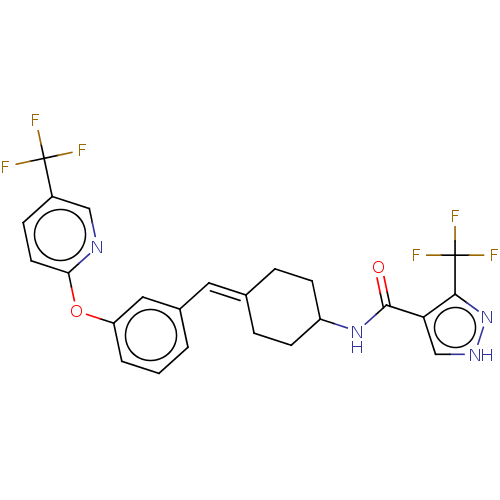 (US9682953, 20.A-8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143391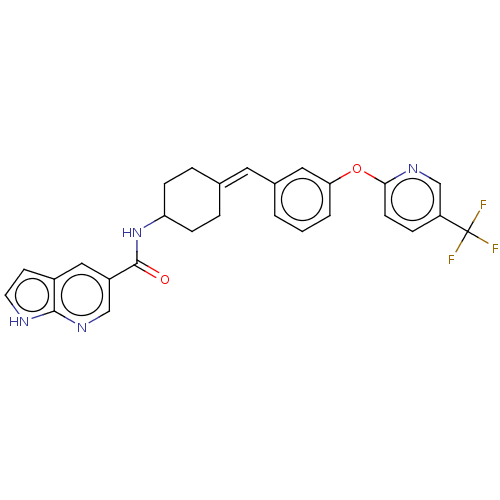 (US9682953, 20.A-14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143352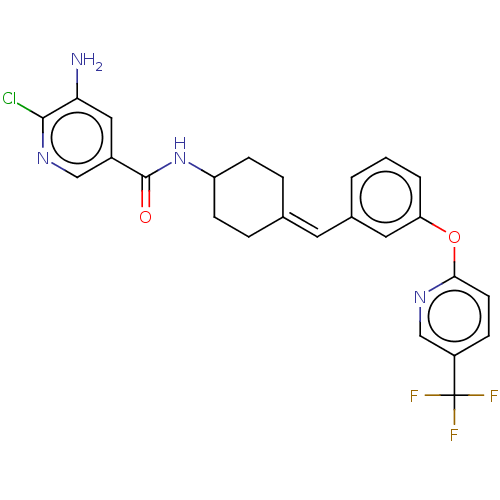 (US9682953, 10.1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143274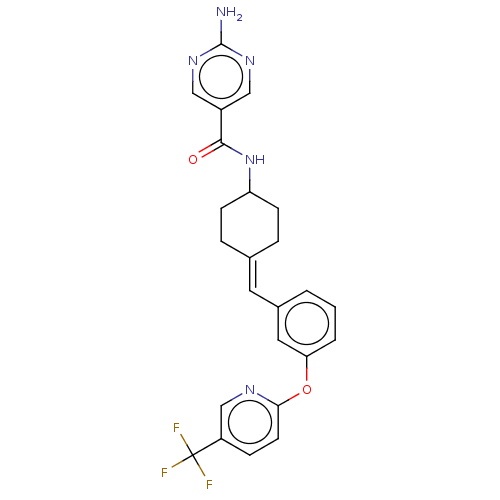 (US9682953, 2.C-6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of src kinase | J Med Chem 39: 2664-71 (1996) Article DOI: 10.1021/jm950588y BindingDB Entry DOI: 10.7270/Q25H7FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50250824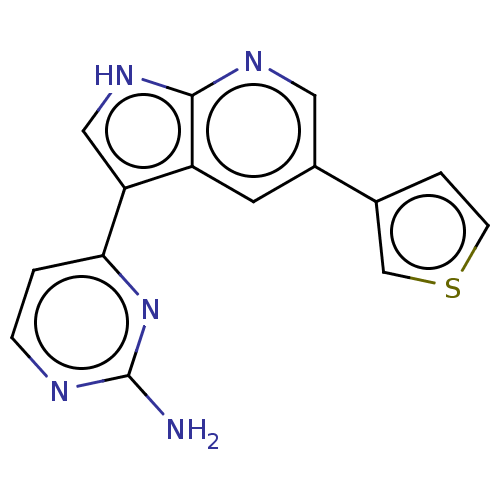 (CHEMBL4101278) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A (unknown origin) using histone H1 as substrate after 30 mins in presence of [33P]-gamma-ATP | J Med Chem 60: 9470-9489 (2017) Article DOI: 10.1021/acs.jmedchem.7b00663 BindingDB Entry DOI: 10.7270/Q2WH2SDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143456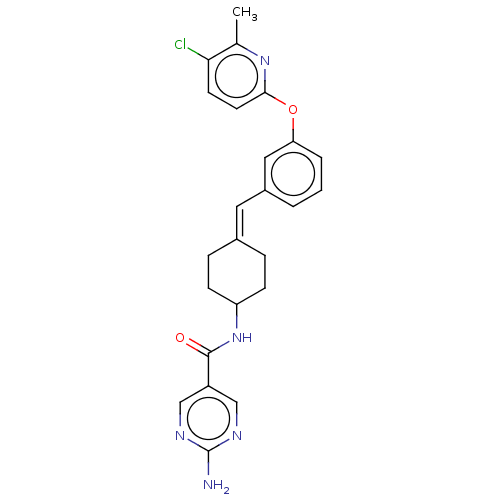 (US9682953, 20.A-21 | US9682953, 20.A-22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143375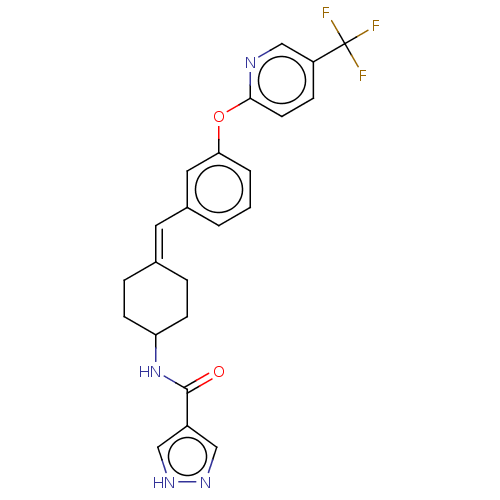 (US9682953, 20.A-11 | US9682953, 20.A-12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143284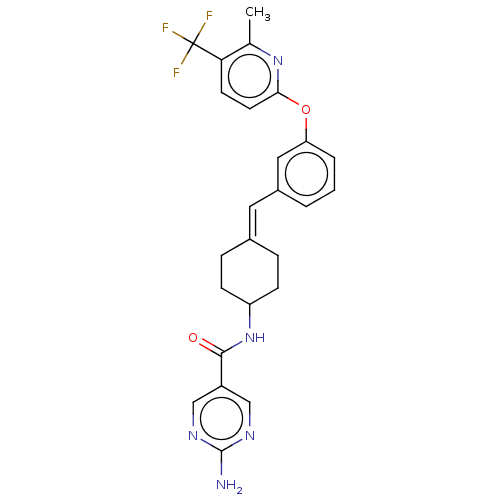 (US9682953, 2.E-22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143117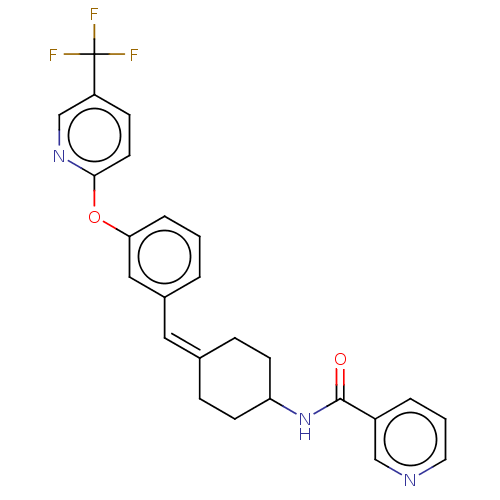 (US9682953, 2.1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143277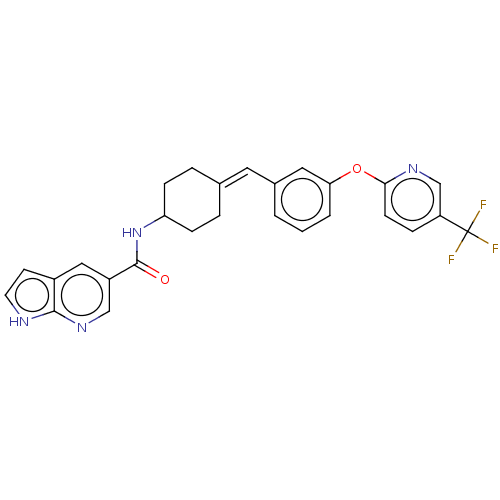 (US9682953, 2.D-5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143166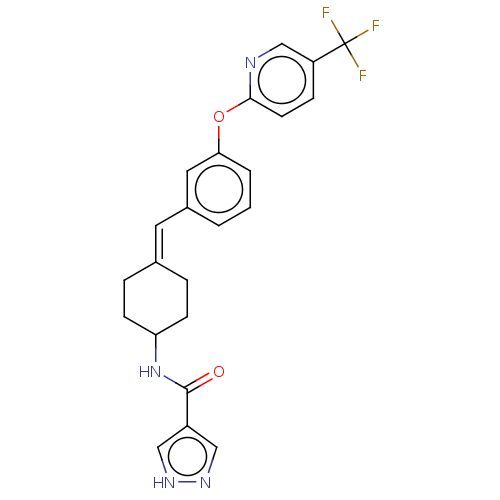 (US9682953, 2.A-9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143257 (US9682953, 2.B-4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143258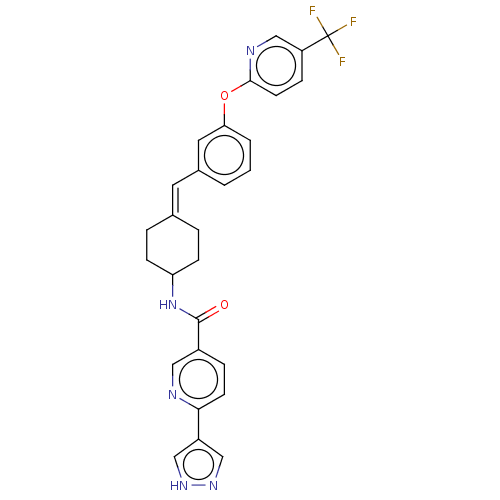 (US9682953, 2.B-5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143265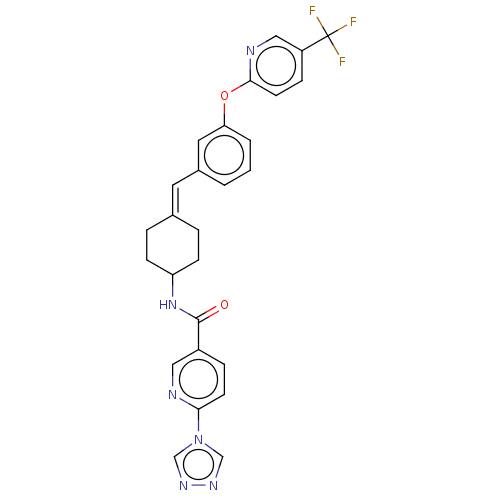 (US9682953, 2.B-13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143266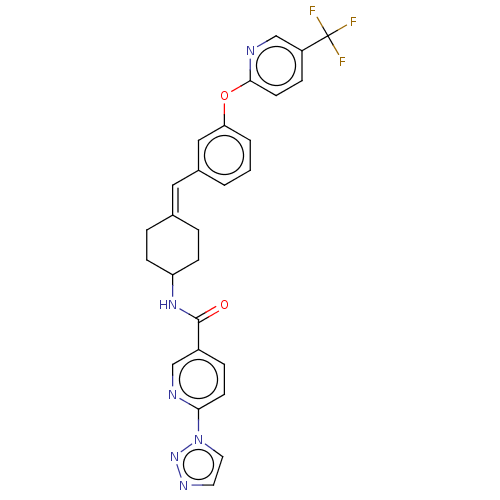 (US9682953, 2.B-14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143280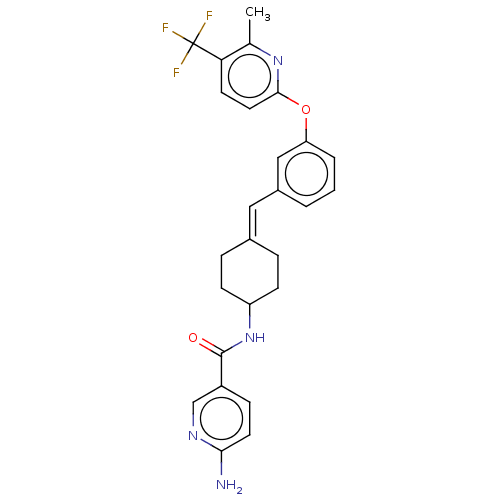 (US9682953, 2.E-17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143239 (US9682953, 2.A-29) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143283 (US9682953, 2.E-20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143267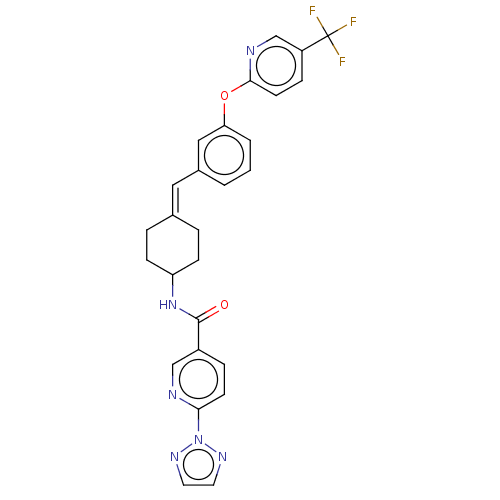 (US9682953, 2.B-15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143293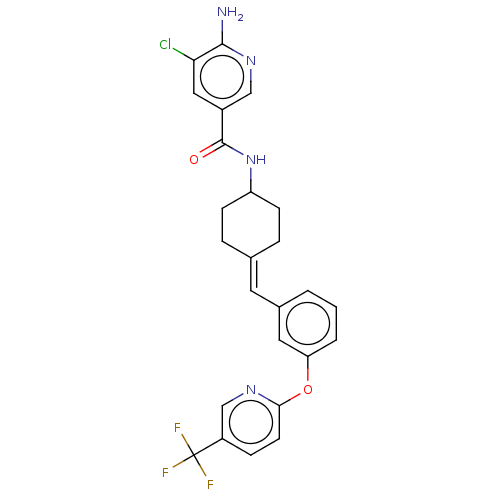 (US9682953, 4.A-3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143186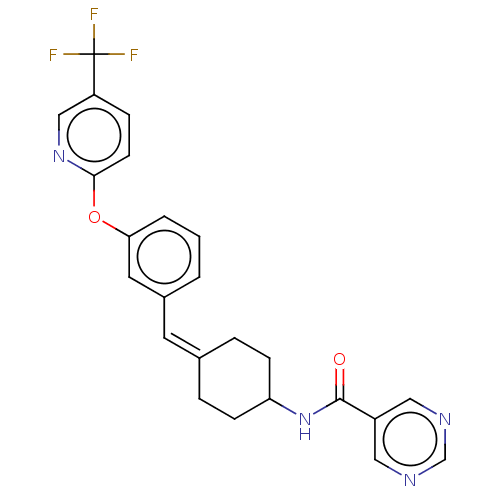 (US9682953, 2.A-12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 415 total ) | Next | Last >> |