

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50095105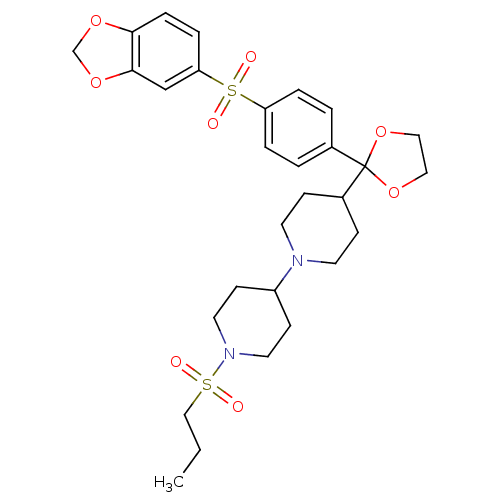 (4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50364330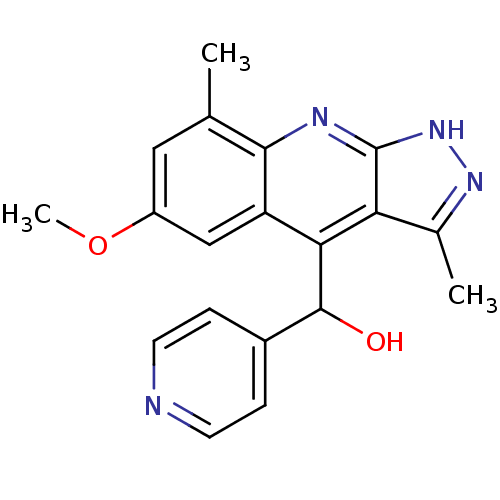 (CHEMBL1949936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 1335-9 (2012) Article DOI: 10.1016/j.bmcl.2011.12.080 BindingDB Entry DOI: 10.7270/Q2RF5VGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50364327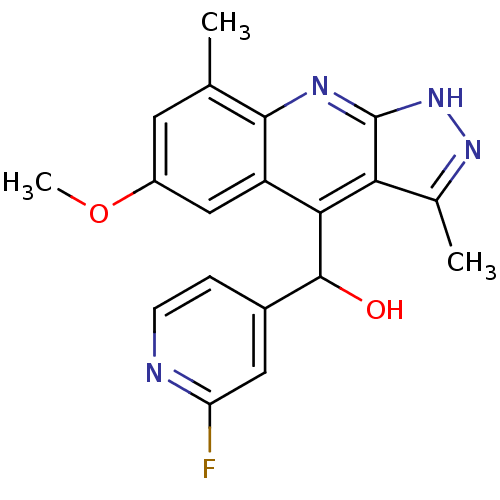 (CHEMBL1949939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 1335-9 (2012) Article DOI: 10.1016/j.bmcl.2011.12.080 BindingDB Entry DOI: 10.7270/Q2RF5VGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202775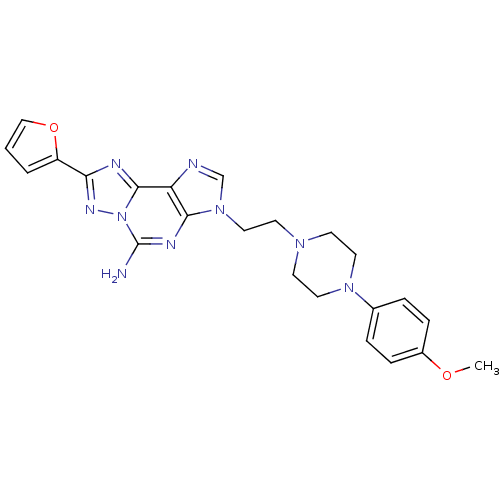 (8-(furan-2-yl)-3-(2-(4-(4-methoxyphenyl)piperazin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103771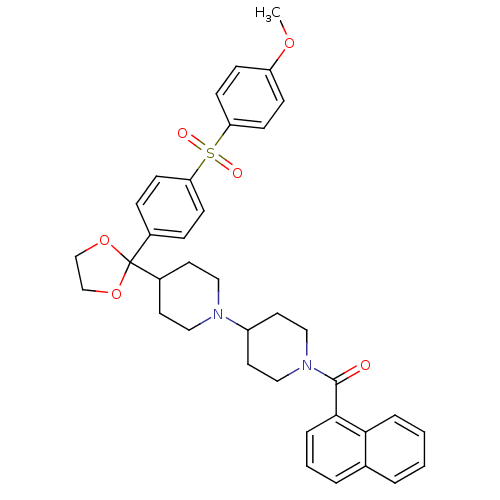 ((4-{2-[4-(4-Methoxy-benzenesulfonyl)-phenyl]-[1,3]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103774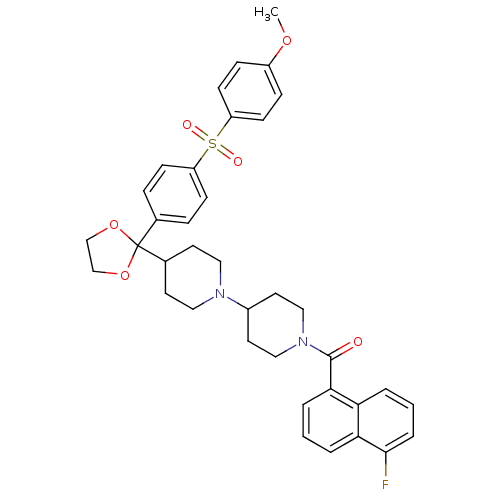 ((5-Fluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50364339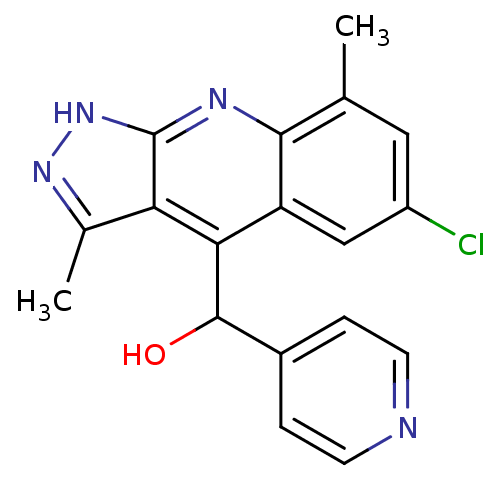 (CHEMBL1950085) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 1335-9 (2012) Article DOI: 10.1016/j.bmcl.2011.12.080 BindingDB Entry DOI: 10.7270/Q2RF5VGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50345941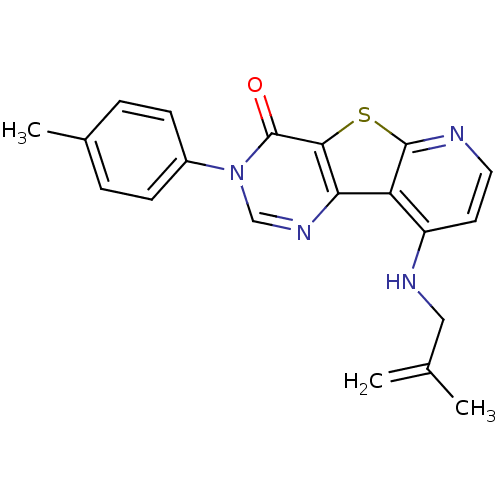 (9-(2-Methyl-allylamino)-3-p-tolyl-3H-pyrido[3',2':...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at rat mGluR1 | Bioorg Med Chem Lett 19: 3199-203 (2009) Article DOI: 10.1016/j.bmcl.2009.04.104 BindingDB Entry DOI: 10.7270/Q27081SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50224406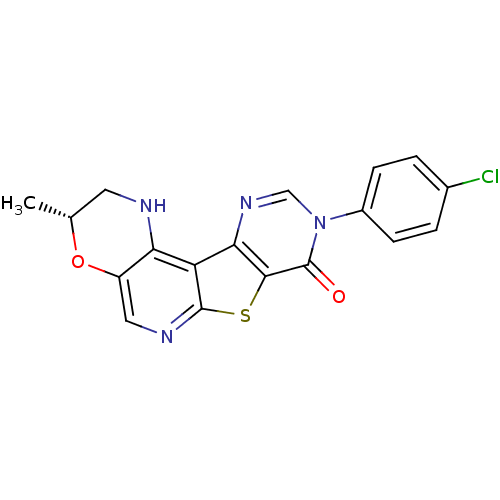 (9-(4-chlorophenyl)-2,3-dihydro-3(R)-methyl-1H-pyri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]9-Dimethylamino-3-(4-methoxy-phenyl)-3H-pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4-one from mGluR1 in rat cerebellum membrane | J Med Chem 50: 5550-3 (2007) Article DOI: 10.1021/jm070590c BindingDB Entry DOI: 10.7270/Q2D50MQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306440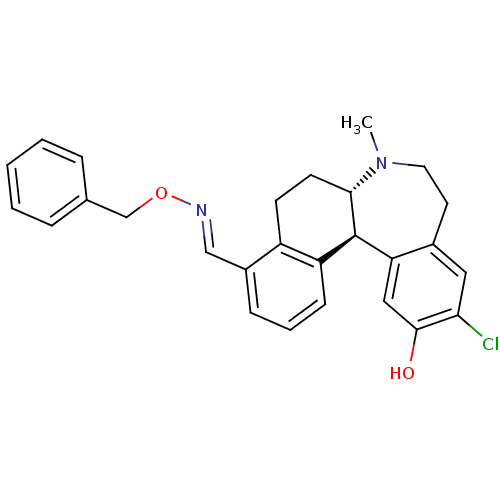 (11-chloro-12-hydroxy-7-methyl-6,6a,7,8,9,13b-hexah...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306442 ((6aS,13bS)-11-chloro-7-methyl-4-phenyl-6,6a,7,8,9,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306314 ((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103772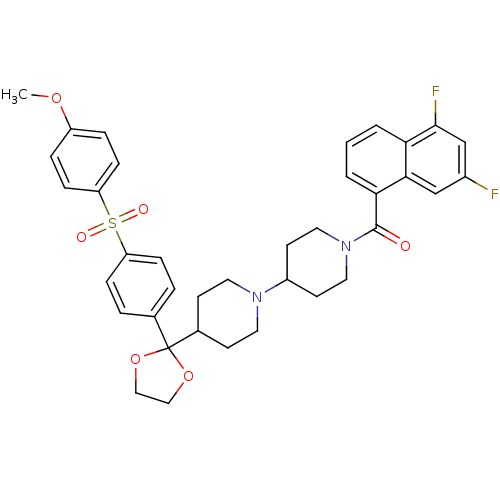 ((5,7-Difluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103767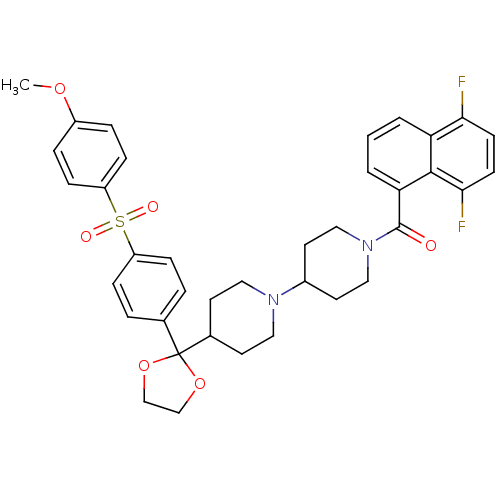 ((5,8-Difluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202777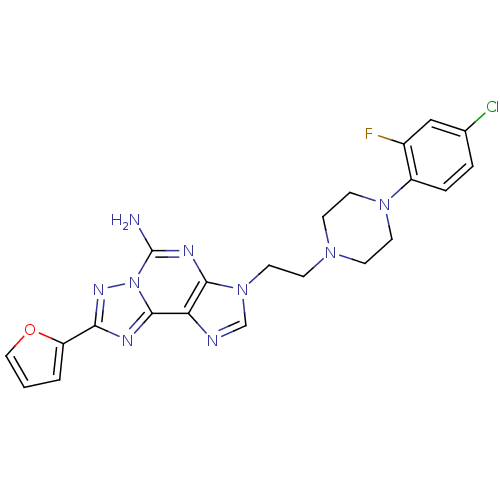 (3-(2-(4-(4-chloro-2-fluorophenyl)piperazin-1-yl)et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50258880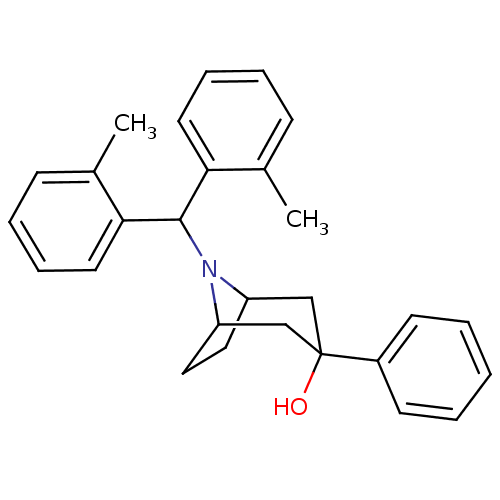 (8-(dio-tolylmethyl)-3-phenyl-8-azabicyclo[3.2.1]oc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human cloned NOP receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 2519-23 (2009) Article DOI: 10.1016/j.bmcl.2009.03.031 BindingDB Entry DOI: 10.7270/Q20V8CPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50364340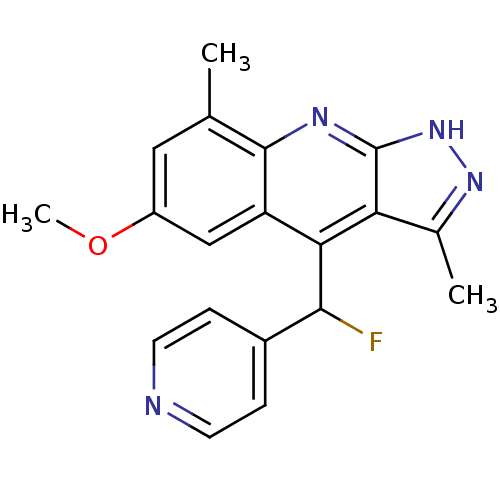 (CHEMBL1950084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 1335-9 (2012) Article DOI: 10.1016/j.bmcl.2011.12.080 BindingDB Entry DOI: 10.7270/Q2RF5VGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50124943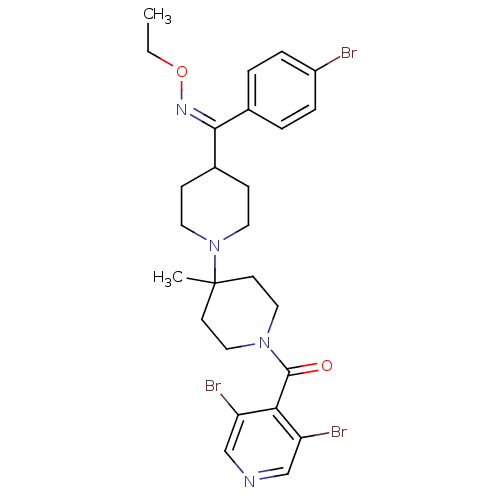 ((4-{(4-Bromo-phenyl)-[(Z)-ethoxyimino]-methyl}-4'-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]RANTES binding to CCR5 receptor. | Bioorg Med Chem Lett 13: 709-12 (2003) BindingDB Entry DOI: 10.7270/Q2VM4CTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50224407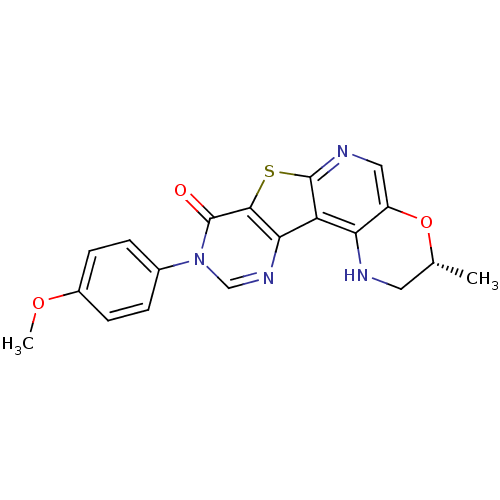 (2,3-dihydro-9-(4-methoxyphenyl)-3(R)-methyl-1H-pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]9-Dimethylamino-3-(4-methoxy-phenyl)-3H-pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4-one from mGluR1 in rat cerebellum membrane | J Med Chem 50: 5550-3 (2007) Article DOI: 10.1021/jm070590c BindingDB Entry DOI: 10.7270/Q2D50MQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50256362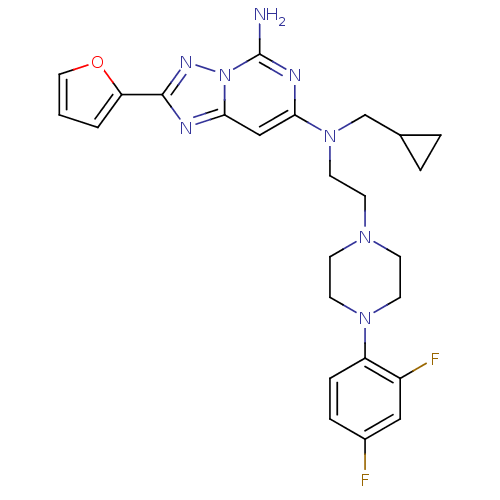 (CHEMBL470942 | N7-(cyclopropylmethyl)-N7-(2-(4-(2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]SCH58261 from human adenosine A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 19: 967-71 (2009) Article DOI: 10.1016/j.bmcl.2008.11.075 BindingDB Entry DOI: 10.7270/Q2CC10KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50256419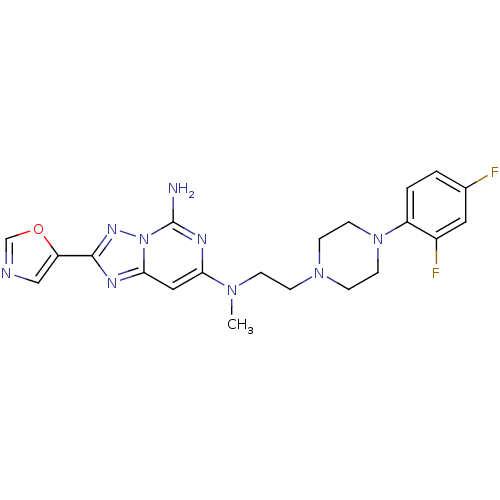 (CHEMBL480542 | N7-(2-(4-(2,4-difluorophenyl)pipera...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]SCH58261 from human adenosine A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 19: 967-71 (2009) Article DOI: 10.1016/j.bmcl.2008.11.075 BindingDB Entry DOI: 10.7270/Q2CC10KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50256492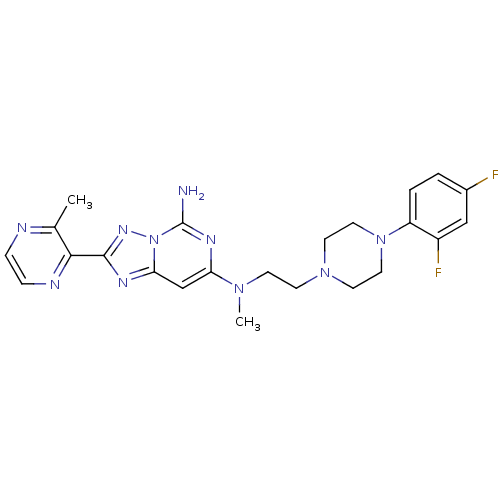 (CHEMBL480570 | N7-(2-(4-(2,4-difluorophenyl)pipera...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]SCH58261 from human adenosine A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 19: 967-71 (2009) Article DOI: 10.1016/j.bmcl.2008.11.075 BindingDB Entry DOI: 10.7270/Q2CC10KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306452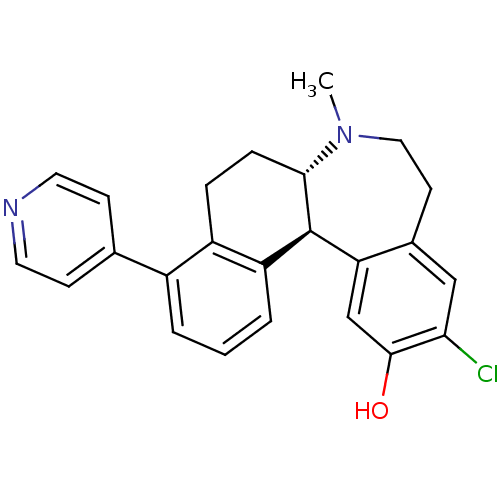 ((6aS,13bS)-11-chloro-7-methyl-4-(pyridin-4-yl)-6,6...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50210235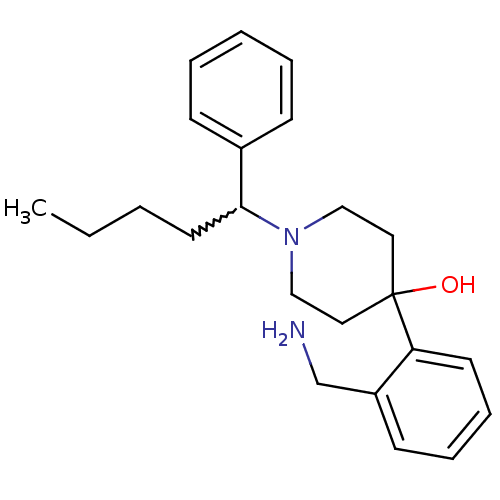 (4-(2-(aminomethyl)phenyl)-1-(1-phenylpentyl)piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin FQ from human NOP receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 3028-33 (2007) Article DOI: 10.1016/j.bmcl.2007.03.062 BindingDB Entry DOI: 10.7270/Q2Z31ZBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202771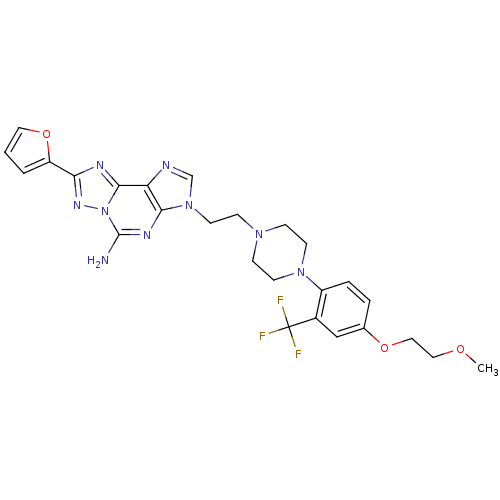 (8-(furan-2-yl)-3-(2-(4-(4-(2-methoxyethoxy)-2-(tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202788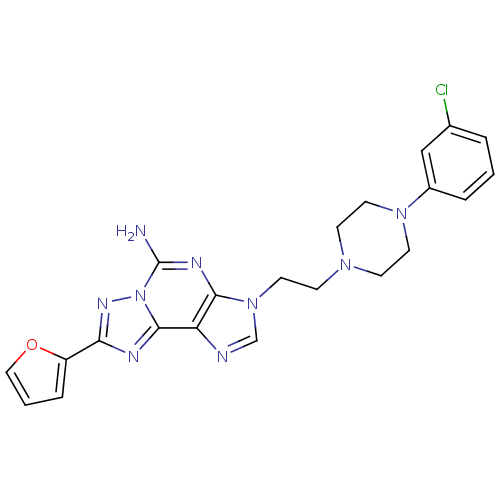 (3-(2-(4-(3-chlorophenyl)piperazin-1-yl)ethyl)-8-(f...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202991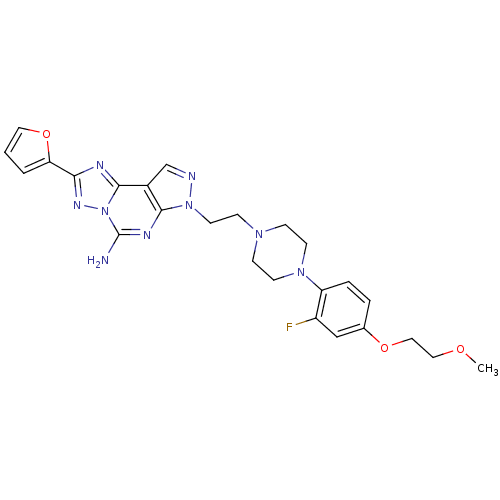 (7-(2-{4-[2-fluoro-4-(2-methoxy-ethoxy)-phenyl]-pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1376-80 (2007) Article DOI: 10.1016/j.bmcl.2006.11.083 BindingDB Entry DOI: 10.7270/Q27H1J8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50373621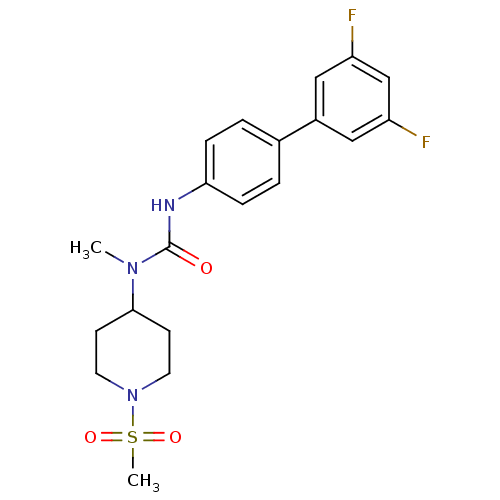 (CHEMBL403414) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125]PYY from human chimeric NPY Y5 receptor expressed in CHOK1 cells | Bioorg Med Chem Lett 18: 1146-50 (2008) Article DOI: 10.1016/j.bmcl.2007.11.132 BindingDB Entry DOI: 10.7270/Q27D2W1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50224391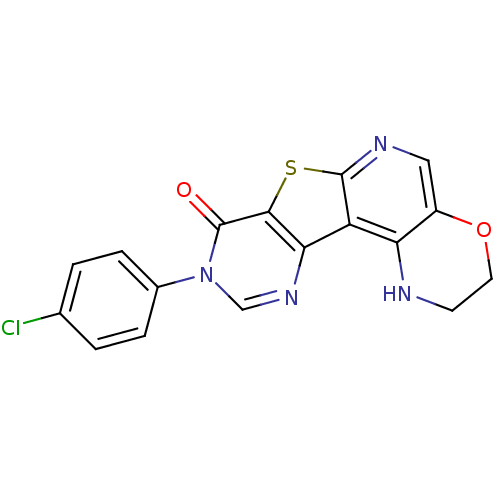 (9-(4-chlorophenyl)-2,3-dihydro-1H-pyrimido[4'',5''...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]9-Dimethylamino-3-(4-methoxy-phenyl)-3H-pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4-one from mGluR1 in rat cerebellum membrane | J Med Chem 50: 5550-3 (2007) Article DOI: 10.1021/jm070590c BindingDB Entry DOI: 10.7270/Q2D50MQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50124953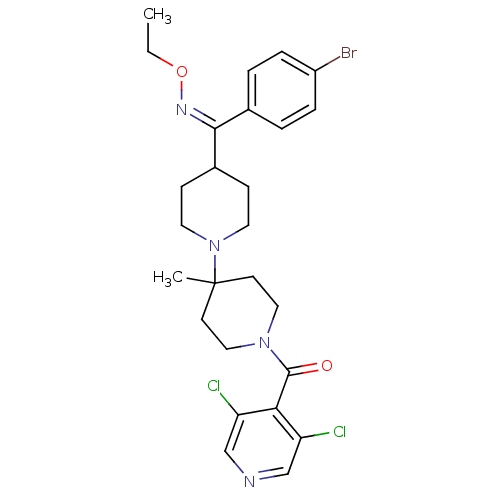 ((4-{(4-Bromo-phenyl)-[(Z)-ethoxyimino]-methyl}-4'-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]RANTES binding to CCR5 receptor. | Bioorg Med Chem Lett 13: 709-12 (2003) BindingDB Entry DOI: 10.7270/Q2VM4CTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50364723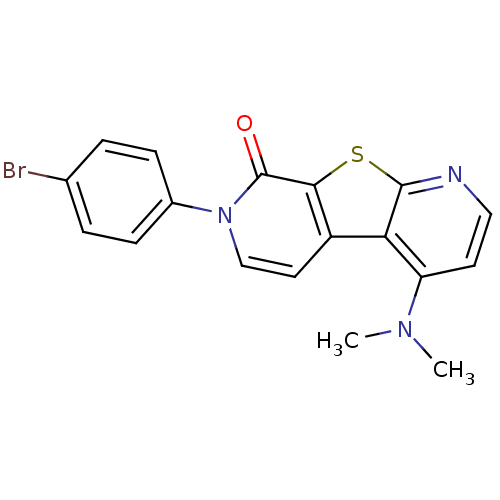 (CHEMBL1951662) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat metabotropic glutamate receptor 1 | Bioorg Med Chem Lett 22: 1575-8 (2012) Article DOI: 10.1016/j.bmcl.2011.12.131 BindingDB Entry DOI: 10.7270/Q2F19063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50345942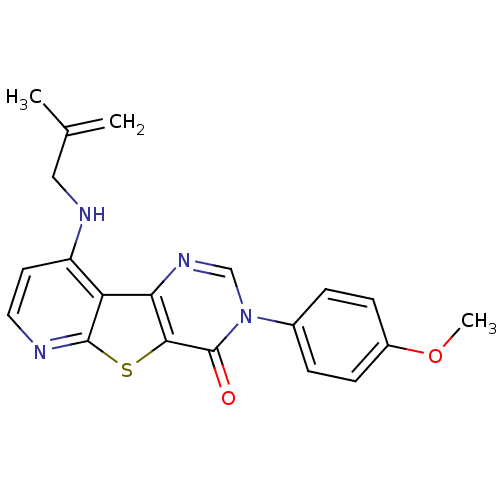 (3-(4-Methoxy-phenyl)-9-(2-methyl-allylamino)-3H-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at rat mGluR1 | Bioorg Med Chem Lett 19: 3199-203 (2009) Article DOI: 10.1016/j.bmcl.2009.04.104 BindingDB Entry DOI: 10.7270/Q27081SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306443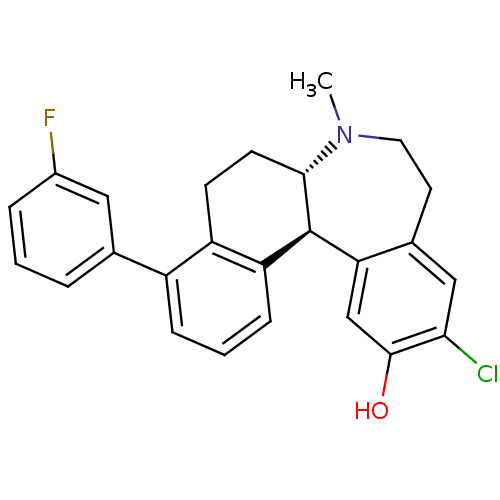 ((6aS,13bS)-11-chloro-4-(3-fluorophenyl)-7-methyl-6...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50364335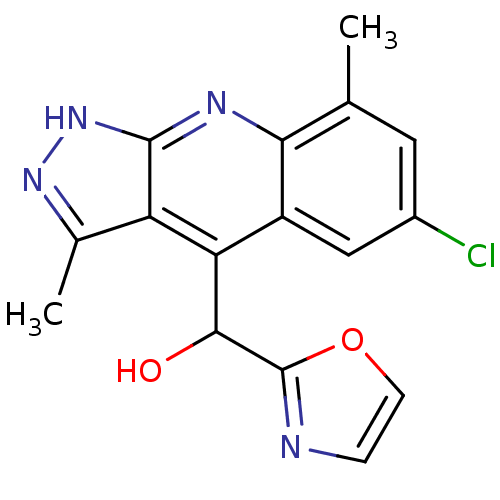 (CHEMBL1950089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 1335-9 (2012) Article DOI: 10.1016/j.bmcl.2011.12.080 BindingDB Entry DOI: 10.7270/Q2RF5VGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50210247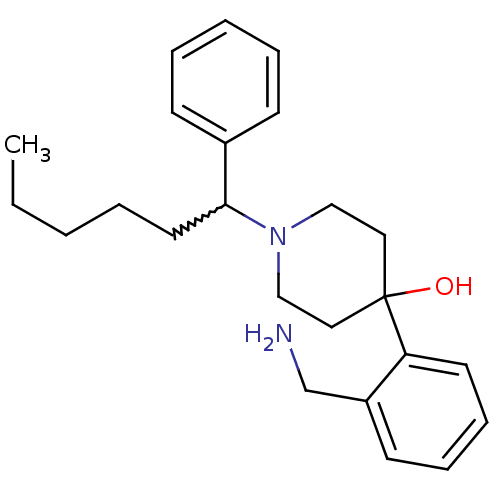 (4-(2-(aminomethyl)phenyl)-1-(1-phenylhexyl)piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin FQ from human NOP receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 3028-33 (2007) Article DOI: 10.1016/j.bmcl.2007.03.062 BindingDB Entry DOI: 10.7270/Q2Z31ZBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50210229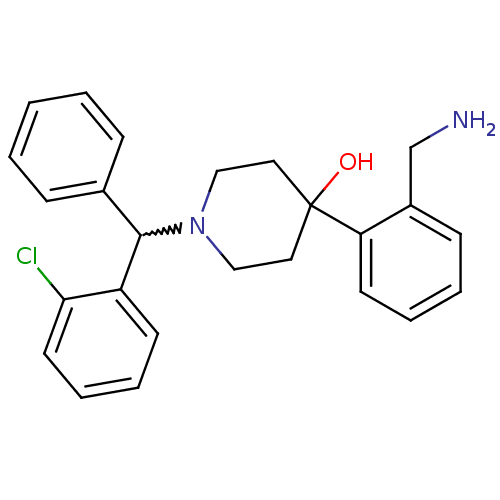 (4-(2-(aminomethyl)phenyl)-1-((2-chlorophenyl)(phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin FQ from human NOP receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 3028-33 (2007) Article DOI: 10.1016/j.bmcl.2007.03.062 BindingDB Entry DOI: 10.7270/Q2Z31ZBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50210212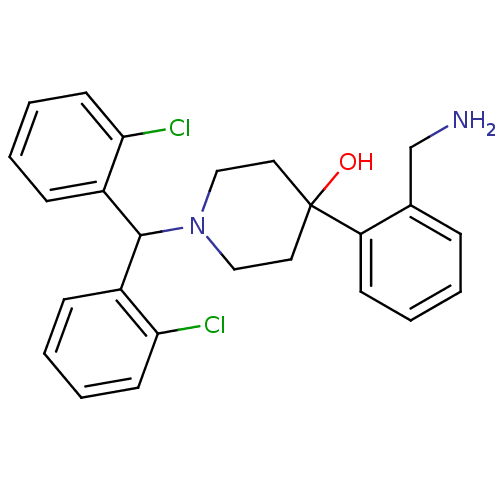 (4-(2-(aminomethyl)phenyl)-1-(bis(2-chlorophenyl)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin FQ from human NOP receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 3028-33 (2007) Article DOI: 10.1016/j.bmcl.2007.03.062 BindingDB Entry DOI: 10.7270/Q2Z31ZBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50210206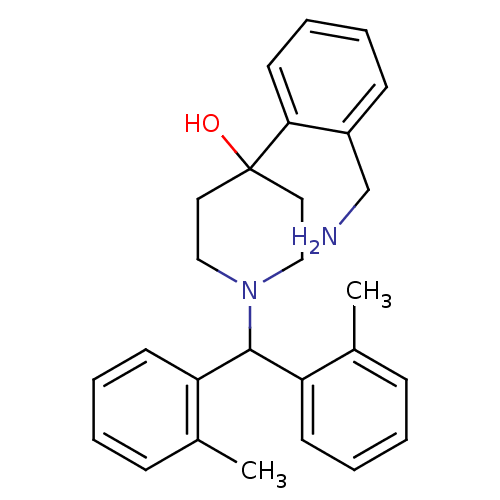 (4-(2-(aminomethyl)phenyl)-1-(dio-tolylmethyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin FQ from human NOP receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 3028-33 (2007) Article DOI: 10.1016/j.bmcl.2007.03.062 BindingDB Entry DOI: 10.7270/Q2Z31ZBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202994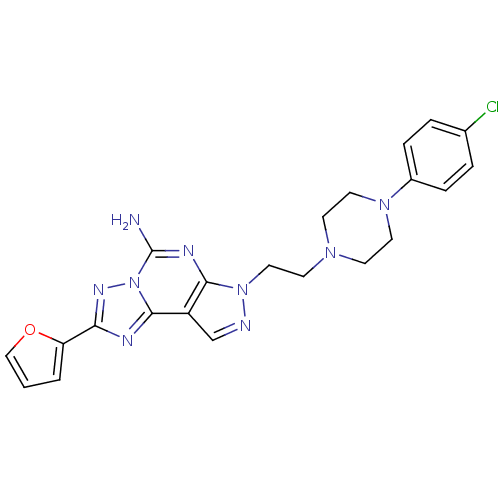 (7-{2-[4-(4-chloro-phenyl)-piperazin-1-yl]-ethyl}-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1376-80 (2007) Article DOI: 10.1016/j.bmcl.2006.11.083 BindingDB Entry DOI: 10.7270/Q27H1J8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50362726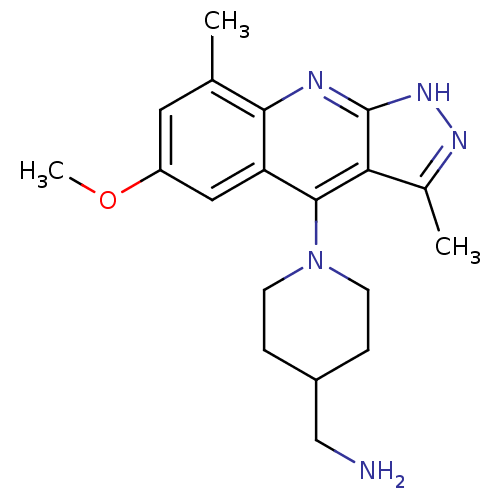 (CHEMBL1939796) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human PDE10A using [3H]cAMP as substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 1019-22 (2012) Article DOI: 10.1016/j.bmcl.2011.11.127 BindingDB Entry DOI: 10.7270/Q2BV7H3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50210242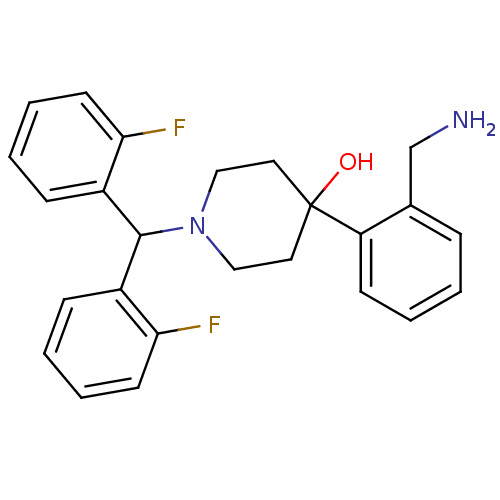 (4-(2-(aminomethyl)phenyl)-1-(bis(2-fluorophenyl)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin FQ from human NOP receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 3028-33 (2007) Article DOI: 10.1016/j.bmcl.2007.03.062 BindingDB Entry DOI: 10.7270/Q2Z31ZBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306316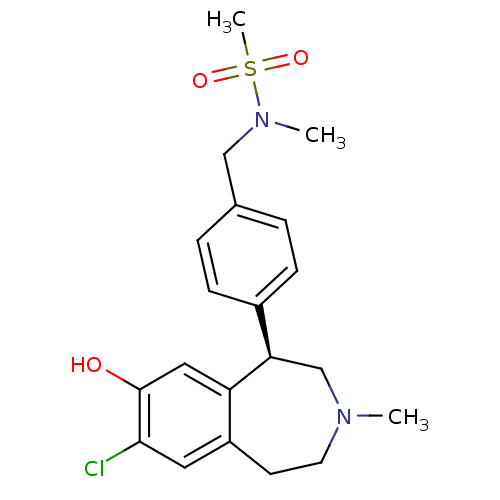 ((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306322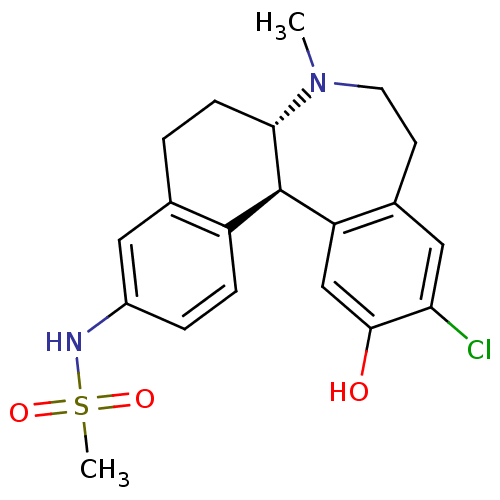 (CHEMBL597909 | N-((6aS,13bR)-11-chloro-12-hydroxy-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50256300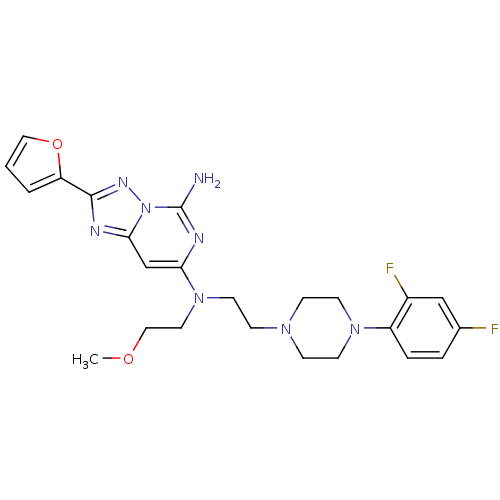 (CHEMBL514939 | N7-(2-(4-(2,4-difluorophenyl)pipera...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]SCH58261 from human adenosine A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 19: 967-71 (2009) Article DOI: 10.1016/j.bmcl.2008.11.075 BindingDB Entry DOI: 10.7270/Q2CC10KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50252010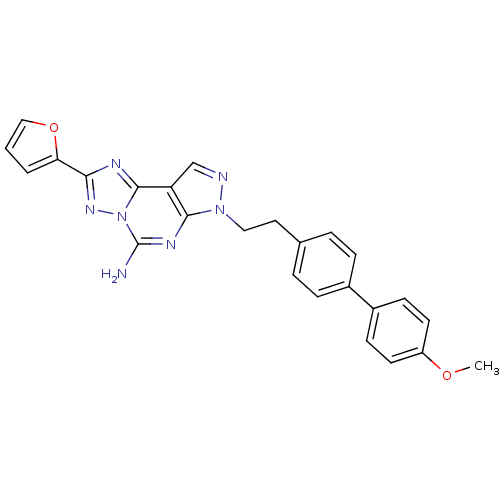 (2-Furan-2-yl-7-[2-(4'-methoxy-biphenyl-4-yl)-ethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 4199-203 (2008) Article DOI: 10.1016/j.bmcl.2008.05.074 BindingDB Entry DOI: 10.7270/Q2X34X89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50224395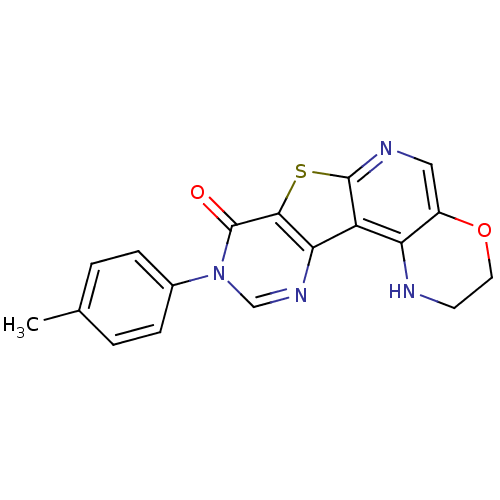 (2,3-dihydro-9-(4-methylphenyl)-1H-pyrimido[4'',5''...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]9-Dimethylamino-3-(4-methoxy-phenyl)-3H-pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4-one from mGluR1 in rat cerebellum membrane | J Med Chem 50: 5550-3 (2007) Article DOI: 10.1021/jm070590c BindingDB Entry DOI: 10.7270/Q2D50MQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50224392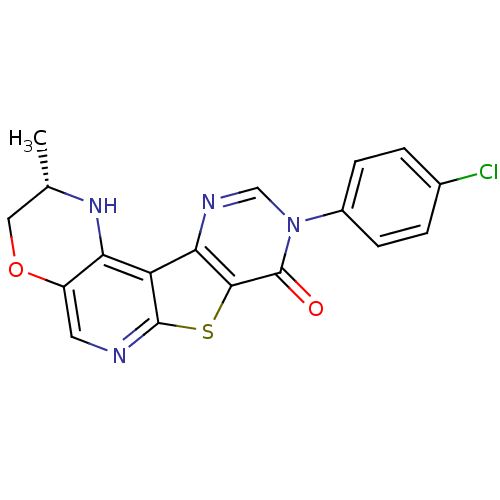 (9-(4-chlorophenyl)-2,3-dihydro-2(S)-methyl-1H-pyri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]9-Dimethylamino-3-(4-methoxy-phenyl)-3H-pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4-one from mGluR1 in rat cerebellum membrane | J Med Chem 50: 5550-3 (2007) Article DOI: 10.1021/jm070590c BindingDB Entry DOI: 10.7270/Q2D50MQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306315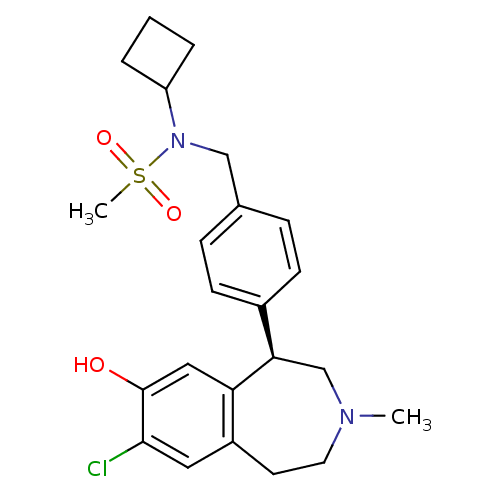 ((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103773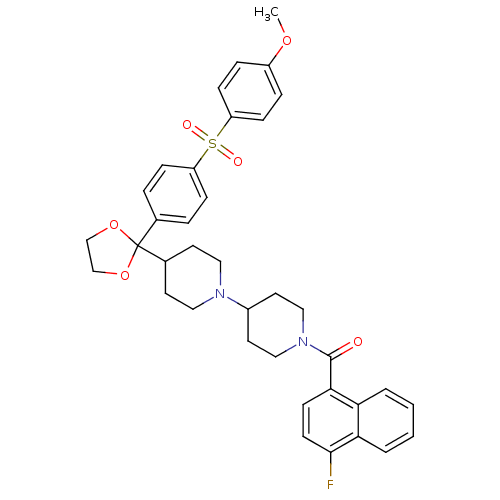 ((4-Fluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for human Muscarinic acetylcholine receptor M2 | J Med Chem 45: 5415-8 (2002) BindingDB Entry DOI: 10.7270/Q2ST7P6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103768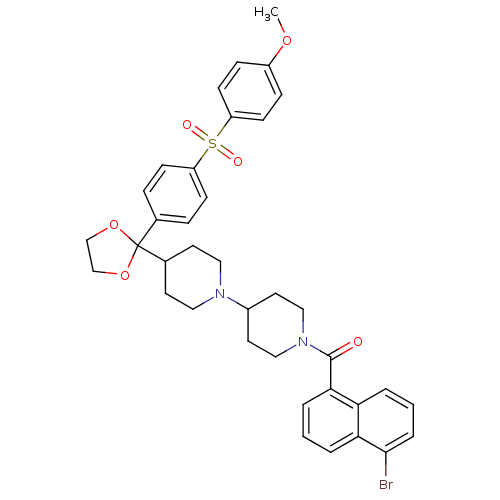 ((5-Bromo-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 6899 total ) | Next | Last >> |