

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50395423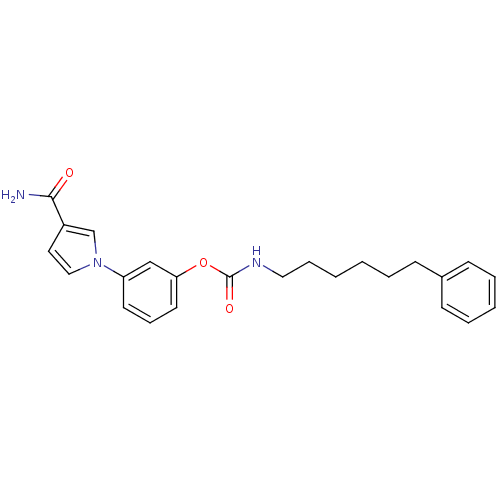 (CHEMBL2165084) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50395424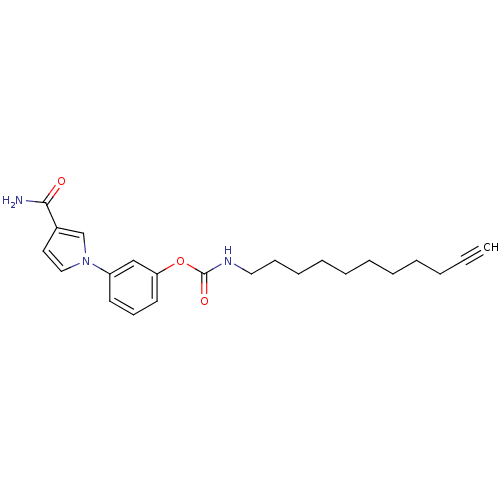 (CHEMBL2165083) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50395421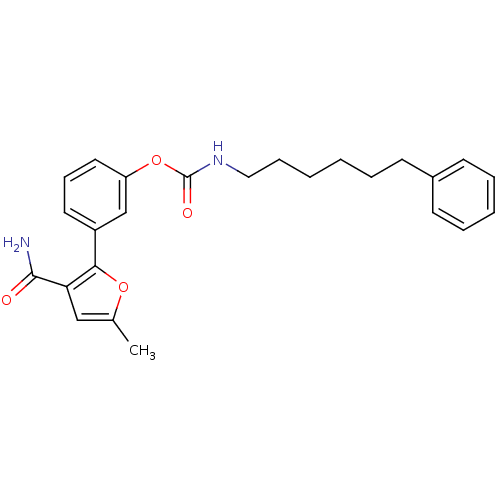 (CHEMBL2165094) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50395420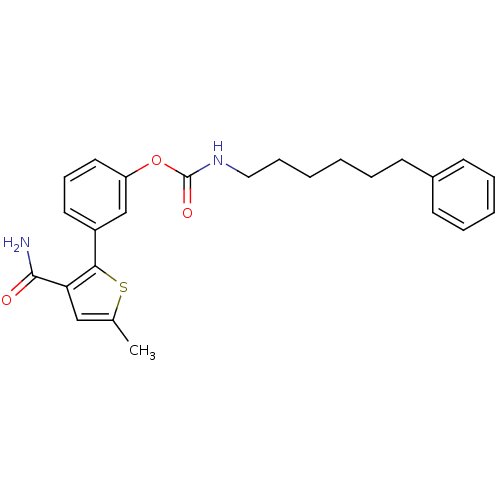 (CHEMBL2165070) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM26739 (3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5-HT7 receptor expressed in HEK293 cells after 30 mins by microbeta counting analysis | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015025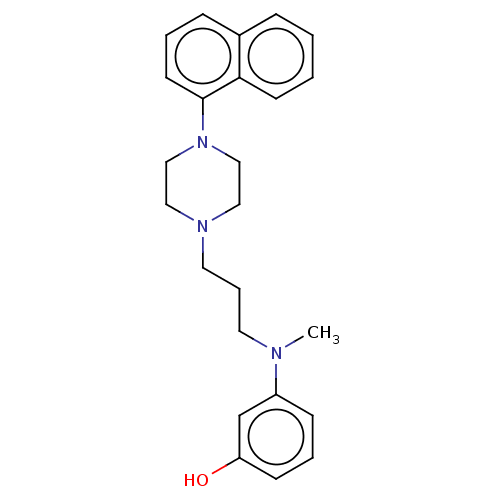 (CHEMBL3262408) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5-HT7 receptor expressed in HEK293 cells after 30 mins by microbeta counting analysis | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015219 (CHEMBL3262410) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5-HT7 receptor expressed in HEK293 cells after 30 mins by microbeta counting analysis | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50395427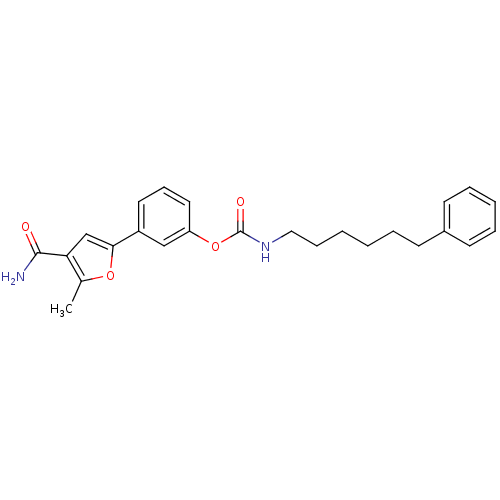 (CHEMBL2165073) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015221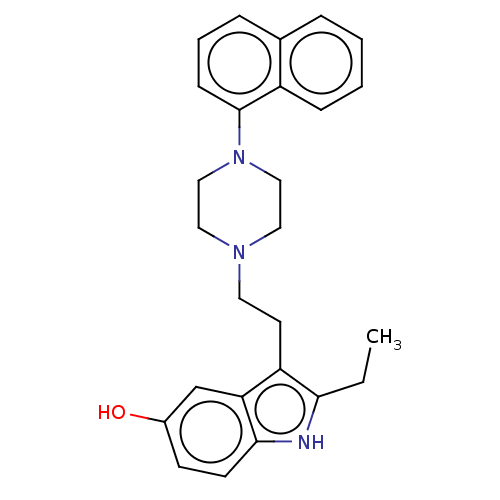 (CHEMBL3262395) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5-HT7 receptor expressed in HEK293 cells after 30 mins by microbeta counting analysis | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50395442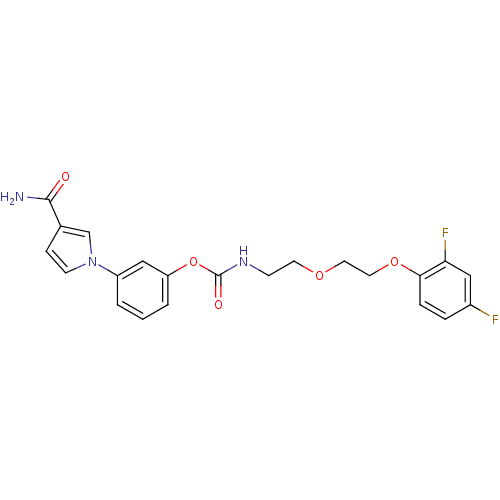 (CHEMBL2165080) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM181012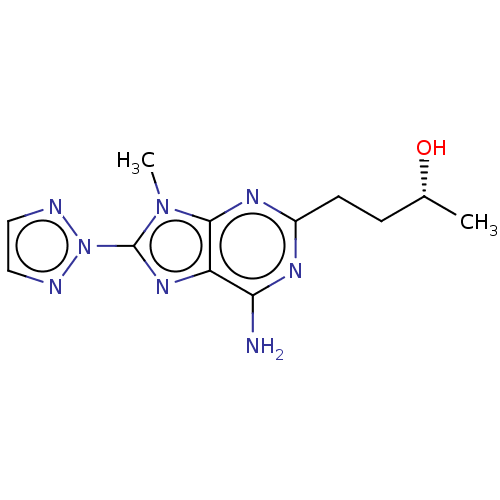 (US9133197, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 1 hr by competitive binding assay | J Med Chem 56: 5456-63 (2013) Article DOI: 10.1021/jm400491x BindingDB Entry DOI: 10.7270/Q2R49TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM181012 (US9133197, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
SIGMA-TAU INDUSTRIE FARMACEUTICHE RIUNITE S.P.A. US Patent | Assay Description The protein concentration of membrane suspension was determined using the Bradford method (Pierce, Rockford, Ill., USA) with bovine albumin as standa... | US Patent US9133197 (2015) BindingDB Entry DOI: 10.7270/Q2542MB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50011300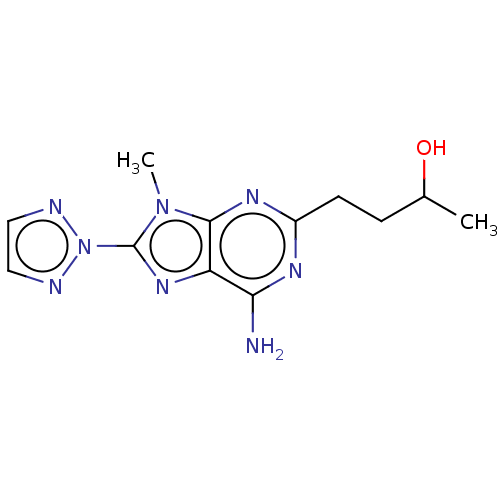 (CHEMBL2398482 | US9133197, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
SIGMA-TAU INDUSTRIE FARMACEUTICHE RIUNITE S.P.A. US Patent | Assay Description The protein concentration of membrane suspension was determined using the Bradford method (Pierce, Rockford, Ill., USA) with bovine albumin as standa... | US Patent US9133197 (2015) BindingDB Entry DOI: 10.7270/Q2542MB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50395444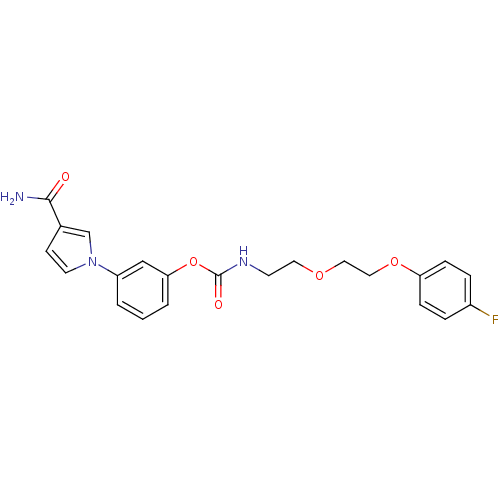 (CHEMBL2165078) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015197 (CHEMBL3262432) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-HT7 receptor expressed in CHO cells after 30 mins by radioligand displacement assay | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015197 (CHEMBL3262432) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5-HT7 receptor expressed in HEK293 cells after 30 mins by microbeta counting analysis | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50011300 (CHEMBL2398482 | US9133197, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 1 hr by competitive binding assay | J Med Chem 56: 5456-63 (2013) Article DOI: 10.1021/jm400491x BindingDB Entry DOI: 10.7270/Q2R49TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50426684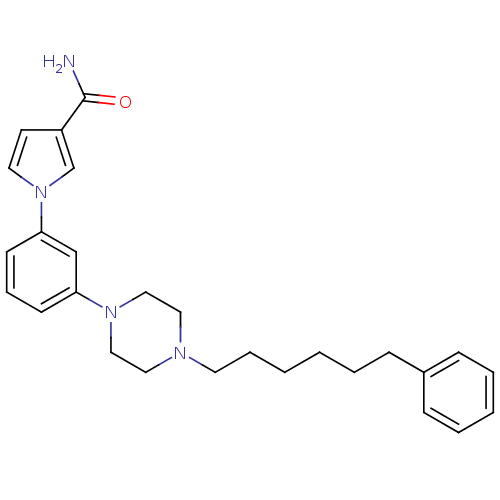 (CHEMBL2326494) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of FAAH in mouse brain homogenate using [3Hethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 23: 492-5 (2012) Article DOI: 10.1016/j.bmcl.2012.11.035 BindingDB Entry DOI: 10.7270/Q22B90BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015024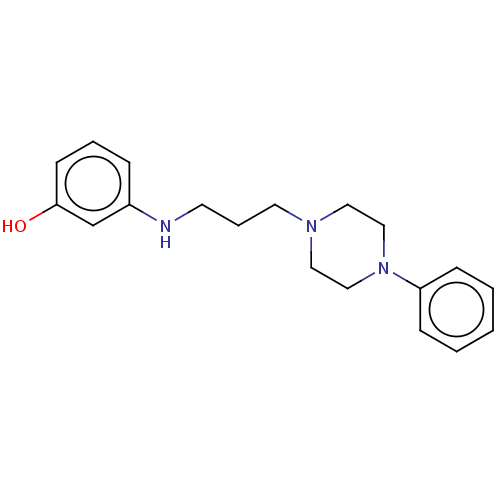 (CHEMBL3262411) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5-HT7 receptor expressed in HEK293 cells after 30 mins by microbeta counting analysis | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50176058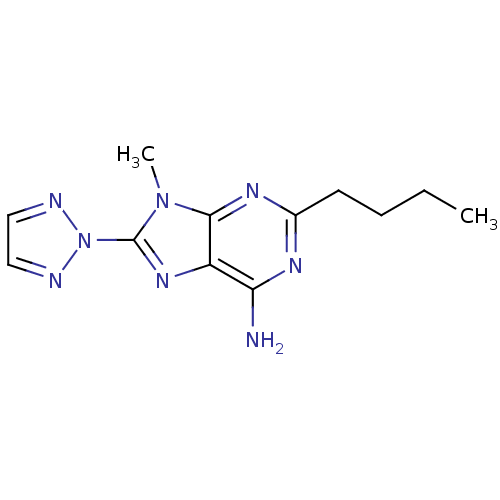 (2-Butyl-9-methyl-8-[1,2,3]triazol-2-yl-9H-purin-6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 1 hr by competitive binding assay | J Med Chem 56: 5456-63 (2013) Article DOI: 10.1021/jm400491x BindingDB Entry DOI: 10.7270/Q2R49TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50395441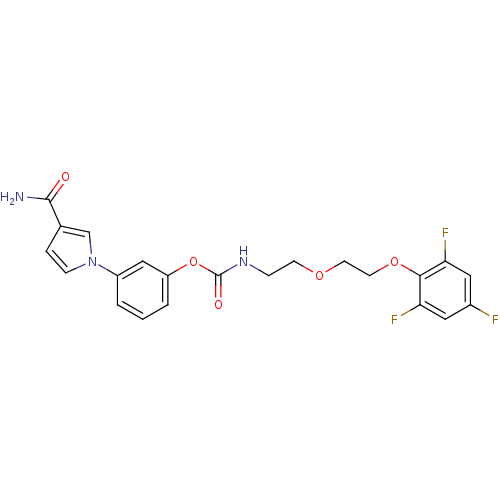 (CHEMBL2165081) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50011301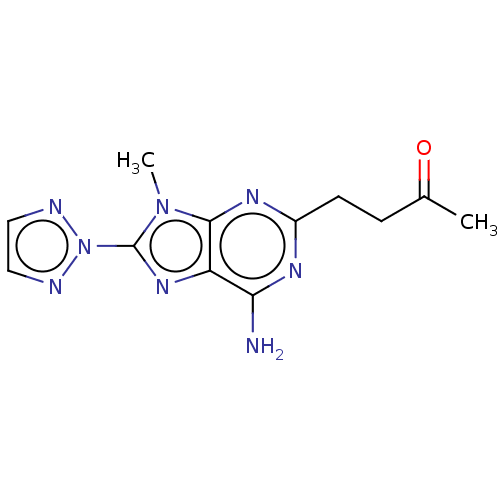 (CHEMBL2398486 | US9133197, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
SIGMA-TAU INDUSTRIE FARMACEUTICHE RIUNITE S.P.A. US Patent | Assay Description The protein concentration of membrane suspension was determined using the Bradford method (Pierce, Rockford, Ill., USA) with bovine albumin as standa... | US Patent US9133197 (2015) BindingDB Entry DOI: 10.7270/Q2542MB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50011301 (CHEMBL2398486 | US9133197, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 1 hr by competitive binding assay | J Med Chem 56: 5456-63 (2013) Article DOI: 10.1021/jm400491x BindingDB Entry DOI: 10.7270/Q2R49TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015220 (CHEMBL3262393) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-HT7 receptor expressed in CHO cells after 30 mins by radioligand displacement assay | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015220 (CHEMBL3262393) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5-HT7 receptor expressed in HEK293 cells after 30 mins by microbeta counting analysis | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015200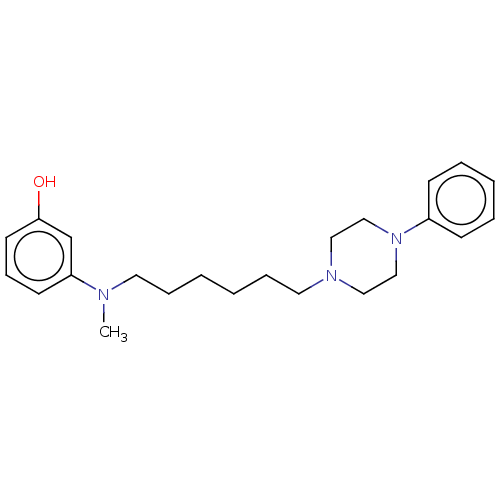 (CHEMBL3262430) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5-HT7 receptor expressed in HEK293 cells after 30 mins by microbeta counting analysis | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015015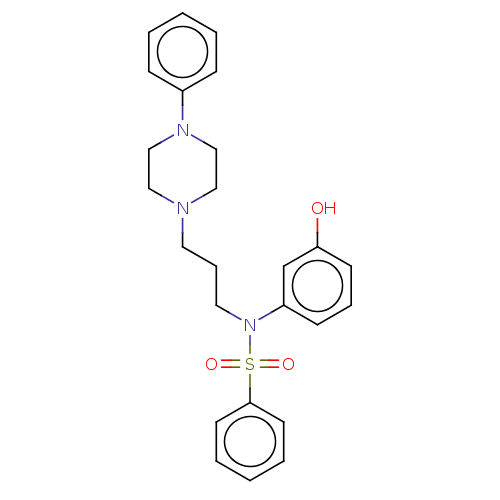 (CHEMBL3262425) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5-HT7 receptor expressed in HEK293 cells after 30 mins by microbeta counting analysis | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015222 (CHEMBL3262394) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5-HT7 receptor expressed in HEK293 cells after 30 mins by microbeta counting analysis | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50426683 (CHEMBL2326500) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of FAAH in mouse brain homogenate using [3Hethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 23: 492-5 (2012) Article DOI: 10.1016/j.bmcl.2012.11.035 BindingDB Entry DOI: 10.7270/Q22B90BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015033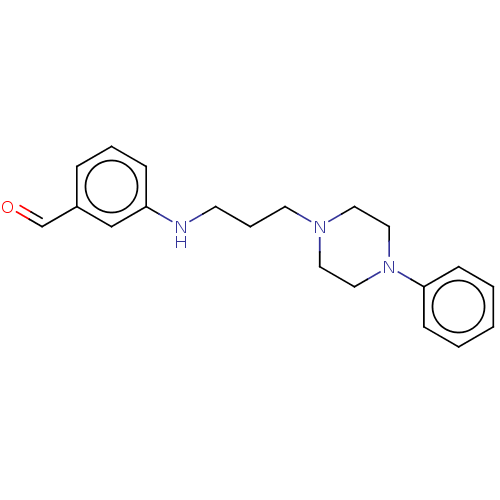 (CHEMBL3262421) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5-HT7 receptor expressed in HEK293 cells after 30 mins by microbeta counting analysis | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015199 (CHEMBL3262429) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5-HT7 receptor expressed in HEK293 cells after 30 mins by microbeta counting analysis | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50395443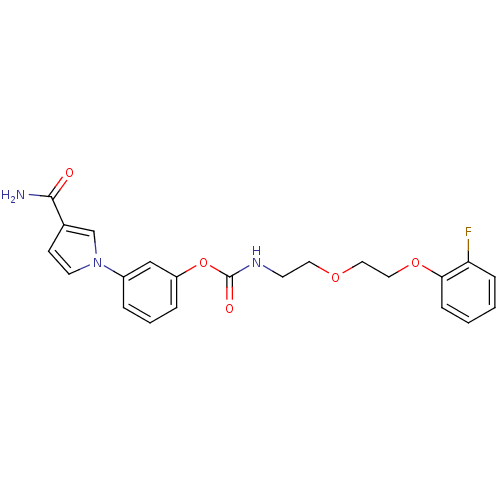 (CHEMBL2165079) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50047166 (8-[(E)-2-(3,4-Dimethoxy-phenyl)-vinyl]-1,3,7-trime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Parma Curated by ChEMBL | Assay Description Binding affinity to A2A receptor in rat brain striatal membrane by radioligand displacement assay | J Med Chem 56: 1247-61 (2013) Article DOI: 10.1021/jm301686s BindingDB Entry DOI: 10.7270/Q2SB472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM181011 (US9133197, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 1 hr by competitive binding assay | J Med Chem 56: 5456-63 (2013) Article DOI: 10.1021/jm400491x BindingDB Entry DOI: 10.7270/Q2R49TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM181014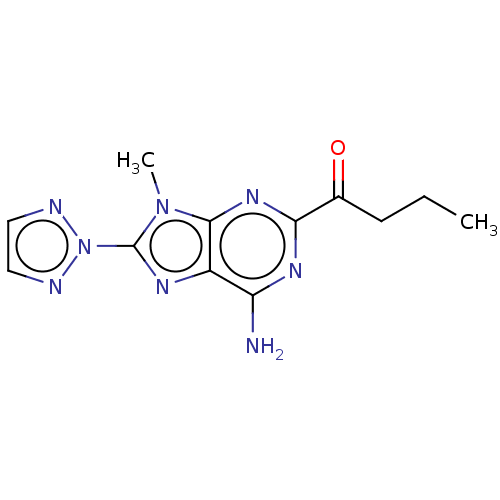 (US9133197, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 19 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
SIGMA-TAU INDUSTRIE FARMACEUTICHE RIUNITE S.P.A. US Patent | Assay Description The protein concentration of membrane suspension was determined using the Bradford method (Pierce, Rockford, Ill., USA) with bovine albumin as standa... | US Patent US9133197 (2015) BindingDB Entry DOI: 10.7270/Q2542MB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM181011 (US9133197, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | 19 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
SIGMA-TAU INDUSTRIE FARMACEUTICHE RIUNITE S.P.A. US Patent | Assay Description The protein concentration of membrane suspension was determined using the Bradford method (Pierce, Rockford, Ill., USA) with bovine albumin as standa... | US Patent US9133197 (2015) BindingDB Entry DOI: 10.7270/Q2542MB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015027 (CHEMBL3262407) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-HT7 receptor expressed in CHO cells after 30 mins by radioligand displacement assay | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015027 (CHEMBL3262407) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5-HT7 receptor expressed in HEK293 cells after 30 mins by microbeta counting analysis | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015012 (CHEMBL3262422) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5-HT7 receptor expressed in HEK293 cells after 30 mins by microbeta counting analysis | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM181013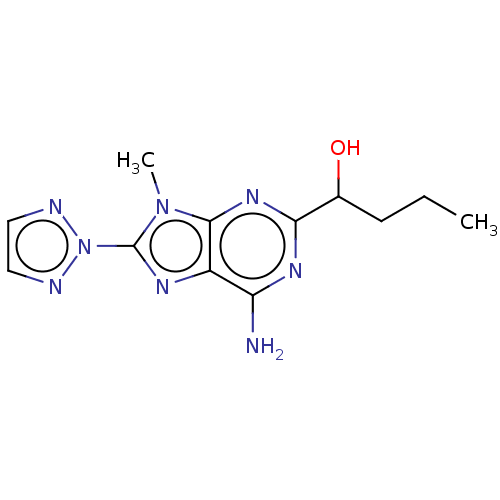 (US9133197, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 1 hr by competitive binding assay | J Med Chem 56: 5456-63 (2013) Article DOI: 10.1021/jm400491x BindingDB Entry DOI: 10.7270/Q2R49TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM181013 (US9133197, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 22 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
SIGMA-TAU INDUSTRIE FARMACEUTICHE RIUNITE S.P.A. US Patent | Assay Description The protein concentration of membrane suspension was determined using the Bradford method (Pierce, Rockford, Ill., USA) with bovine albumin as standa... | US Patent US9133197 (2015) BindingDB Entry DOI: 10.7270/Q2542MB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50395445 (CHEMBL2165077) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426683 (CHEMBL2326500) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of human FAAH using [3Hethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition measured after 15 mins by beta coun... | Bioorg Med Chem Lett 23: 492-5 (2012) Article DOI: 10.1016/j.bmcl.2012.11.035 BindingDB Entry DOI: 10.7270/Q22B90BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50011300 (CHEMBL2398482 | US9133197, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | 27 | -43.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
SIGMA-TAU INDUSTRIE FARMACEUTICHE RIUNITE S.P.A. US Patent | Assay Description Competition binding experiments have been performed incubating membranes from CHO-K1 cells stably transfected with the human adenosine A1 receptor (c... | US Patent US9133197 (2015) BindingDB Entry DOI: 10.7270/Q2542MB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50015198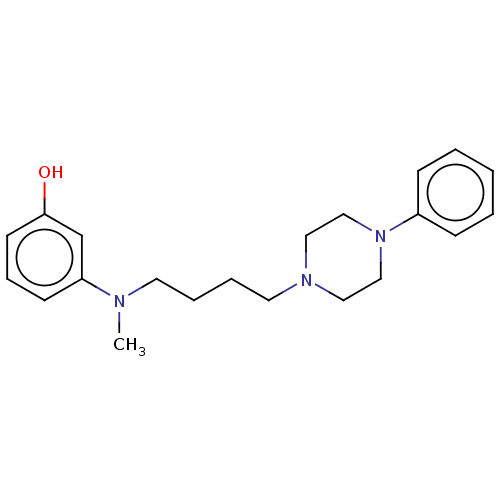 (CHEMBL3262428) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5-HT7 receptor expressed in HEK293 cells after 30 mins by microbeta counting analysis | Eur J Med Chem 80: 8-35 (2014) Article DOI: 10.1016/j.ejmech.2014.04.034 BindingDB Entry DOI: 10.7270/Q2HT2QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50395425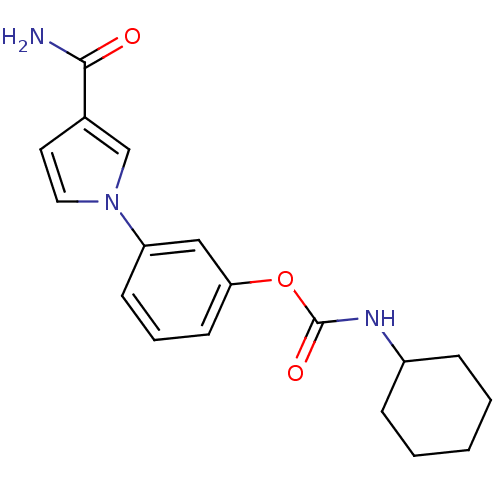 (CHEMBL2165082) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50011300 (CHEMBL2398482 | US9133197, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human recombinant adenosine A1 receptor expressed in CHO-K1 cells after 60 mins | J Med Chem 56: 5456-63 (2013) Article DOI: 10.1021/jm400491x BindingDB Entry DOI: 10.7270/Q2R49TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM181012 (US9133197, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human recombinant adenosine A1 receptor expressed in CHO-K1 cells after 60 mins | J Med Chem 56: 5456-63 (2013) Article DOI: 10.1021/jm400491x BindingDB Entry DOI: 10.7270/Q2R49TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50061950 (8-((E)-2-Benzo[1,3]dioxol-5-yl-vinyl)-3,7-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Parma Curated by ChEMBL | Assay Description Binding affinity to A2A receptor in rat brain striatal membrane by radioligand displacement assay | J Med Chem 56: 1247-61 (2013) Article DOI: 10.1021/jm301686s BindingDB Entry DOI: 10.7270/Q2SB472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1400 total ) | Next | Last >> |