 Found 4893 hits with Last Name = 'chan' and Initial = 'v'
Found 4893 hits with Last Name = 'chan' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50586388

(CHEMBL5081557)Show SMILES CS(=O)(=O)c1ccc(CNC(=O)c2cc3CCOC4(CCN(Cc5ccccc5Cl)CC4)c3s2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM498074
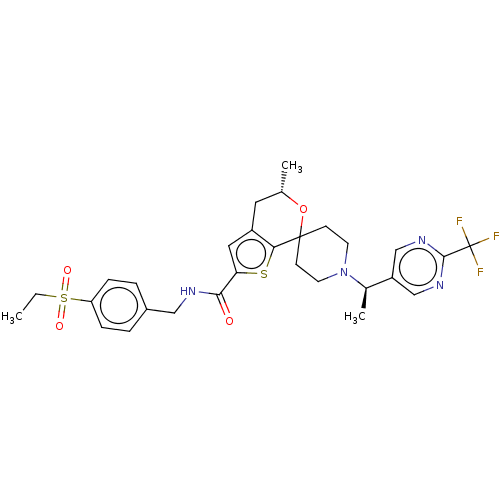
((5′S)óN-[4-(Ethylsulfonyl)benzyl]-5′-m...)Show SMILES CCS(=O)(=O)c1ccc(CNC(=O)c2cc3C[C@H](C)OC4(CCN(CC4)[C@H](C)c4cnc(nc4)C(F)(F)F)c3s2)cc1 |r| Show InChI InChI=1S/C29H33F3N4O4S2/c1-4-42(38,39)23-7-5-20(6-8-23)15-33-26(37)24-14-21-13-18(2)40-28(25(21)41-24)9-11-36(12-10-28)19(3)22-16-34-27(35-17-22)29(30,31)32/h5-8,14,16-19H,4,9-13,15H2,1-3H3,(H,33,37)/t18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM21342
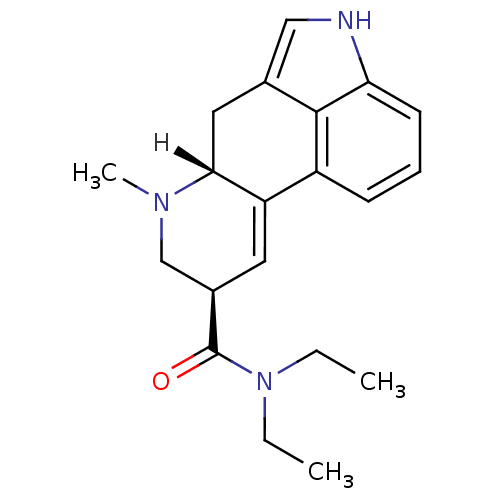
((4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclo[...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)C(=O)N(CC)CC)c34 |c:12| Show InChI InChI=1S/C20H25N3O/c1-4-23(5-2)20(24)14-9-16-15-7-6-8-17-19(15)13(11-21-17)10-18(16)22(3)12-14/h6-9,11,14,18,21H,4-5,10,12H2,1-3H3/t14-,18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2 receptor from rat frontal cortex using [125]-(R)-DOI as radioligand |
J Med Chem 38: 3593-601 (1995)
Article DOI: 10.1021/jm00018a019
BindingDB Entry DOI: 10.7270/Q2PC3535 |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50470580
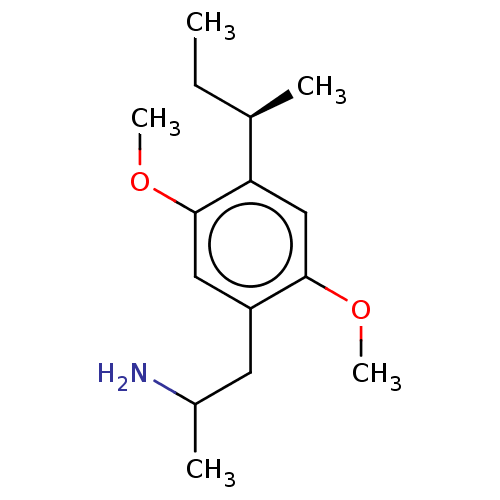
(CHEMBL119924)Show InChI InChI=1S/C15H25NO2/c1-6-10(2)13-9-14(17-4)12(7-11(3)16)8-15(13)18-5/h8-11H,6-7,16H2,1-5H3/t10-,11?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2 receptor from rat frontal cortex using [125]-(R)-DOI as radioligand |
J Med Chem 38: 3593-601 (1995)
Article DOI: 10.1021/jm00018a019
BindingDB Entry DOI: 10.7270/Q2PC3535 |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50470391
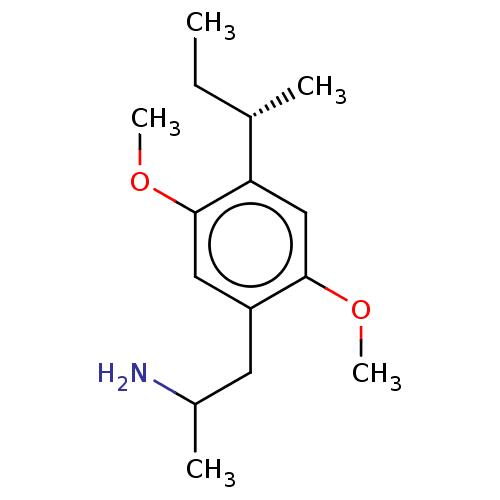
(CHEMBL120116)Show InChI InChI=1S/C15H25NO2/c1-6-10(2)13-9-14(17-4)12(7-11(3)16)8-15(13)18-5/h8-11H,6-7,16H2,1-5H3/t10-,11?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2 receptor from rat frontal cortex using [125]-(R)-DOI as radioligand |
J Med Chem 38: 3593-601 (1995)
Article DOI: 10.1021/jm00018a019
BindingDB Entry DOI: 10.7270/Q2PC3535 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50586394

(CHEMBL5078351)Show SMILES CCS(=O)(=O)c1ccc(CNC(=O)c2cc3CCOC4(CCN(CC4)[C@H](C)c4ccc(cc4)C#N)c3s2)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50586395

(CHEMBL5069696)Show SMILES CCS(=O)(=O)c1ccc(CNC(=O)c2cc3CCOC4(CCN(CC4)[C@H](C)c4ccc(cc4)C(F)(F)F)c3s2)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50586396

(CHEMBL5079705)Show SMILES CCS(=O)(=O)c1ccc(CNC(=O)c2cc3CCOC4(CCN(CC4)[C@H](C)c4cnc(nc4)C(F)(F)F)c3s2)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM302860
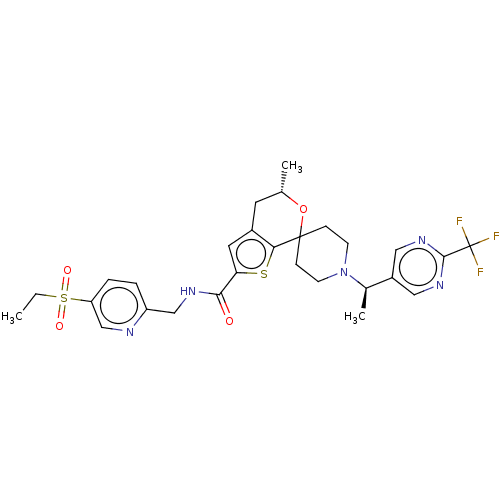
((2S)-1-(3-Thienyl)propan-2-ol | US9598431, 3)Show SMILES CCS(=O)(=O)c1ccc(CNC(=O)c2cc3C[C@H](C)OC4(CCN(CC4)[C@H](C)c4cnc(nc4)C(F)(F)F)c3s2)nc1 |r| Show InChI InChI=1S/C28H32F3N5O4S2/c1-4-42(38,39)22-6-5-21(32-16-22)15-33-25(37)23-12-19-11-17(2)40-27(24(19)41-23)7-9-36(10-8-27)18(3)20-13-34-26(35-14-20)28(29,30)31/h5-6,12-14,16-18H,4,7-11,15H2,1-3H3,(H,33,37)/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50005265
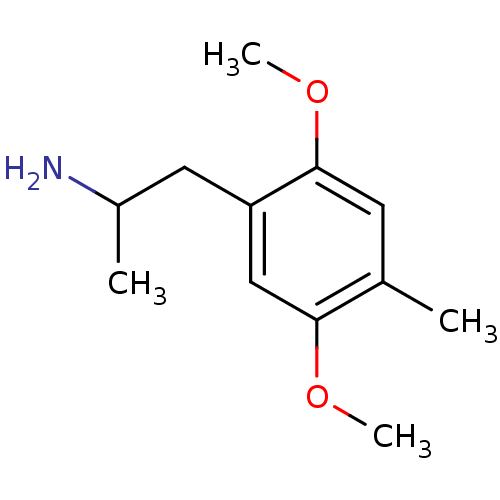
((+/-)2-(2,5-Dimethoxy-4-methyl-phenyl)-1-methyl-et...)Show InChI InChI=1S/C12H19NO2/c1-8-5-12(15-4)10(6-9(2)13)7-11(8)14-3/h5,7,9H,6,13H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2 receptor from rat frontal cortex using [125]-(R)-DOI as radioligand |
J Med Chem 38: 3593-601 (1995)
Article DOI: 10.1021/jm00018a019
BindingDB Entry DOI: 10.7270/Q2PC3535 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50398211
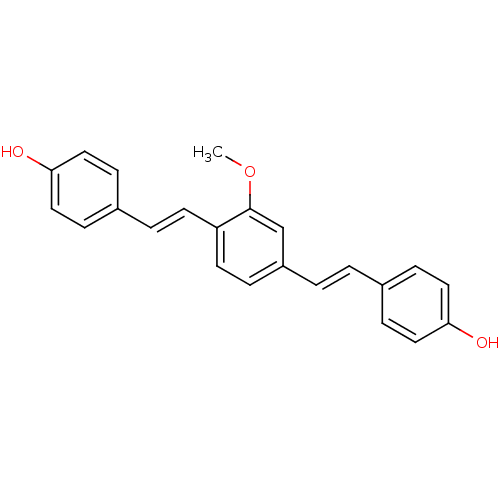
(CHEMBL2181036)Show SMILES COc1cc(\C=C\c2ccc(O)cc2)ccc1\C=C\c1ccc(O)cc1 Show InChI InChI=1S/C23H20O3/c1-26-23-16-19(3-2-17-6-12-21(24)13-7-17)5-11-20(23)10-4-18-8-14-22(25)15-9-18/h2-16,24-25H,1H3/b3-2+,10-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]chrysamine G from fibrillar amyloid beta (1 to 40) (unknown origin) after 1 hr by liquid scintillation spectrophotometric analysi... |
Bioorg Med Chem 22: 2469-81 (2014)
Article DOI: 10.1016/j.bmc.2014.02.054
BindingDB Entry DOI: 10.7270/Q2DB83C0 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50586389

(CHEMBL5093568)Show SMILES CS(=O)(=O)c1ccc(CNC(=O)c2cc3CCOC4(CCN(Cc5ccc(Cl)cc5Cl)CC4)c3s2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50002834
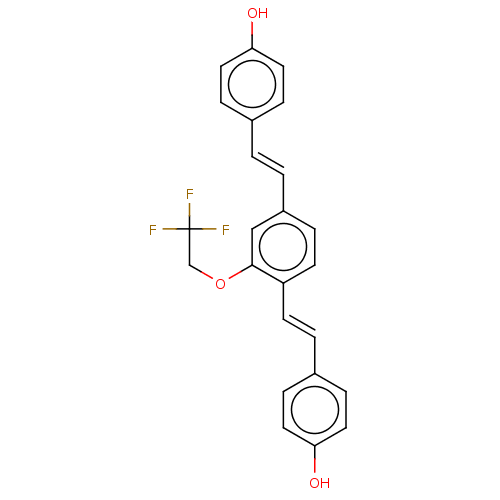
(CHEMBL3233658)Show SMILES Oc1ccc(\C=C\c2ccc(\C=C\c3ccc(O)cc3)c(OCC(F)(F)F)c2)cc1 Show InChI InChI=1S/C24H19F3O3/c25-24(26,27)16-30-23-15-19(2-1-17-5-11-21(28)12-6-17)4-10-20(23)9-3-18-7-13-22(29)14-8-18/h1-15,28-29H,16H2/b2-1+,9-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]chrysamine G from fibrillar amyloid beta (1 to 40) (unknown origin) after 1 hr by liquid scintillation spectrophotometric analysi... |
Bioorg Med Chem 22: 2469-81 (2014)
Article DOI: 10.1016/j.bmc.2014.02.054
BindingDB Entry DOI: 10.7270/Q2DB83C0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50002835
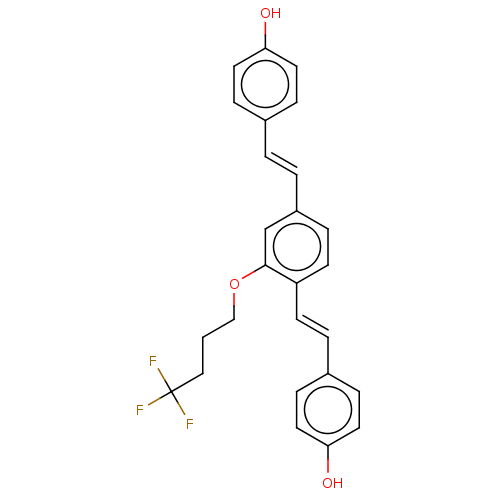
(CHEMBL3233659)Show SMILES Oc1ccc(\C=C\c2ccc(\C=C\c3ccc(O)cc3)c(OCCCC(F)(F)F)c2)cc1 Show InChI InChI=1S/C26H23F3O3/c27-26(28,29)16-1-17-32-25-18-21(3-2-19-6-12-23(30)13-7-19)5-11-22(25)10-4-20-8-14-24(31)15-9-20/h2-15,18,30-31H,1,16-17H2/b3-2+,10-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]chrysamine G from fibrillar amyloid beta (1 to 40) (unknown origin) after 1 hr by liquid scintillation spectrophotometric analysi... |
Bioorg Med Chem 22: 2469-81 (2014)
Article DOI: 10.1016/j.bmc.2014.02.054
BindingDB Entry DOI: 10.7270/Q2DB83C0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50002836

(CHEMBL3233660)Show SMILES Oc1ccc(\C=C\c2ccc(\C=C\c3ccc(O)cc3)c(OC(C(F)(F)F)(C(F)(F)F)C(F)(F)F)c2)cc1 Show InChI InChI=1S/C26H17F9O3/c27-24(28,29)23(25(30,31)32,26(33,34)35)38-22-15-18(2-1-16-5-11-20(36)12-6-16)4-10-19(22)9-3-17-7-13-21(37)14-8-17/h1-15,36-37H/b2-1+,9-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]chrysamine G from fibrillar amyloid beta (1 to 40) (unknown origin) after 1 hr by liquid scintillation spectrophotometric analysi... |
Bioorg Med Chem 22: 2469-81 (2014)
Article DOI: 10.1016/j.bmc.2014.02.054
BindingDB Entry DOI: 10.7270/Q2DB83C0 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50586393

(CHEMBL5077159)Show SMILES C[C@@H](N1CCC2(CC1)OCCc1cc(sc21)C(=O)NCc1ccc(cc1)S(C)(=O)=O)c1ccc(cc1)C#N |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50586397
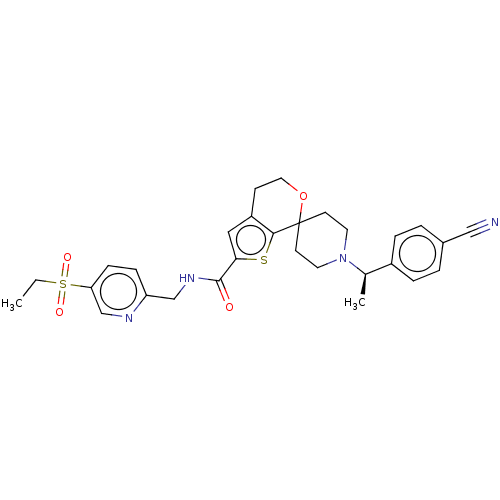
(CHEMBL5092218)Show SMILES CCS(=O)(=O)c1ccc(CNC(=O)c2cc3CCOC4(CCN(CC4)[C@H](C)c4ccc(cc4)C#N)c3s2)nc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50002833

(CHEMBL3233657)Show SMILES Oc1ccc(\C=C\c2ccc(\C=C\c3ccc(O)cc3)c(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C23H17F3O2/c24-23(25,26)22-15-18(2-1-16-5-11-20(27)12-6-16)4-10-19(22)9-3-17-7-13-21(28)14-8-17/h1-15,27-28H/b2-1+,9-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]chrysamine G from fibrillar amyloid beta (1 to 40) (unknown origin) after 1 hr by liquid scintillation spectrophotometric analysi... |
Bioorg Med Chem 22: 2469-81 (2014)
Article DOI: 10.1016/j.bmc.2014.02.054
BindingDB Entry DOI: 10.7270/Q2DB83C0 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50586391

(CHEMBL5085419)Show SMILES CS(=O)(=O)c1ccc(CNC(=O)c2cc3CCOC4(CCN(Cc5ccc(cc5)C(F)(F)F)CC4)c3s2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 249 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50586390

(CHEMBL5081257)Show SMILES CS(=O)(=O)c1ccc(CNC(=O)c2cc3CCOC4(CCN(Cc5ccc(Cl)cc5)CC4)c3s2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 249 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50586392

(CHEMBL5082586)Show SMILES CS(=O)(=O)c1ccc(CNC(=O)c2cc3CCOC4(CCN(Cc5ccc(cc5)C#N)CC4)c3s2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 278 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50586398
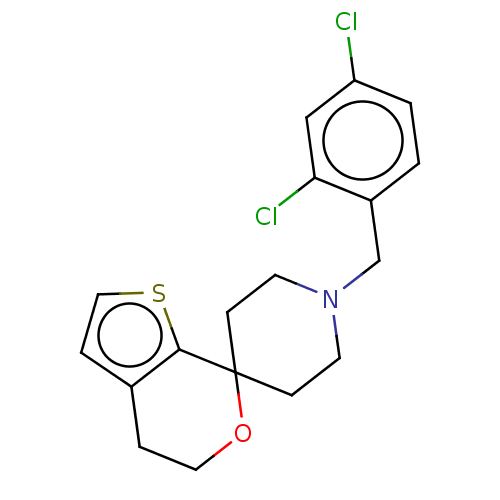
(CHEMBL4226176) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-alpha
(Homo sapiens (Human)) | BDBM302860

((2S)-1-(3-Thienyl)propan-2-ol | US9598431, 3)Show SMILES CCS(=O)(=O)c1ccc(CNC(=O)c2cc3C[C@H](C)OC4(CCN(CC4)[C@H](C)c4cnc(nc4)C(F)(F)F)c3s2)nc1 |r| Show InChI InChI=1S/C28H32F3N5O4S2/c1-4-42(38,39)22-6-5-21(32-16-22)15-33-25(37)23-12-19-11-17(2)40-27(24(19)41-23)7-9-36(10-8-27)18(3)20-13-34-26(35-14-20)28(29,30)31/h5-6,12-14,16-18H,4,7-11,15H2,1-3H3,(H,33,37)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to RORalpha (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-beta
(Homo sapiens (Human)) | BDBM302860

((2S)-1-(3-Thienyl)propan-2-ol | US9598431, 3)Show SMILES CCS(=O)(=O)c1ccc(CNC(=O)c2cc3C[C@H](C)OC4(CCN(CC4)[C@H](C)c4cnc(nc4)C(F)(F)F)c3s2)nc1 |r| Show InChI InChI=1S/C28H32F3N5O4S2/c1-4-42(38,39)22-6-5-21(32-16-22)15-33-25(37)23-12-19-11-17(2)40-27(24(19)41-23)7-9-36(10-8-27)18(3)20-13-34-26(35-14-20)28(29,30)31/h5-6,12-14,16-18H,4,7-11,15H2,1-3H3,(H,33,37)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to RORbeta (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50403048
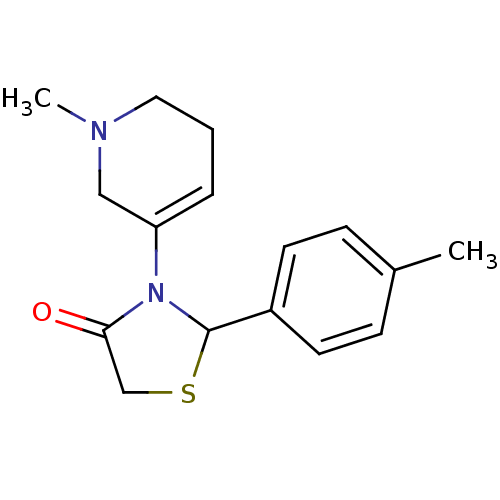
(CHEMBL2216805)Show InChI InChI=1S/C16H20N2OS/c1-12-5-7-13(8-6-12)16-18(15(19)11-20-16)14-4-3-9-17(2)10-14/h4-8,16H,3,9-11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Hari Singh Gour University
Curated by ChEMBL
| Assay Description
Displacement of [3H]QNB from M1 receptor in Wistar rat cerebral cortex homogenate |
Bioorg Med Chem 20: 3378-95 (2012)
Article DOI: 10.1016/j.bmc.2012.03.069
BindingDB Entry DOI: 10.7270/Q2222VX4 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356075
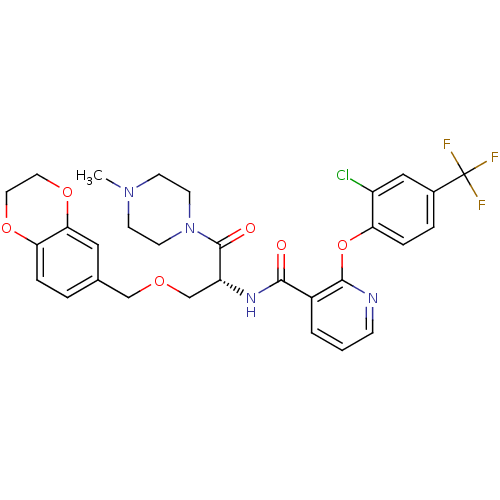
(CHEMBL1911818)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccc2OCCOc2c1)NC(=O)c1cccnc1Oc1ccc(cc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C30H30ClF3N4O6/c1-37-9-11-38(12-10-37)29(40)23(18-41-17-19-4-6-25-26(15-19)43-14-13-42-25)36-27(39)21-3-2-8-35-28(21)44-24-7-5-20(16-22(24)31)30(32,33)34/h2-8,15-16,23H,9-14,17-18H2,1H3,(H,36,39)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402421
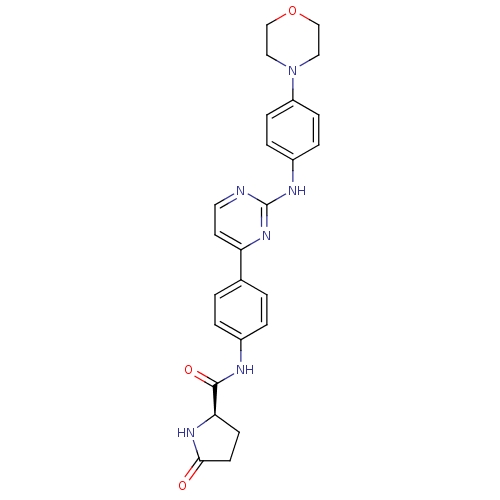
(CHEMBL2208035)Show SMILES O=C(Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1)[C@H]1CCC(=O)N1 |r| Show InChI InChI=1S/C25H26N6O3/c32-23-10-9-22(29-23)24(33)27-18-3-1-17(2-4-18)21-11-12-26-25(30-21)28-19-5-7-20(8-6-19)31-13-15-34-16-14-31/h1-8,11-12,22H,9-10,13-16H2,(H,27,33)(H,29,32)(H,26,28,30)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356075

(CHEMBL1911818)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccc2OCCOc2c1)NC(=O)c1cccnc1Oc1ccc(cc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C30H30ClF3N4O6/c1-37-9-11-38(12-10-37)29(40)23(18-41-17-19-4-6-25-26(15-19)43-14-13-42-25)36-27(39)21-3-2-8-35-28(21)44-24-7-5-20(16-22(24)31)30(32,33)34/h2-8,15-16,23H,9-14,17-18H2,1H3,(H,36,39)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356076
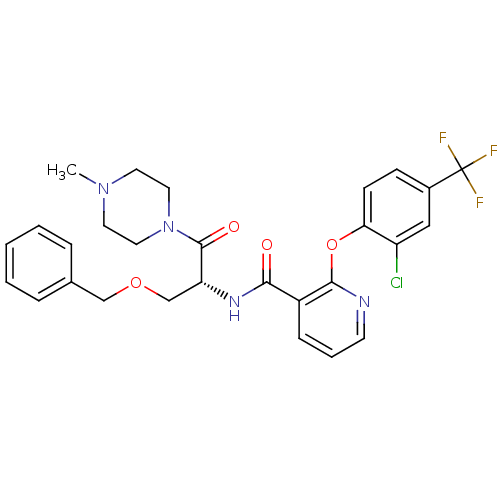
(CHEMBL1911817)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccccc1)NC(=O)c1cccnc1Oc1ccc(cc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C28H28ClF3N4O4/c1-35-12-14-36(15-13-35)27(38)23(18-39-17-19-6-3-2-4-7-19)34-25(37)21-8-5-11-33-26(21)40-24-10-9-20(16-22(24)29)28(30,31)32/h2-11,16,23H,12-15,17-18H2,1H3,(H,34,37)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50272192
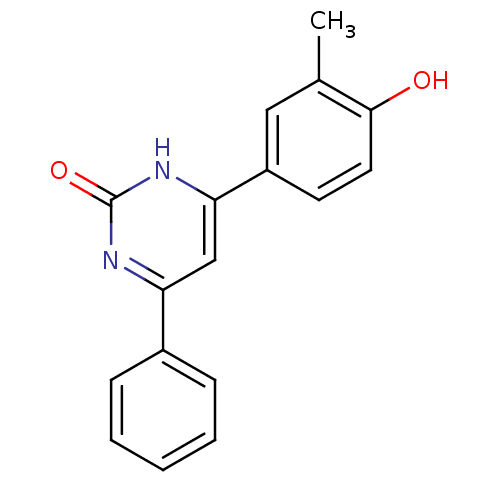
(4-(4-hydroxy-3-methylphenyl)-6-phenylpyrimidin-2(1...)Show InChI InChI=1S/C17H14N2O2/c1-11-9-13(7-8-16(11)20)15-10-14(18-17(21)19-15)12-5-3-2-4-6-12/h2-10,20H,1H3,(H,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402413

(CHEMBL2208032)Show SMILES C[C@@H](N)C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C23H26N6O2/c1-16(24)22(30)26-18-4-2-17(3-5-18)21-10-11-25-23(28-21)27-19-6-8-20(9-7-19)29-12-14-31-15-13-29/h2-11,16H,12-15,24H2,1H3,(H,26,30)(H,25,27,28)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356077
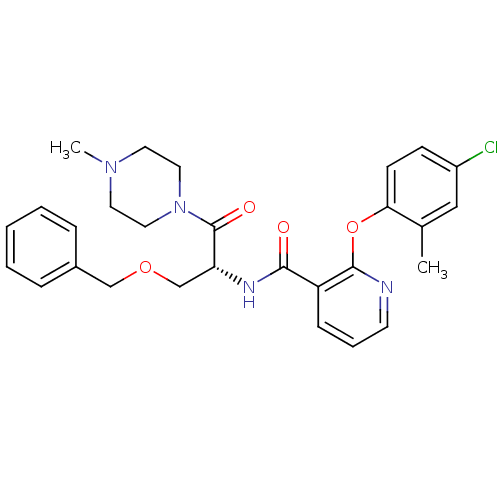
(CHEMBL1911816)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccccc1)NC(=O)c1cccnc1Oc1ccc(Cl)cc1C |r| Show InChI InChI=1S/C28H31ClN4O4/c1-20-17-22(29)10-11-25(20)37-27-23(9-6-12-30-27)26(34)31-24(19-36-18-21-7-4-3-5-8-21)28(35)33-15-13-32(2)14-16-33/h3-12,17,24H,13-16,18-19H2,1-2H3,(H,31,34)/t24-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
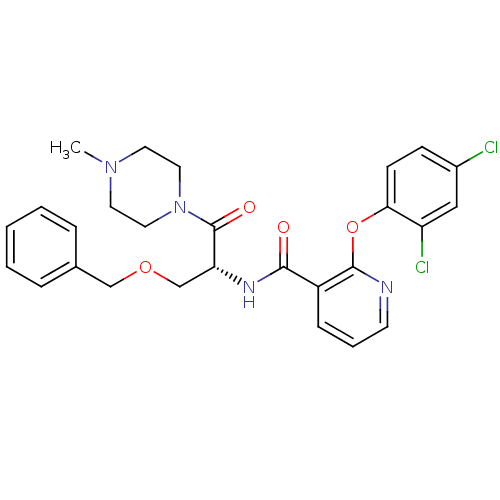
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356078
(CHEMBL1911815)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccccc1)NC(=O)c1cccnc1Oc1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C27H28Cl2N4O4/c1-32-12-14-33(15-13-32)27(35)23(18-36-17-19-6-3-2-4-7-19)31-25(34)21-8-5-11-30-26(21)37-24-10-9-20(28)16-22(24)29/h2-11,16,23H,12-15,17-18H2,1H3,(H,31,34)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
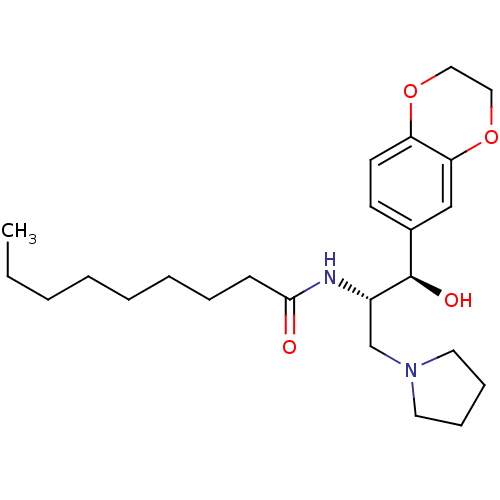
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356090
(CHEMBL1911678)Show SMILES CCCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C24H38N2O4/c1-2-3-4-5-6-7-10-23(27)25-20(18-26-13-8-9-14-26)24(28)19-11-12-21-22(17-19)30-16-15-29-21/h11-12,17,20,24,28H,2-10,13-16,18H2,1H3,(H,25,27)/t20-,24+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
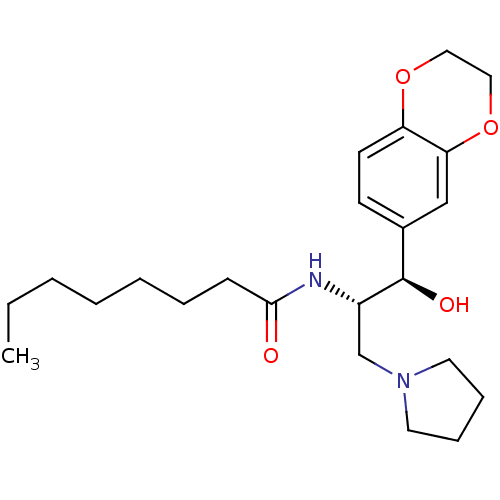
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356091
(CHEMBL1911679)Show SMILES CCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C23H36N2O4/c1-2-3-4-5-6-9-22(26)24-19(17-25-12-7-8-13-25)23(27)18-10-11-20-21(16-18)29-15-14-28-20/h10-11,16,19,23,27H,2-9,12-15,17H2,1H3,(H,24,26)/t19-,23+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356076

(CHEMBL1911817)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccccc1)NC(=O)c1cccnc1Oc1ccc(cc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C28H28ClF3N4O4/c1-35-12-14-36(15-13-35)27(38)23(18-39-17-19-6-3-2-4-7-19)34-25(37)21-8-5-11-33-26(21)40-24-10-9-20(16-22(24)29)28(30,31)32/h2-11,16,23H,12-15,17-18H2,1H3,(H,34,37)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394804
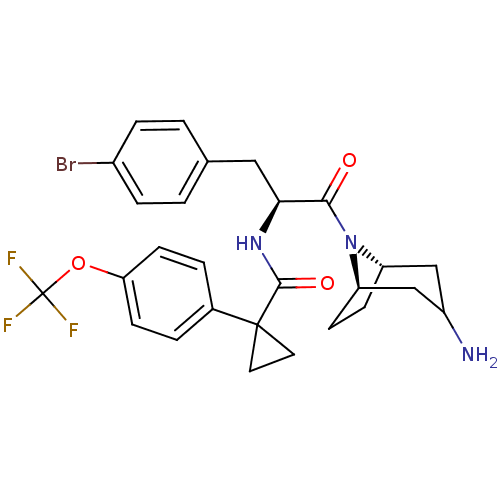
(CHEMBL2163828)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(OC(F)(F)F)cc1 |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C27H29BrF3N3O3/c28-18-5-1-16(2-6-18)13-23(24(35)34-20-7-8-21(34)15-19(32)14-20)33-25(36)26(11-12-26)17-3-9-22(10-4-17)37-27(29,30)31/h1-6,9-10,19-21,23H,7-8,11-15,32H2,(H,33,36)/t19?,20-,21+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394807
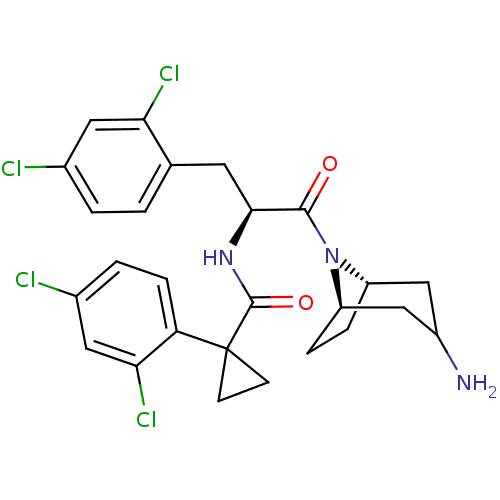
(CHEMBL2163825)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H27Cl4N3O2/c27-15-2-1-14(21(29)10-15)9-23(24(34)33-18-4-5-19(33)13-17(31)12-18)32-25(35)26(7-8-26)20-6-3-16(28)11-22(20)30/h1-3,6,10-11,17-19,23H,4-5,7-9,12-13,31H2,(H,32,35)/t17?,18-,19+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394812
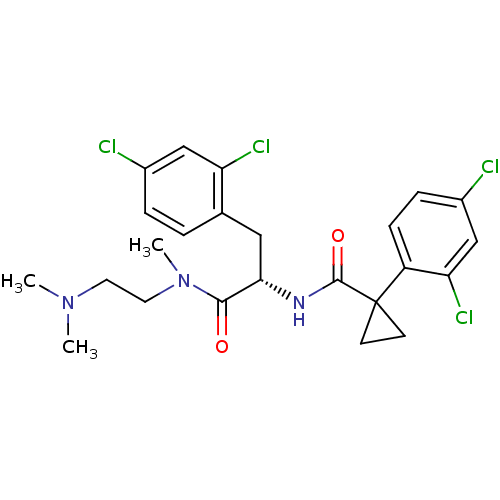
(CHEMBL2163838)Show SMILES CN(C)CCN(C)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H27Cl4N3O2/c1-30(2)10-11-31(3)22(32)21(12-15-4-5-16(25)13-19(15)27)29-23(33)24(8-9-24)18-7-6-17(26)14-20(18)28/h4-7,13-14,21H,8-12H2,1-3H3,(H,29,33)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356090

(CHEMBL1911678)Show SMILES CCCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C24H38N2O4/c1-2-3-4-5-6-7-10-23(27)25-20(18-26-13-8-9-14-26)24(28)19-11-12-21-22(17-19)30-16-15-29-21/h11-12,17,20,24,28H,2-10,13-16,18H2,1H3,(H,25,27)/t20-,24+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394820
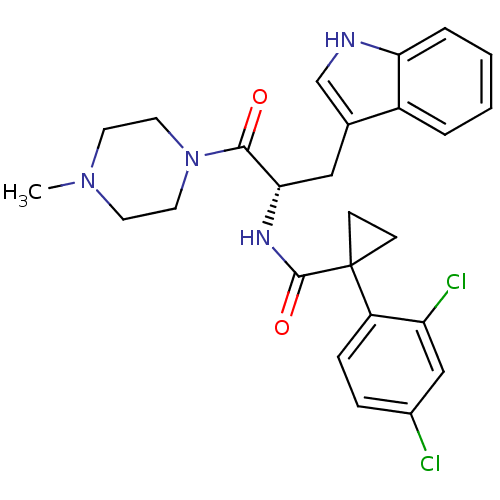
(CHEMBL2163829)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O2/c1-31-10-12-32(13-11-31)24(33)23(14-17-16-29-22-5-3-2-4-19(17)22)30-25(34)26(8-9-26)20-7-6-18(27)15-21(20)28/h2-7,15-16,23,29H,8-14H2,1H3,(H,30,34)/t23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356091

(CHEMBL1911679)Show SMILES CCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C23H36N2O4/c1-2-3-4-5-6-9-22(26)24-19(17-25-12-7-8-13-25)23(27)18-10-11-20-21(16-18)29-15-14-28-20/h10-11,16,19,23,27H,2-9,12-15,17H2,1H3,(H,24,26)/t19-,23+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402409
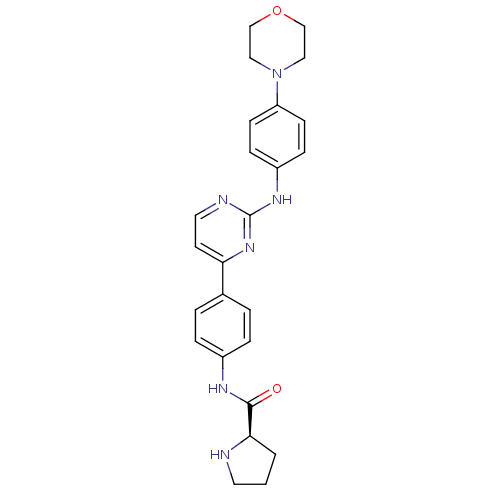
(CHEMBL2208034)Show SMILES O=C(Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1)[C@H]1CCCN1 |r| Show InChI InChI=1S/C25H28N6O2/c32-24(23-2-1-12-26-23)28-19-5-3-18(4-6-19)22-11-13-27-25(30-22)29-20-7-9-21(10-8-20)31-14-16-33-17-15-31/h3-11,13,23,26H,1-2,12,14-17H2,(H,28,32)(H,27,29,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402412
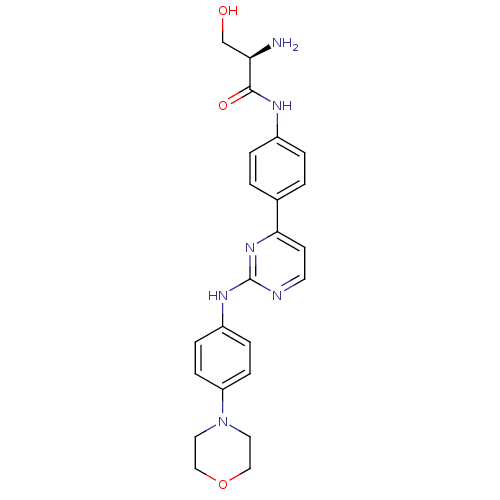
(CHEMBL2208033)Show SMILES N[C@H](CO)C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C23H26N6O3/c24-20(15-30)22(31)26-17-3-1-16(2-4-17)21-9-10-25-23(28-21)27-18-5-7-19(8-6-18)29-11-13-32-14-12-29/h1-10,20,30H,11-15,24H2,(H,26,31)(H,25,27,28)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394805
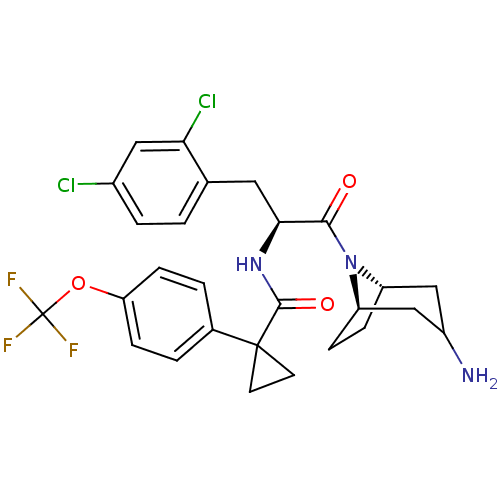
(CHEMBL2163827)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(OC(F)(F)F)cc1 |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C27H28Cl2F3N3O3/c28-17-4-1-15(22(29)12-17)11-23(24(36)35-19-5-6-20(35)14-18(33)13-19)34-25(37)26(9-10-26)16-2-7-21(8-3-16)38-27(30,31)32/h1-4,7-8,12,18-20,23H,5-6,9-11,13-14,33H2,(H,34,37)/t18?,19-,20+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385084

(CHEMBL2035636)Show SMILES CN1C[C@@H]2CC[C@H]1CN2Cc1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 |r| Show InChI InChI=1S/C18H19BrN4O2/c1-22-7-12-4-3-11(22)8-23(12)9-15-20-16-13-6-10(19)2-5-14(13)25-17(16)18(24)21-15/h2,5-6,11-12H,3-4,7-9H2,1H3,(H,20,21,24)/t11-,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385078
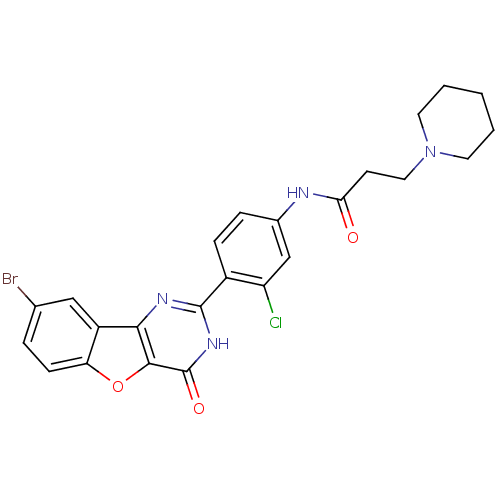
(CHEMBL2035629)Show SMILES Clc1cc(NC(=O)CCN2CCCCC2)ccc1-c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 Show InChI InChI=1S/C24H22BrClN4O3/c25-14-4-7-19-17(12-14)21-22(33-19)24(32)29-23(28-21)16-6-5-15(13-18(16)26)27-20(31)8-11-30-9-2-1-3-10-30/h4-7,12-13H,1-3,8-11H2,(H,27,31)(H,28,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394809
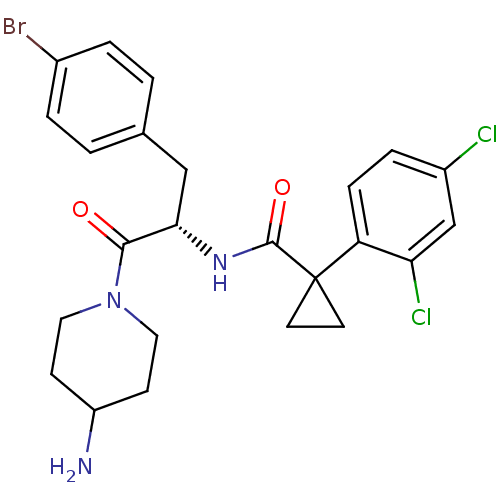
(CHEMBL2163823)Show SMILES NC1CCN(CC1)C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H26BrCl2N3O2/c25-16-3-1-15(2-4-16)13-21(22(31)30-11-7-18(28)8-12-30)29-23(32)24(9-10-24)19-6-5-17(26)14-20(19)27/h1-6,14,18,21H,7-13,28H2,(H,29,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394806

(CHEMBL2163826)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H28BrCl2N3O2/c27-16-3-1-15(2-4-16)11-23(24(33)32-19-6-7-20(32)14-18(30)13-19)31-25(34)26(9-10-26)21-8-5-17(28)12-22(21)29/h1-5,8,12,18-20,23H,6-7,9-11,13-14,30H2,(H,31,34)/t18?,19-,20+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394816
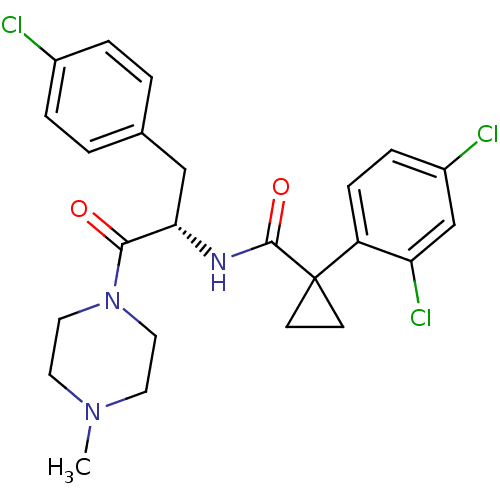
(CHEMBL2163833)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H26Cl3N3O2/c1-29-10-12-30(13-11-29)22(31)21(14-16-2-4-17(25)5-3-16)28-23(32)24(8-9-24)19-7-6-18(26)15-20(19)27/h2-7,15,21H,8-14H2,1H3,(H,28,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data