

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094650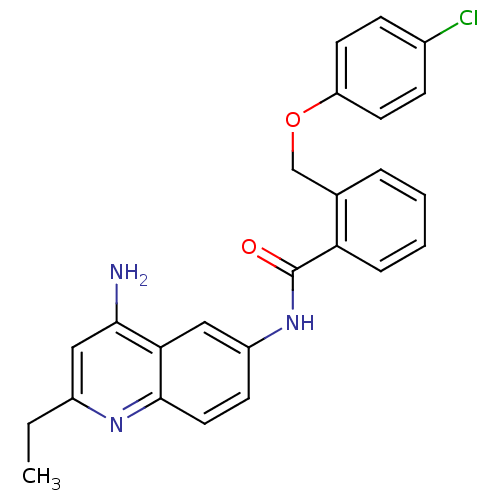 (CHEMBL434060 | N-(4-Amino-2-ethyl-quinolin-6-yl)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094648 (CHEMBL139776 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094640 (CHEMBL140640 | N-(4-Amino-2-propyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094651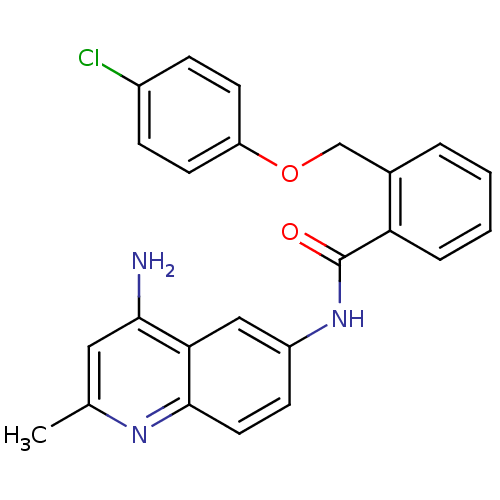 (CHEMBL342580 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094638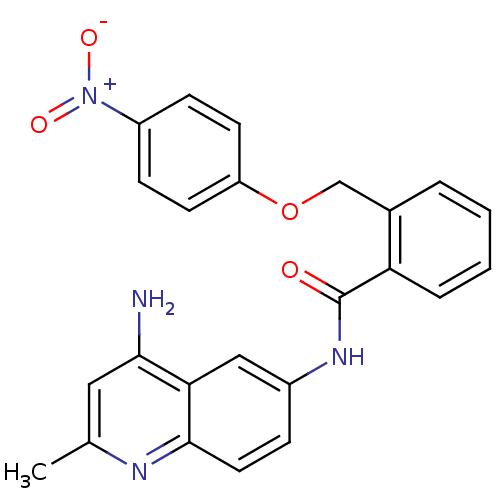 (CHEMBL337128 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094642 (CHEMBL142454 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094646 (CHEMBL139934 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094636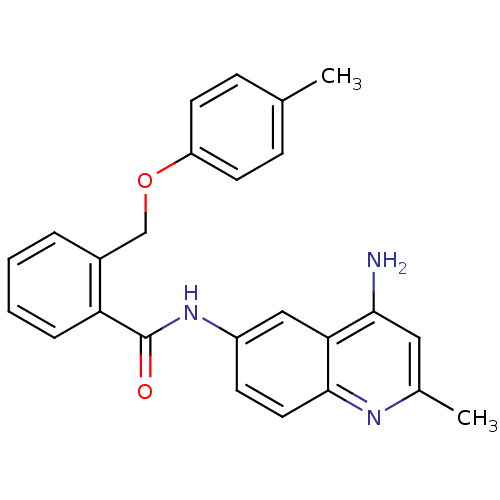 (CHEMBL140103 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094634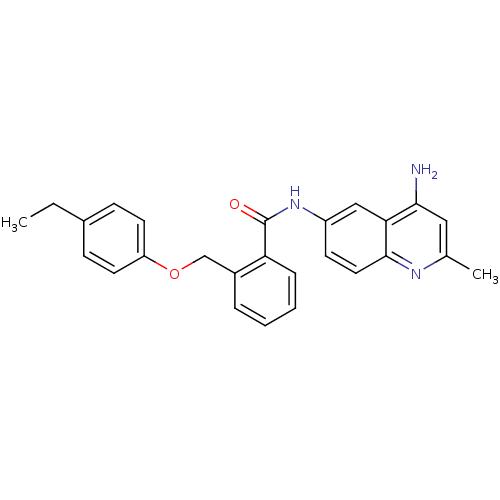 (CHEMBL140979 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay | Bioorg Med Chem 21: 979-92 (2013) Article DOI: 10.1016/j.bmc.2012.11.058 BindingDB Entry DOI: 10.7270/Q23F4R13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094644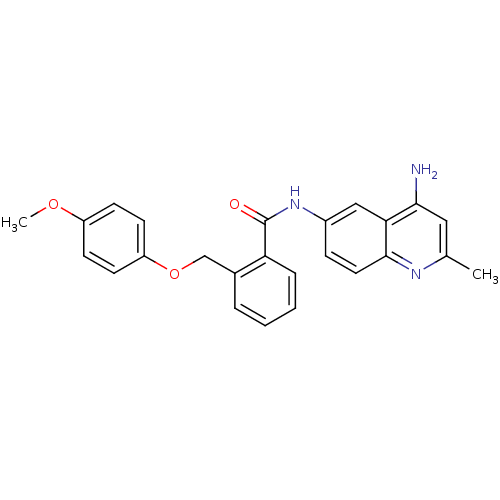 (CHEMBL140519 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094639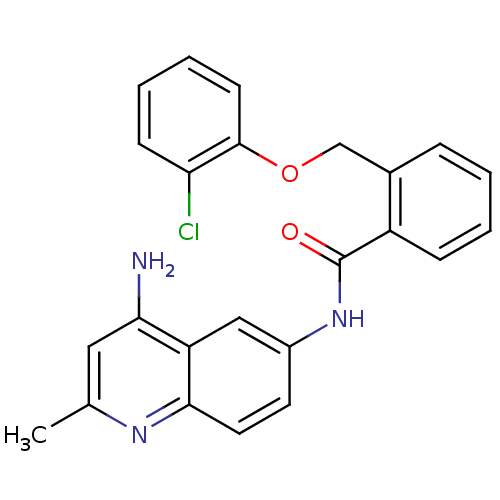 (CHEMBL142999 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094647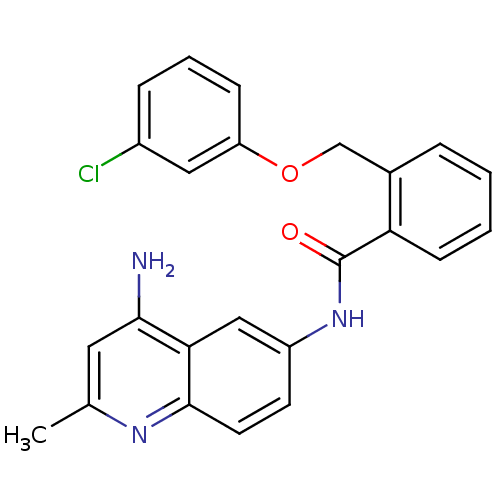 (CHEMBL143605 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094649 (CHEMBL140580 | N-(1-Amino-3-methyl-isoquinolin-7-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094637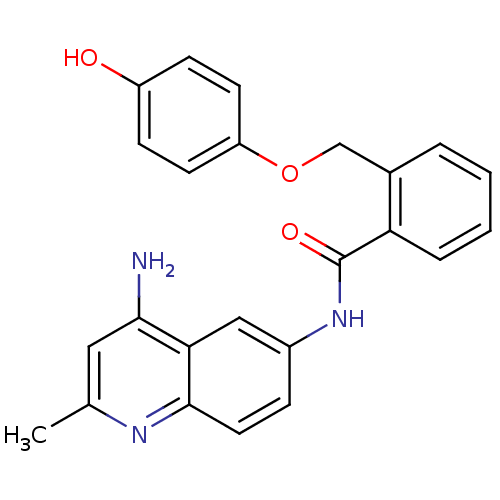 (CHEMBL139566 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094635 (CHEMBL336238 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]-nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50513317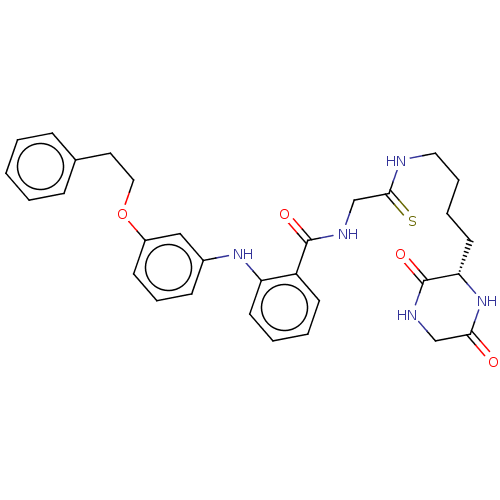 (CHEMBL4465620) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... | J Med Chem 62: 5844-5862 (2019) Article DOI: 10.1021/acs.jmedchem.9b00255 BindingDB Entry DOI: 10.7270/Q2JH3QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094643 (CHEMBL358306 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]-nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094641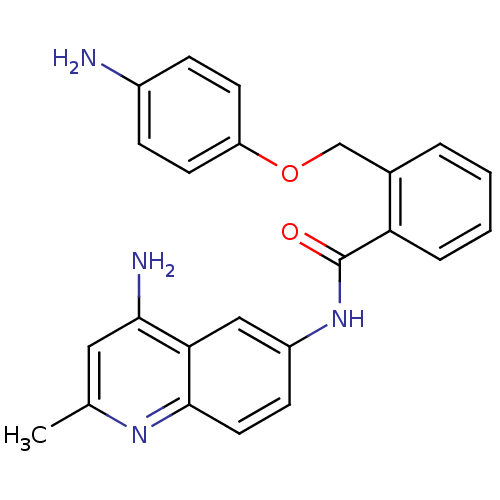 (CHEMBL422641 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094645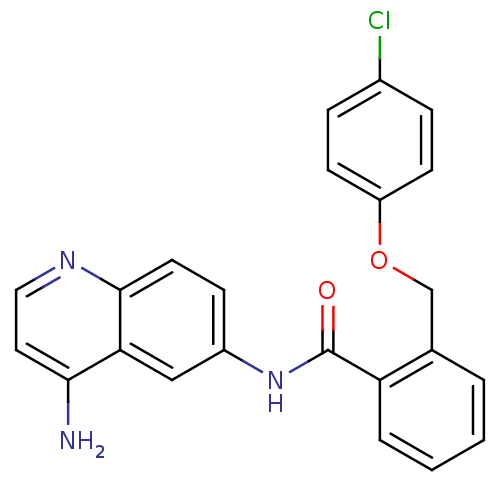 (CHEMBL143243 | N-(4-Amino-quinolin-6-yl)-2-(4-chlo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094652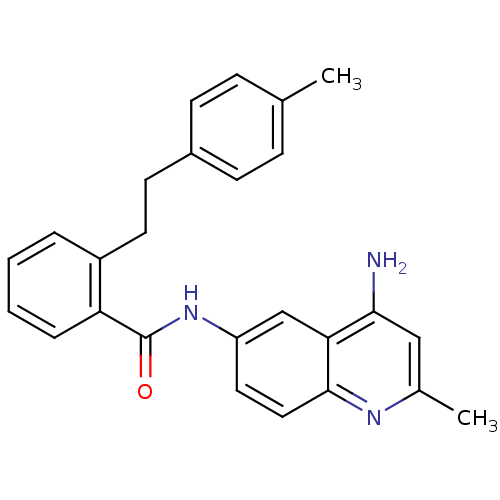 (CHEMBL343424 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50094634 (CHEMBL140979 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine (0.33 nM) binding from human Opioid receptor mu 1 expressed in CHO-K1 cells. | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094653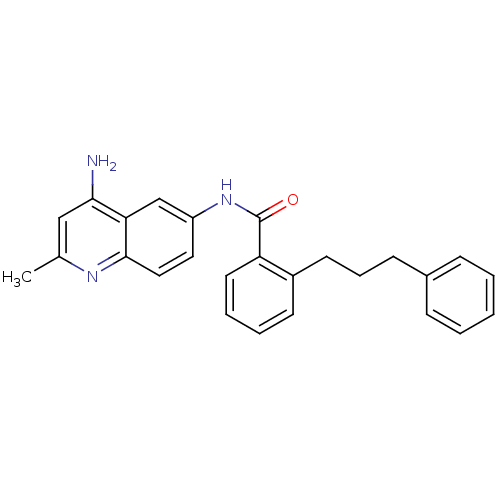 (CHEMBL141078 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094654 (Biphenyl-2-carboxylic acid (4-amino-2-methyl-quino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 369 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50513318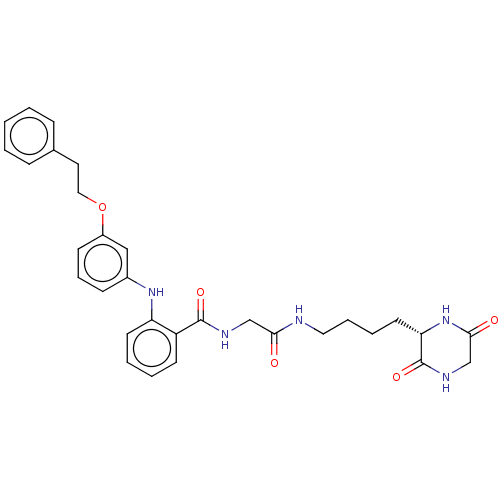 (CHEMBL4516553) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... | J Med Chem 62: 5844-5862 (2019) Article DOI: 10.1021/acs.jmedchem.9b00255 BindingDB Entry DOI: 10.7270/Q2JH3QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50094634 (CHEMBL140979 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]naltrindole (0.55 nM) binding from human Opioid receptor kappa 1 | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50094634 (CHEMBL140979 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 8.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]naltrindole (0.55 nM) binding from human Opioid receptor delta 1 expressed in CHO-K1 cells. | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557765 (CHEMBL4791414) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557761 (CHEMBL4758580) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557764 (CHEMBL4787891) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557766 (CHEMBL4751832) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557753 (CHEMBL4748097) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50428877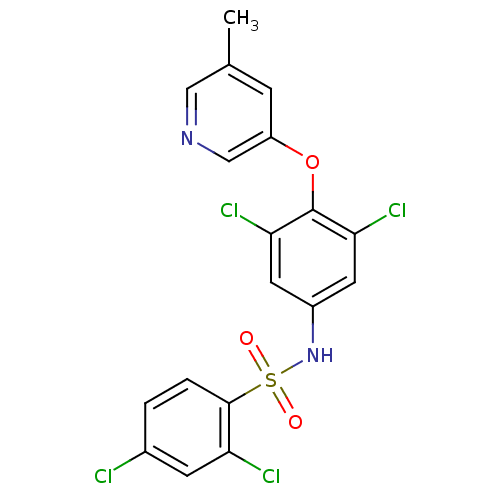 (CHEMBL2338480) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem 21: 979-92 (2013) Article DOI: 10.1016/j.bmc.2012.11.058 BindingDB Entry DOI: 10.7270/Q23F4R13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557742 (CHEMBL4752199) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557760 (CHEMBL4749011) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557756 (CHEMBL4779273) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557755 (CHEMBL4782729) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557744 (CHEMBL4741185) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557763 (CHEMBL4744618) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557767 (CHEMBL4764221) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557746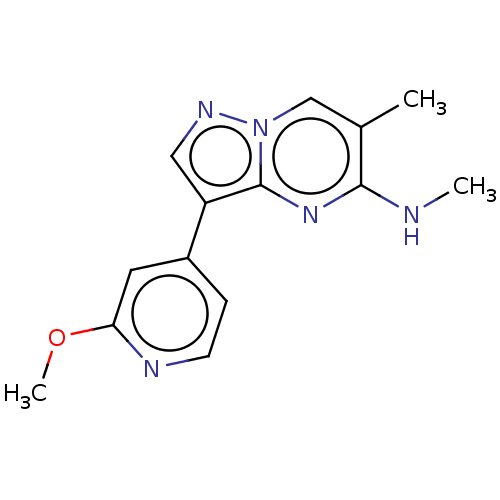 (CHEMBL4779579) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557759 (CHEMBL4763627) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557757 (CHEMBL4792464) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557745 (CHEMBL4741913) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557754 (CHEMBL4764416) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557758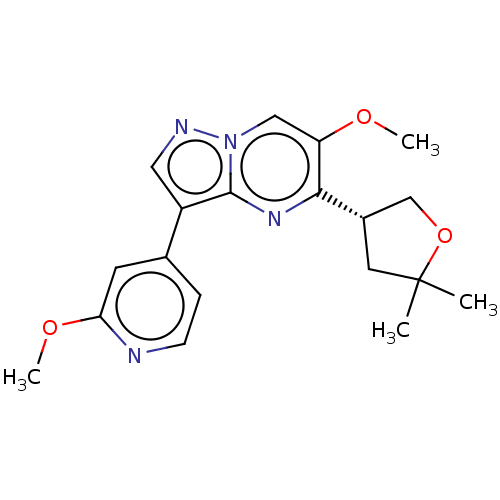 (CHEMBL4749061) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557743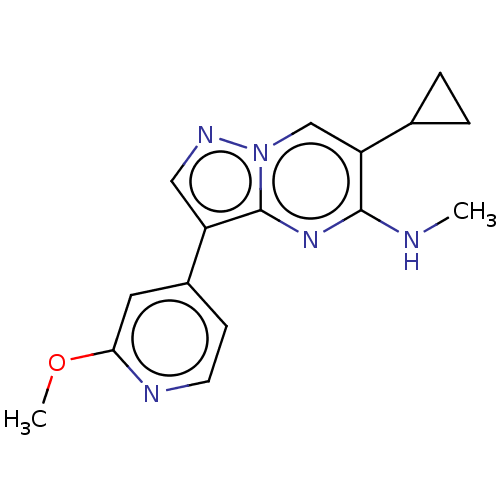 (CHEMBL4744988) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557747 (CHEMBL4795223) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557739 (CHEMBL4798505) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50557762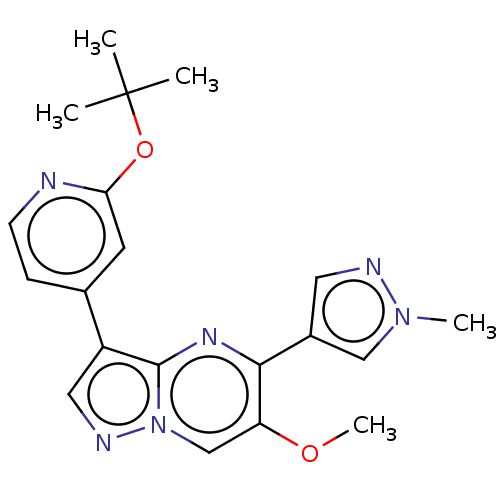 (CHEMBL4747422) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00679 BindingDB Entry DOI: 10.7270/Q2PG1WD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 252 total ) | Next | Last >> |