

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079375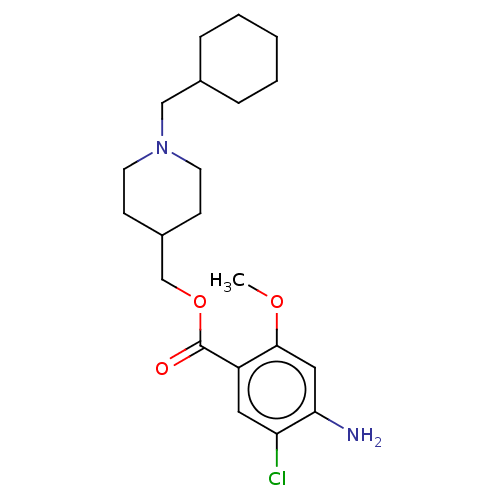 (CHEMBL3417008 | US9663465, 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50106249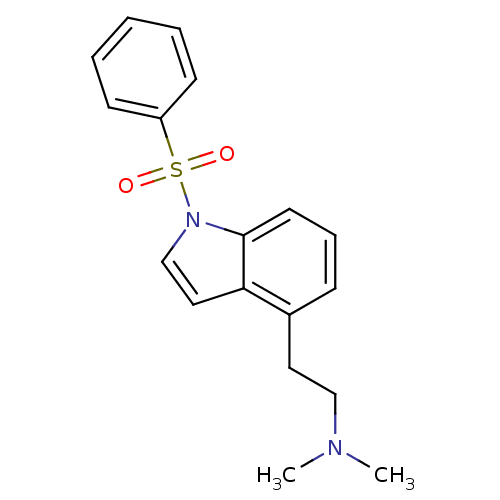 (CHEMBL92139 | [2-(1-Benzenesulfonyl-1H-indol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Displacement of [3H]lysergic acid diethylamide from human 5-HT6 receptor transfected in HeLa cells after 60 mins by scintillation spectrometry | J Med Chem 58: 7901-12 (2015) Article DOI: 10.1021/acs.jmedchem.5b00179 BindingDB Entry DOI: 10.7270/Q2M90BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Displacement of [3H]lysergic acid diethylamide from human 5-HT6 receptor expressed in HeLa cells after 120 mins by scintillation counter | J Med Chem 58: 7901-12 (2015) Article DOI: 10.1021/acs.jmedchem.5b00179 BindingDB Entry DOI: 10.7270/Q2M90BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM34141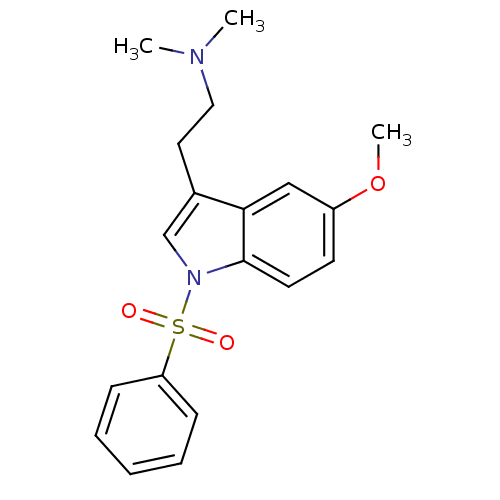 (CHEMBL76237 | MS-245) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Displacement of [3H]lysergic acid diethylamide from human 5-HT6 receptor transfected in HEK293 cells after 60 mins by liquid scintillation spectromet... | J Med Chem 58: 7901-12 (2015) Article DOI: 10.1021/acs.jmedchem.5b00179 BindingDB Entry DOI: 10.7270/Q2M90BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079377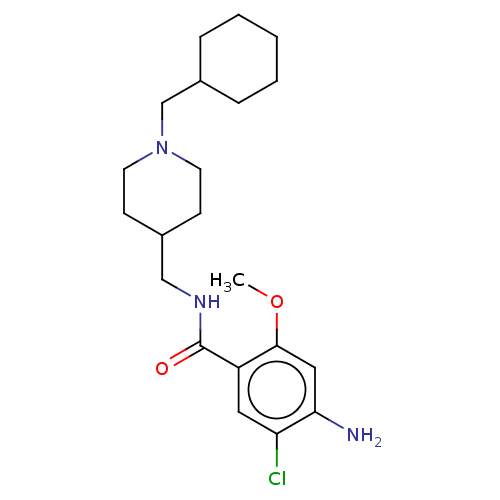 (CHEMBL3417007) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079367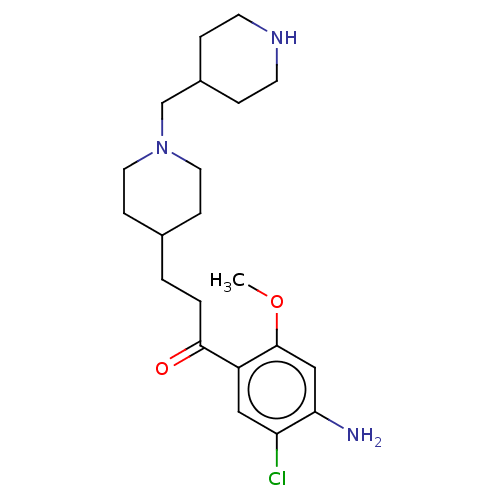 (CHEMBL3416998) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50106250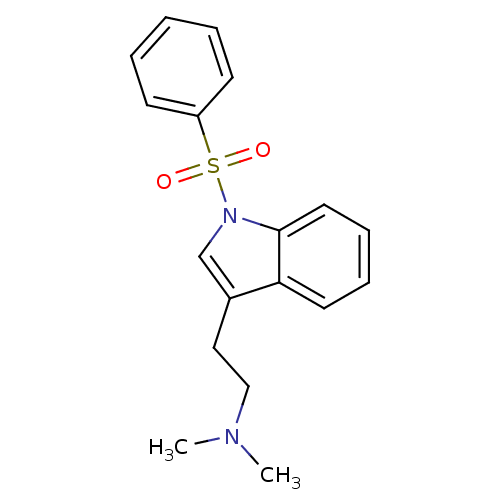 (CHEMBL93868 | N,N-dimethyl-2-(1-(phenylsulfonyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Displacement of [3H]lysergic acid diethylamide from human 5-HT6 receptor transfected in HeLa cells after 60 mins by scintillation spectrometry | J Med Chem 58: 7901-12 (2015) Article DOI: 10.1021/acs.jmedchem.5b00179 BindingDB Entry DOI: 10.7270/Q2M90BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079365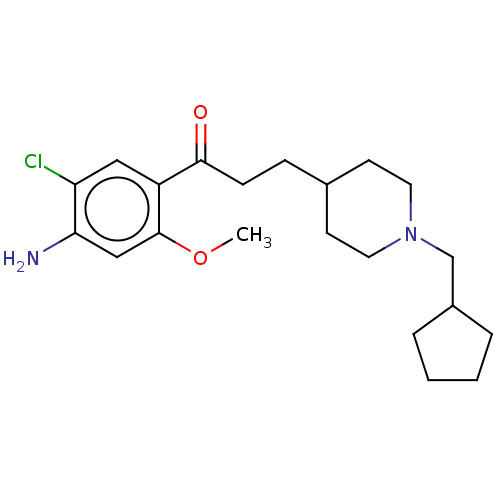 (CHEMBL3416996 | US9663465, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079366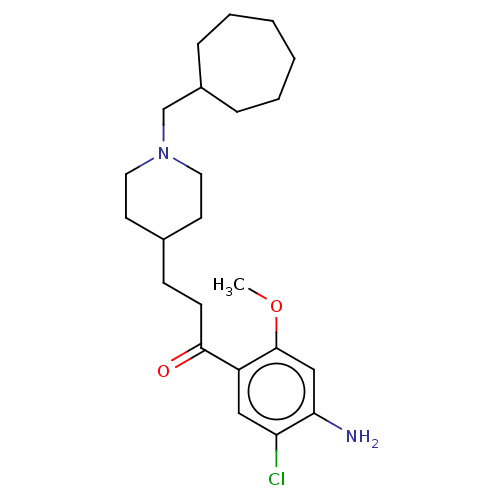 (CHEMBL3416997 | US9663465, 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300828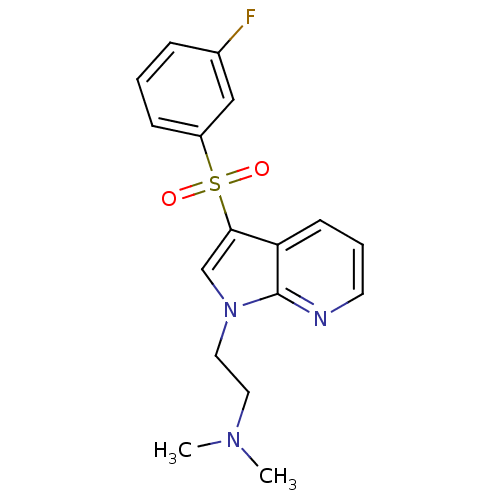 (2-(3-(3-fluorophenylsulfonyl)-1H-pyrrolo[2,3-b]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Displacement of [3H]lysergic acid diethylamide from human 5-HT6 receptor expressed in HeLa cells by scintillation counter | J Med Chem 58: 7901-12 (2015) Article DOI: 10.1021/acs.jmedchem.5b00179 BindingDB Entry DOI: 10.7270/Q2M90BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079369 (CHEMBL3417000 | US9663465, 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21354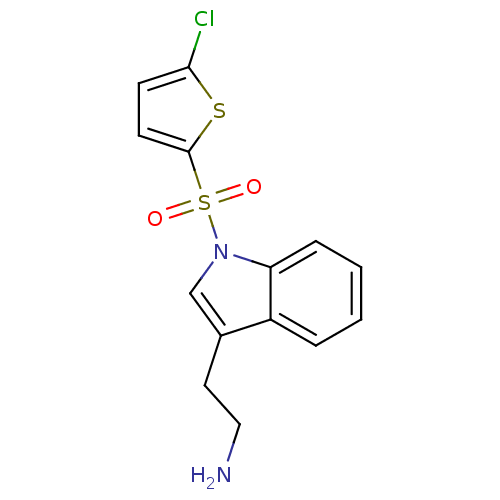 (2-{1-[(5-chlorothiophene-2-)sulfonyl]-1H-indol-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Displacement of [3H]lysergic acid diethylamide from human 5-HT6 receptor expressed in HeLa cells after 120 mins by scintillation counter | J Med Chem 58: 7901-12 (2015) Article DOI: 10.1021/acs.jmedchem.5b00179 BindingDB Entry DOI: 10.7270/Q2M90BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079368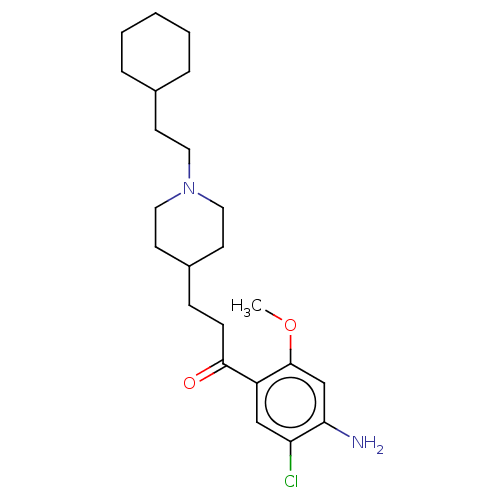 (CHEMBL3416999 | US9663465, 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079364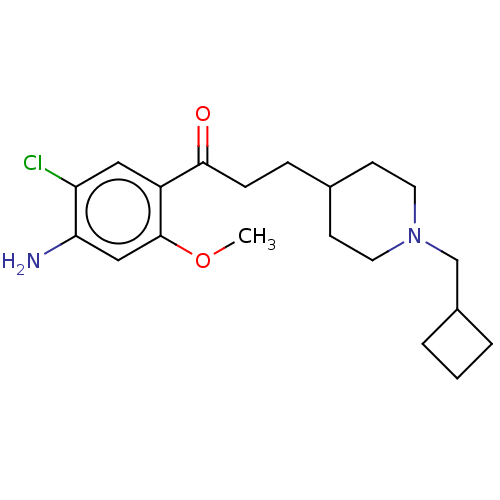 (CHEMBL3416995 | US9663465, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM84950 (CAS_183782 | NSC_183782 | RS 67333) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21366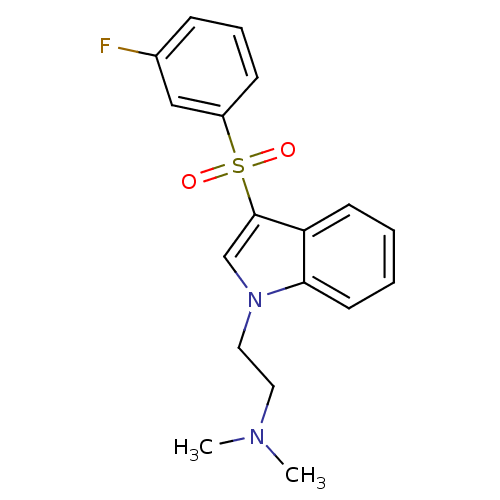 ((2-{3-[(3-fluorobenzene)sulfonyl]-1H-indol-1-yl}et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Displacement of radioligand from human 5-HT6 receptor expressed in HeLa cells by scintillation counter | J Med Chem 58: 7901-12 (2015) Article DOI: 10.1021/acs.jmedchem.5b00179 BindingDB Entry DOI: 10.7270/Q2M90BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079370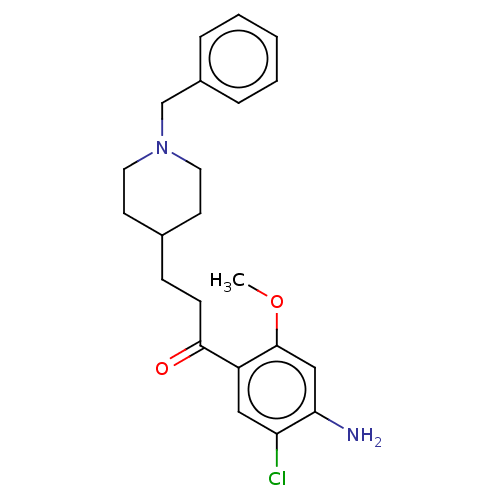 (CHEMBL3417001) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079363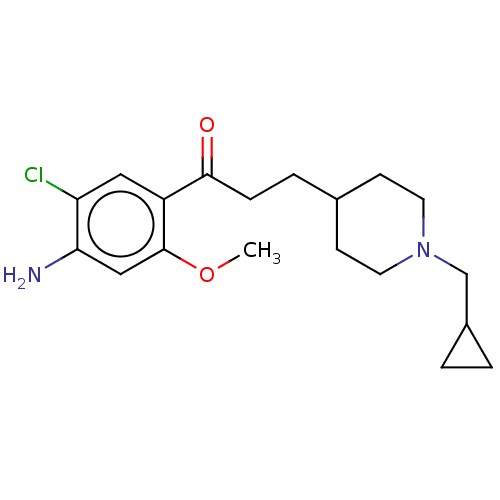 (CHEMBL3414597 | US9663465, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM34142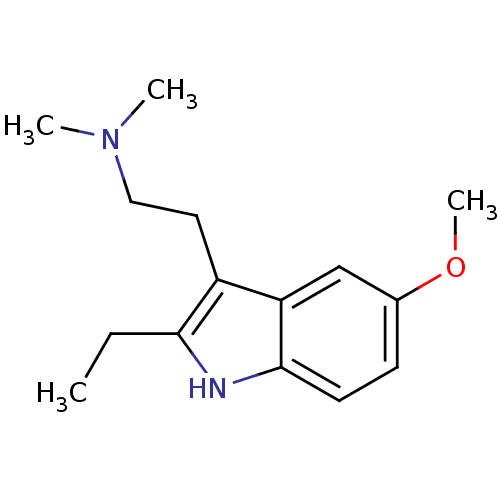 (2-ethyl-5-methoxy-N,N-dimethyltryptamine | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Displacement of [3H]lysergic acid diethylamide from human 5-HT6 receptor transfected in HEK293 cells after 60 mins by liquid scintillation spectromet... | J Med Chem 58: 7901-12 (2015) Article DOI: 10.1021/acs.jmedchem.5b00179 BindingDB Entry DOI: 10.7270/Q2M90BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079371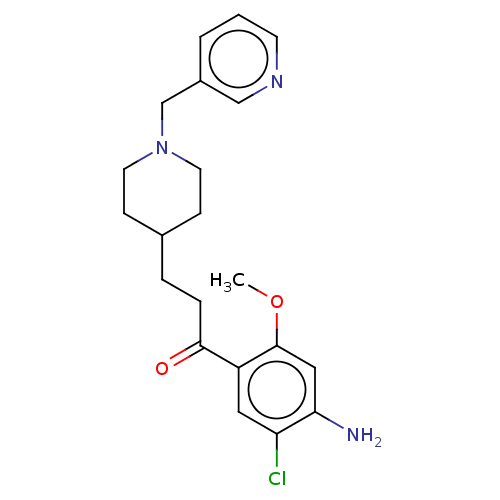 (CHEMBL3417002) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50085970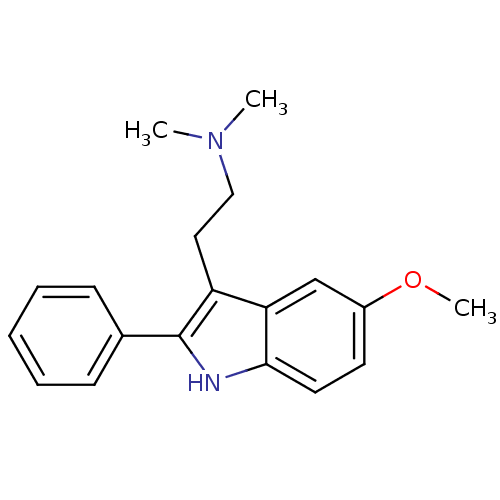 (CHEMBL7318 | [2-(5-Methoxy-2-phenyl-1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Displacement of [3H]lysergic acid diethylamide from human 5-HT6 receptor transfected in HEK293 cells after 60 mins by liquid scintillation spectromet... | J Med Chem 58: 7901-12 (2015) Article DOI: 10.1021/acs.jmedchem.5b00179 BindingDB Entry DOI: 10.7270/Q2M90BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079372 (CHEMBL3417003) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (RAT) | BDBM50171234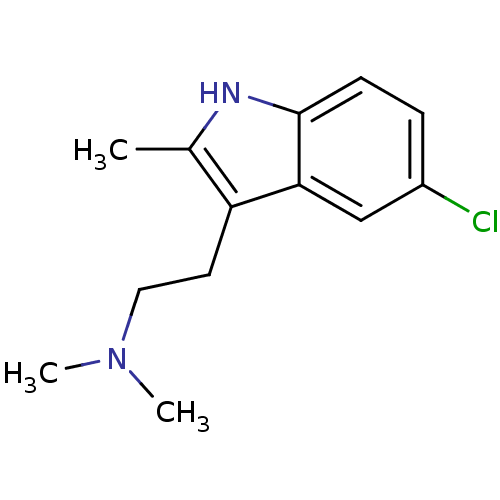 (CHEMBL365751 | [2-(5-Chloro-2-methyl-1H-indol-3-yl...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Displacement of [3H] lysergic acid diethylamide from rat 5-HT6 receptor transfected in HEK293 cells after 60 mins by scintillation counter | J Med Chem 58: 7901-12 (2015) Article DOI: 10.1021/acs.jmedchem.5b00179 BindingDB Entry DOI: 10.7270/Q2M90BGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079376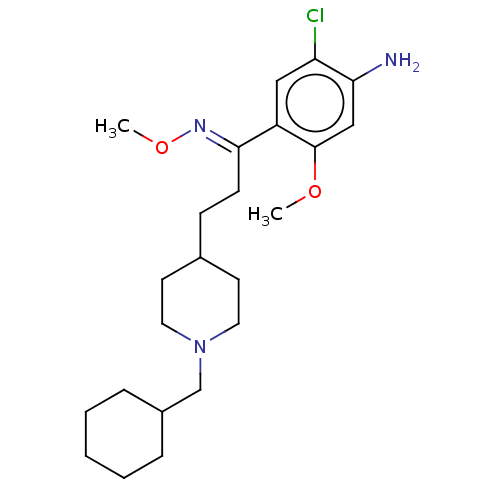 (CHEMBL3417005) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079374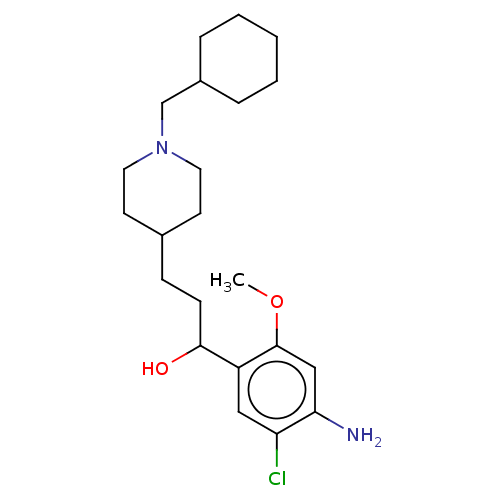 (CHEMBL3417006) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079373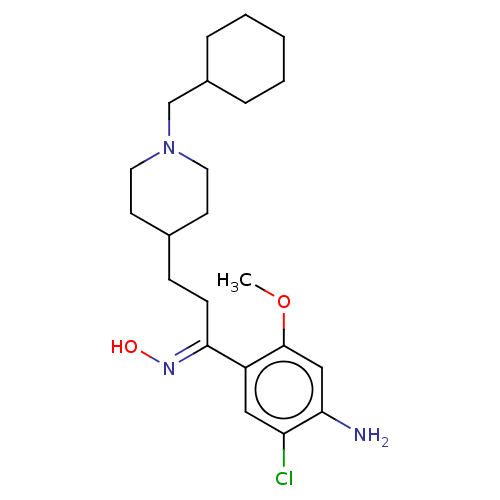 (CHEMBL3417004) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50171234 (CHEMBL365751 | [2-(5-Chloro-2-methyl-1H-indol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Binding affinity to 5-HT2B receptor (unknown origin) | J Med Chem 58: 7901-12 (2015) Article DOI: 10.1021/acs.jmedchem.5b00179 BindingDB Entry DOI: 10.7270/Q2M90BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50171234 (CHEMBL365751 | [2-(5-Chloro-2-methyl-1H-indol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Binding affinity to 5-HT7 receptor (unknown origin) | J Med Chem 58: 7901-12 (2015) Article DOI: 10.1021/acs.jmedchem.5b00179 BindingDB Entry DOI: 10.7270/Q2M90BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50171234 (CHEMBL365751 | [2-(5-Chloro-2-methyl-1H-indol-3-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Binding affinity to human recombinant alpha2 adrenergic receptor | J Med Chem 58: 7901-12 (2015) Article DOI: 10.1021/acs.jmedchem.5b00179 BindingDB Entry DOI: 10.7270/Q2M90BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079370 (CHEMBL3417001) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079373 (CHEMBL3417004) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079371 (CHEMBL3417002) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079366 (CHEMBL3416997 | US9663465, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079365 (CHEMBL3416996 | US9663465, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079367 (CHEMBL3416998) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079372 (CHEMBL3417003) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079368 (CHEMBL3416999 | US9663465, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 222 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079369 (CHEMBL3417000 | US9663465, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 321 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM84950 (CAS_183782 | NSC_183782 | RS 67333) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 403 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079364 (CHEMBL3416995 | US9663465, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 577 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079363 (CHEMBL3414597 | US9663465, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 937 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079374 (CHEMBL3417006) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50079370 (CHEMBL3417001) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE pre-incubated for 5 mins before butyrylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079375 (CHEMBL3417008 | US9663465, 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE pre-incubated for 5 mins before butyrylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50079374 (CHEMBL3417006) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE pre-incubated for 5 mins before butyrylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50079366 (CHEMBL3416997 | US9663465, 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE pre-incubated for 5 mins before butyrylthiocholine iodide substrate by Ellman' method | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 62 total ) | Next | Last >> |