 Found 740 hits with Last Name = 'sang' and Initial = 'y'
Found 740 hits with Last Name = 'sang' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126960
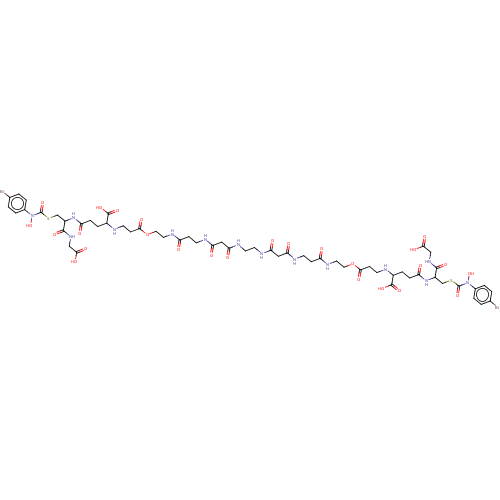
(CHEMBL3629116)Show SMILES ON(C(=O)SCC(NC(=O)CCC(NCCC(=O)OCCNC(=O)CCNC(=O)CC(=O)NCCNC(=O)CC(=O)NCCC(=O)NCCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Br)cc1 Show InChI InChI=1S/C58H78Br2N14O26S2/c59-33-1-5-35(6-2-33)73(97)57(95)101-31-39(53(89)69-29-49(83)84)71-43(77)11-9-37(55(91)92)61-19-15-51(87)99-25-23-67-41(75)13-17-63-45(79)27-47(81)65-21-22-66-48(82)28-46(80)64-18-14-42(76)68-24-26-100-52(88)16-20-62-38(56(93)94)10-12-44(78)72-40(54(90)70-30-50(85)86)32-102-58(96)74(98)36-7-3-34(60)4-8-36/h1-8,37-40,61-62,97-98H,9-32H2,(H,63,79)(H,64,80)(H,65,81)(H,66,82)(H,67,75)(H,68,76)(H,69,89)(H,70,90)(H,71,77)(H,72,78)(H,83,84)(H,85,86)(H,91,92)(H,93,94) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of 6-His tagged recombinant human glyoxalase 1 transfected in Escherichia coli BL21 (DE3) assessed as S-D-lactoylglutathione formation by ... |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His6-tagged Aurora A (62 to 344 residues) (unknown origin) expressed in baculovirus expression system |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126961
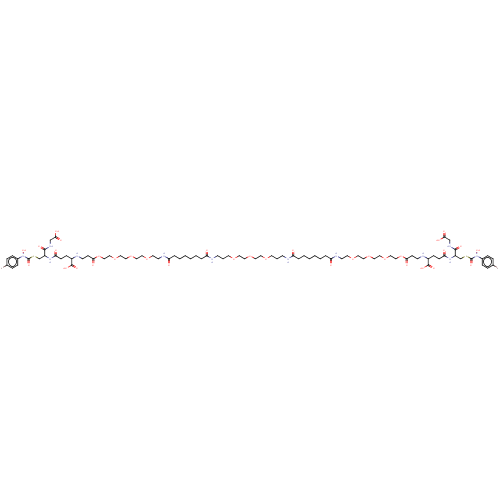
(CHEMBL3629115)Show SMILES ON(C(=O)SCC(NC(=O)CCC(NCCC(=O)OCCOCCOCCOCCNC(=O)CCCCCCC(=O)NCCCOCCOCCOCCCNC(=O)CCCCCCC(=O)NCCOCCOCCOCCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Br)cc1 Show InChI InChI=1S/C82H128Br2N12O33S2/c83-59-15-19-61(20-16-59)95(117)81(115)130-57-65(77(109)91-55-73(103)104)93-71(101)25-23-63(79(111)112)85-31-27-75(107)128-53-51-126-49-47-124-45-41-121-37-33-89-69(99)13-7-3-1-5-11-67(97)87-29-9-35-119-39-43-123-44-40-120-36-10-30-88-68(98)12-6-2-4-8-14-70(100)90-34-38-122-42-46-125-48-50-127-52-54-129-76(108)28-32-86-64(80(113)114)24-26-72(102)94-66(78(110)92-56-74(105)106)58-131-82(116)96(118)62-21-17-60(84)18-22-62/h15-22,63-66,85-86,117-118H,1-14,23-58H2,(H,87,97)(H,88,98)(H,89,99)(H,90,100)(H,91,109)(H,92,110)(H,93,101)(H,94,102)(H,103,104)(H,105,106)(H,111,112)(H,113,114) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of 6-His tagged recombinant human glyoxalase 1 transfected in Escherichia coli BL21 (DE3) assessed as S-D-lactoylglutathione formation by ... |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His6-tagged Aurora C (1 to 309 residues) (unknown origin) expressed in baculovirus expression system |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50092826
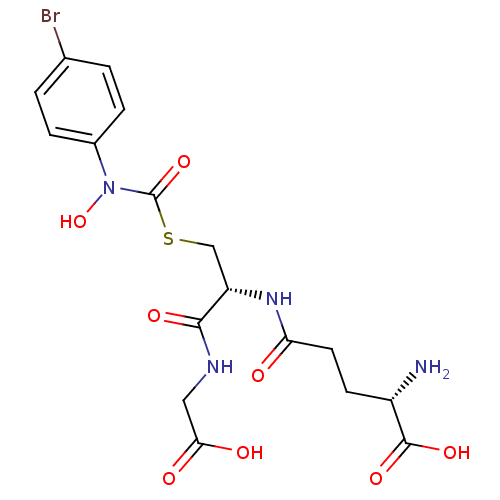
((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H21BrN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His6-tagged Aurora B (1 to 403 residues) (unknown origin) expressed in baculovirus expression system |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126957
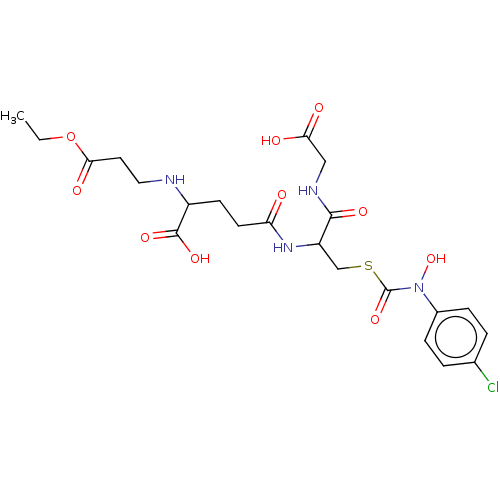
(CHEMBL3629119)Show SMILES CCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C22H29ClN4O10S/c1-2-37-19(31)9-10-24-15(21(33)34)7-8-17(28)26-16(20(32)25-11-18(29)30)12-38-22(35)27(36)14-5-3-13(23)4-6-14/h3-6,15-16,24,36H,2,7-12H2,1H3,(H,25,32)(H,26,28)(H,29,30)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50092824
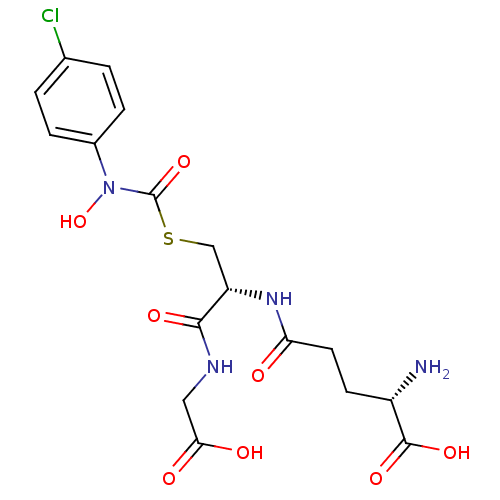
(CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H21ClN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Hydroxyacylglutathione hydrolase, mitochondrial
(Bos taurus) | BDBM50126960

(CHEMBL3629116)Show SMILES ON(C(=O)SCC(NC(=O)CCC(NCCC(=O)OCCNC(=O)CCNC(=O)CC(=O)NCCNC(=O)CC(=O)NCCC(=O)NCCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Br)cc1 Show InChI InChI=1S/C58H78Br2N14O26S2/c59-33-1-5-35(6-2-33)73(97)57(95)101-31-39(53(89)69-29-49(83)84)71-43(77)11-9-37(55(91)92)61-19-15-51(87)99-25-23-67-41(75)13-17-63-45(79)27-47(81)65-21-22-66-48(82)28-46(80)64-18-14-42(76)68-24-26-100-52(88)16-20-62-38(56(93)94)10-12-44(78)72-40(54(90)70-30-50(85)86)32-102-58(96)74(98)36-7-3-34(60)4-8-36/h1-8,37-40,61-62,97-98H,9-32H2,(H,63,79)(H,64,80)(H,65,81)(H,66,82)(H,67,75)(H,68,76)(H,69,89)(H,70,90)(H,71,77)(H,72,78)(H,83,84)(H,85,86)(H,91,92)(H,93,94) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver glyoxalase 2 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126958
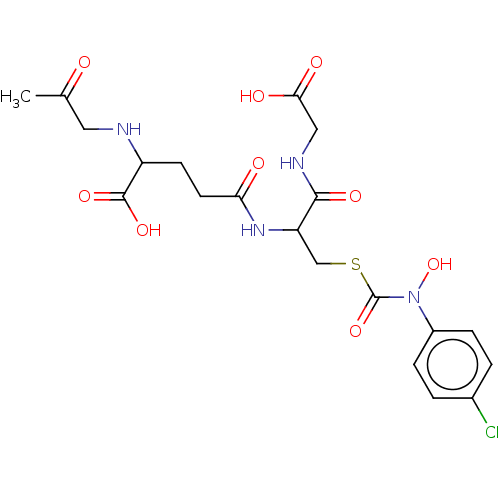
(CHEMBL3629118)Show SMILES CC(=O)CNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C20H25ClN4O9S/c1-11(26)8-22-14(19(31)32)6-7-16(27)24-15(18(30)23-9-17(28)29)10-35-20(33)25(34)13-4-2-12(21)3-5-13/h2-5,14-15,22,34H,6-10H2,1H3,(H,23,30)(H,24,27)(H,28,29)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Hydroxyacylglutathione hydrolase, mitochondrial
(Bos taurus) | BDBM50126961
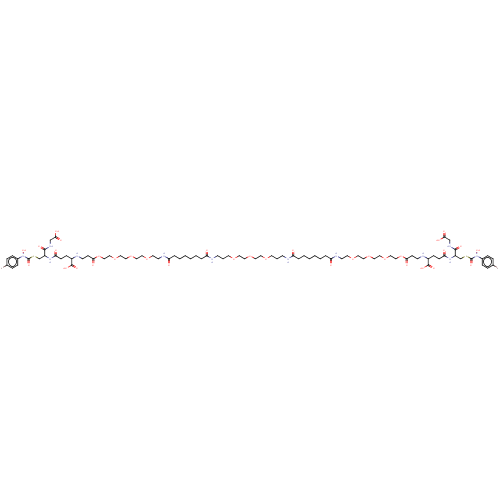
(CHEMBL3629115)Show SMILES ON(C(=O)SCC(NC(=O)CCC(NCCC(=O)OCCOCCOCCOCCNC(=O)CCCCCCC(=O)NCCCOCCOCCOCCCNC(=O)CCCCCCC(=O)NCCOCCOCCOCCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Br)cc1 Show InChI InChI=1S/C82H128Br2N12O33S2/c83-59-15-19-61(20-16-59)95(117)81(115)130-57-65(77(109)91-55-73(103)104)93-71(101)25-23-63(79(111)112)85-31-27-75(107)128-53-51-126-49-47-124-45-41-121-37-33-89-69(99)13-7-3-1-5-11-67(97)87-29-9-35-119-39-43-123-44-40-120-36-10-30-88-68(98)12-6-2-4-8-14-70(100)90-34-38-122-42-46-125-48-50-127-52-54-129-76(108)28-32-86-64(80(113)114)24-26-72(102)94-66(78(110)92-56-74(105)106)58-131-82(116)96(118)62-21-17-60(84)18-22-62/h15-22,63-66,85-86,117-118H,1-14,23-58H2,(H,87,97)(H,88,98)(H,89,99)(H,90,100)(H,91,109)(H,92,110)(H,93,101)(H,94,102)(H,103,104)(H,105,106)(H,111,112)(H,113,114) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver glyoxalase 2 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126959
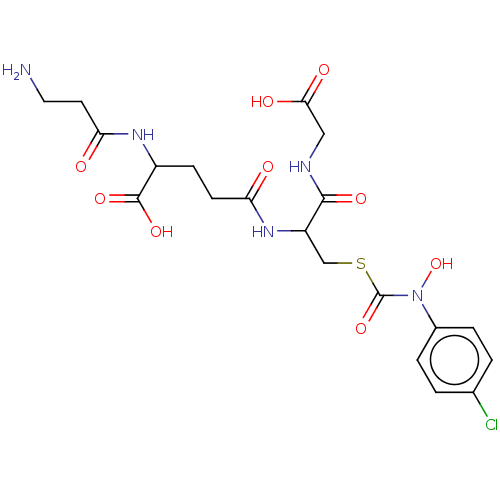
(CHEMBL3629117)Show SMILES NCCC(=O)NC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C20H26ClN5O9S/c21-11-1-3-12(4-2-11)26(35)20(34)36-10-14(18(31)23-9-17(29)30)25-15(27)6-5-13(19(32)33)24-16(28)7-8-22/h1-4,13-14,35H,5-10,22H2,(H,23,31)(H,24,28)(H,25,27)(H,29,30)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50472300
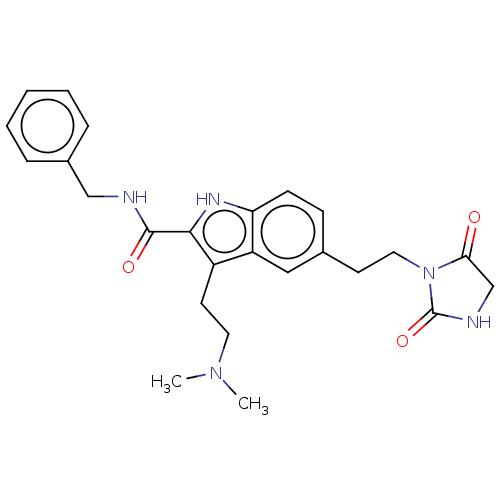
(CHEMBL84165)Show SMILES CN(C)CCc1c([nH]c2ccc(CCN3C(=O)CNC3=O)cc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C25H29N5O3/c1-29(2)12-11-19-20-14-17(10-13-30-22(31)16-27-25(30)33)8-9-21(20)28-23(19)24(32)26-15-18-6-4-3-5-7-18/h3-9,14,28H,10-13,15-16H2,1-2H3,(H,26,32)(H,27,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 1D receptor in calf caudate homogenates. |
J Med Chem 42: 2504-26 (1999)
Article DOI: 10.1021/jm9706325
BindingDB Entry DOI: 10.7270/Q2VD7257 |
More data for this
Ligand-Target Pair | |
Hydroxyacylglutathione hydrolase, mitochondrial
(Bos taurus) | BDBM50092824

(CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H21ClN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver glyoxalase 2 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50472300

(CHEMBL84165)Show SMILES CN(C)CCc1c([nH]c2ccc(CCN3C(=O)CNC3=O)cc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C25H29N5O3/c1-29(2)12-11-19-20-14-17(10-13-30-22(31)16-27-25(30)33)8-9-21(20)28-23(19)24(32)26-15-18-6-4-3-5-7-18/h3-9,14,28H,10-13,15-16H2,1-2H3,(H,26,32)(H,27,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2C receptor in rat cortex homogenates. |
J Med Chem 42: 2504-26 (1999)
Article DOI: 10.1021/jm9706325
BindingDB Entry DOI: 10.7270/Q2VD7257 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50472301
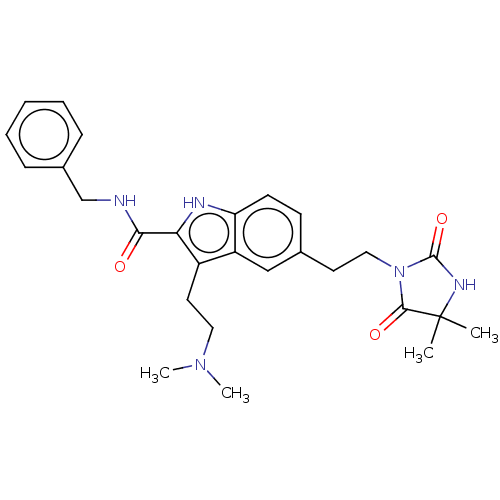
(CHEMBL86223)Show SMILES CN(C)CCc1c([nH]c2ccc(CCN3C(=O)NC(C)(C)C3=O)cc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C27H33N5O3/c1-27(2)25(34)32(26(35)30-27)15-12-18-10-11-22-21(16-18)20(13-14-31(3)4)23(29-22)24(33)28-17-19-8-6-5-7-9-19/h5-11,16,29H,12-15,17H2,1-4H3,(H,28,33)(H,30,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2C receptor in rat cortex homogenates. |
J Med Chem 42: 2504-26 (1999)
Article DOI: 10.1021/jm9706325
BindingDB Entry DOI: 10.7270/Q2VD7257 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM14712
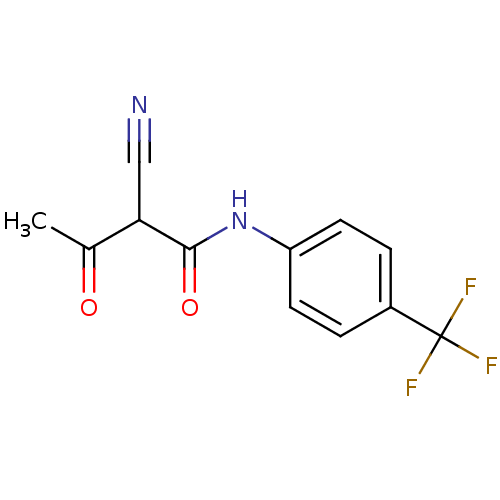
((2Z)-2-cyano-3-hydroxy-N-[4-(trifluoromethyl)pheny...)Show InChI InChI=1S/C12H9F3N2O2/c1-7(18)10(6-16)11(19)17-9-4-2-8(3-5-9)12(13,14)15/h2-5,10H,1H3,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of dihydroorotate dehydrogenase |
J Med Chem 44: 281-97 (2001)
BindingDB Entry DOI: 10.7270/Q2NP2541 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50472301

(CHEMBL86223)Show SMILES CN(C)CCc1c([nH]c2ccc(CCN3C(=O)NC(C)(C)C3=O)cc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C27H33N5O3/c1-27(2)25(34)32(26(35)30-27)15-12-18-10-11-22-21(16-18)20(13-14-31(3)4)23(29-22)24(33)28-17-19-8-6-5-7-9-19/h5-11,16,29H,12-15,17H2,1-4H3,(H,28,33)(H,30,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 1D receptor in calf caudate homogenates. |
J Med Chem 42: 2504-26 (1999)
Article DOI: 10.1021/jm9706325
BindingDB Entry DOI: 10.7270/Q2VD7257 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50227263
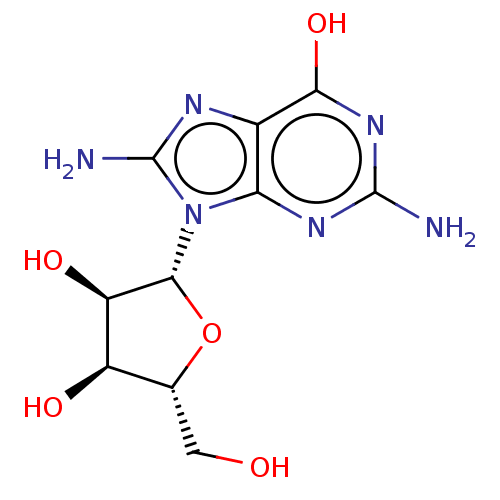
(CHEMBL2021376)Show SMILES Nc1nc2c(O)nc(N)nc2n1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14N6O5/c11-9-14-6-3(7(20)15-9)13-10(12)16(6)8-5(19)4(18)2(1-17)21-8/h2,4-5,8,17-19H,1H2,(H2,12,13)(H3,11,14,15,20)/t2-,4-,5-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nucleic Acid Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against purine nucleoside phosphorylase (PNPase ) |
J Med Chem 31: 330-5 (1988)
BindingDB Entry DOI: 10.7270/Q25H7GV0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for 5-hydroxytryptamine 2C receptor was measured in rat cortex homogenates. |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for Alpha-2 adrenergic receptor was measured in rat cortex homogenates. |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for 5-hydroxytryptamine 2A receptor was measured in rat cortex homogenates |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for 5-hydroxytryptamine 1A receptor was measured in rat cortex homogenates. |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50022506

(5-Amino-1-(3,4-dihydroxy-5-hydroxymethyl-tetrahydr...)Show InChI InChI=1S/C8H14N6O4/c9-5(10)6-12-8(11)14(13-6)7-4(17)3(16)2(1-15)18-7/h2-4,7,15-17H,1H2,(H3,9,10)(H2,11,12,13)/t2?,3?,4-,7?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nucleic Acid Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against purine nucleoside phosphorylase (PNPase ) |
J Med Chem 31: 330-5 (1988)
BindingDB Entry DOI: 10.7270/Q25H7GV0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for 5-hydroxytryptamine 1D receptor was measured in calf caudate homogenate |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for Dopamine receptor D2 was measured in rat cortex homogenates |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for Alpha-1 adrenergic receptor was measured in rat cortex homogenates |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50472301

(CHEMBL86223)Show SMILES CN(C)CCc1c([nH]c2ccc(CCN3C(=O)NC(C)(C)C3=O)cc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C27H33N5O3/c1-27(2)25(34)32(26(35)30-27)15-12-18-10-11-22-21(16-18)20(13-14-31(3)4)23(29-22)24(33)28-17-19-8-6-5-7-9-19/h5-11,16,29H,12-15,17H2,1-4H3,(H,28,33)(H,30,35) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 1A receptor in rat cortex homogenates. |
J Med Chem 42: 2504-26 (1999)
Article DOI: 10.1021/jm9706325
BindingDB Entry DOI: 10.7270/Q2VD7257 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50367673
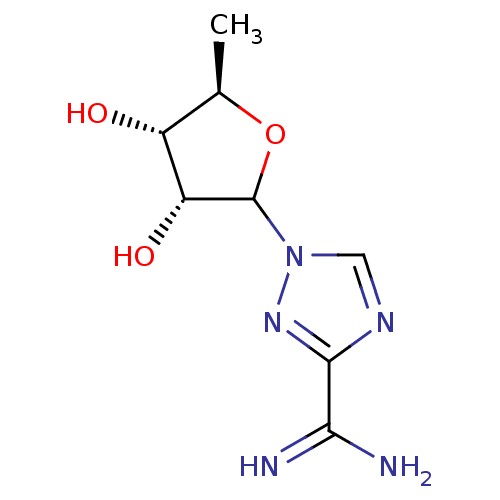
(CHEMBL608050)Show SMILES C[C@H]1OC([C@H](O)[C@@H]1O)n1cnc(n1)C(N)=N |r| Show InChI InChI=1S/C8H13N5O3/c1-3-4(14)5(15)8(16-3)13-2-11-7(12-13)6(9)10/h2-5,8,14-15H,1H3,(H3,9,10)/t3-,4-,5-,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nucleic Acid Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against purine nucleoside phosphorylase (PNPase ) |
J Med Chem 31: 330-5 (1988)
BindingDB Entry DOI: 10.7270/Q25H7GV0 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50142711
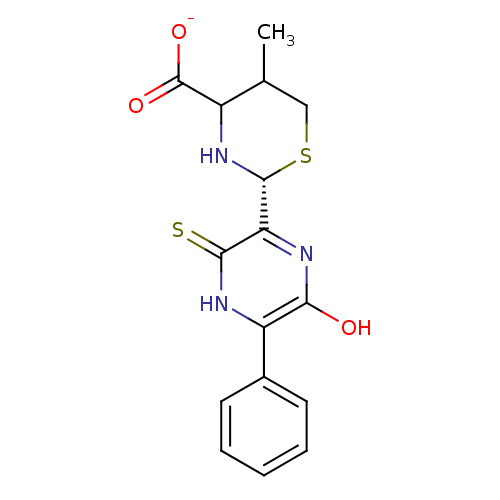
((R)-5-Methyl-2-(6-oxo-5-phenyl-3-thioxo-piperazin-...)Show SMILES CC1CS[C@@H](NC1C([O-])=O)c1nc(O)c([nH]c1=S)-c1ccccc1 Show InChI InChI=1S/C16H17N3O3S2/c1-8-7-24-15(19-10(8)16(21)22)12-14(23)18-11(13(20)17-12)9-5-3-2-4-6-9/h2-6,8,10,15,19H,7H2,1H3,(H,17,20)(H,18,23)(H,21,22)/p-1/t8?,10?,15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Huddersfield
Curated by ChEMBL
| Assay Description
Binding affinity against metallo-beta-lactamase bacillus cereus(BCII) |
Bioorg Med Chem Lett 14: 1737-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.047
BindingDB Entry DOI: 10.7270/Q22R3S7M |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50022505
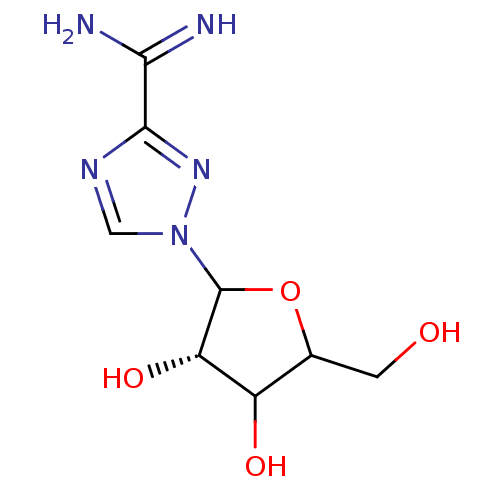
(1-(3,4-Dihydroxy-5-hydroxymethyl-tetrahydro-furan-...)Show InChI InChI=1S/C8H13N5O4/c9-6(10)7-11-2-13(12-7)8-5(16)4(15)3(1-14)17-8/h2-5,8,14-16H,1H2,(H3,9,10)/t3?,4?,5-,8?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nucleic Acid Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against purine nucleoside phosphorylase (PNPase ) |
J Med Chem 31: 330-5 (1988)
BindingDB Entry DOI: 10.7270/Q25H7GV0 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for Dopamine receptor D1 was measured in rat cortex homogenates. |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for Beta adrenergic receptor was measured in rat cortex homogenates |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50235611
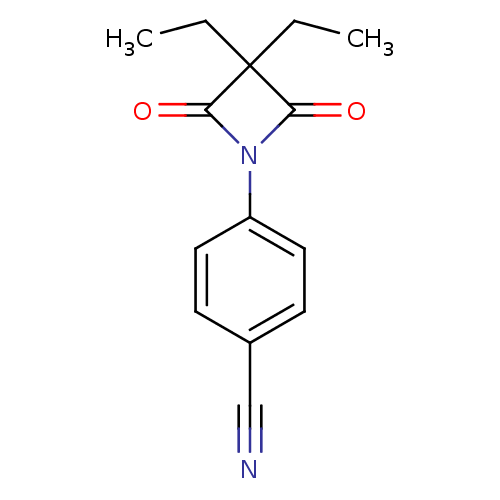
(4-(3,3-diethyl-2,4-dioxoazetidin-1-yl)benzonitrile...)Show InChI InChI=1S/C14H14N2O2/c1-3-14(4-2)12(17)16(13(14)18)11-7-5-10(9-15)6-8-11/h5-8H,3-4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase |
J Med Chem 51: 1783-90 (2008)
Article DOI: 10.1021/jm701257h
BindingDB Entry DOI: 10.7270/Q2ST7QPB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50472300

(CHEMBL84165)Show SMILES CN(C)CCc1c([nH]c2ccc(CCN3C(=O)CNC3=O)cc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C25H29N5O3/c1-29(2)12-11-19-20-14-17(10-13-30-22(31)16-27-25(30)33)8-9-21(20)28-23(19)24(32)26-15-18-6-4-3-5-7-18/h3-9,14,28H,10-13,15-16H2,1-2H3,(H,26,32)(H,27,33) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 1A receptor in rat cortex homogenates. |
J Med Chem 42: 2504-26 (1999)
Article DOI: 10.1021/jm9706325
BindingDB Entry DOI: 10.7270/Q2VD7257 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50367674
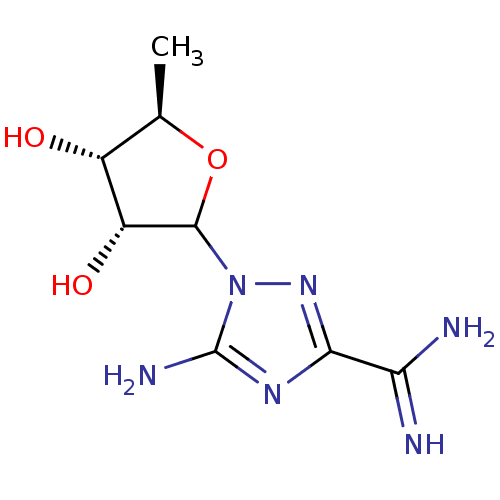
(CHEMBL611275)Show SMILES C[C@H]1OC([C@H](O)[C@@H]1O)n1nc(nc1N)C(N)=N |r| Show InChI InChI=1S/C8H14N6O3/c1-2-3(15)4(16)7(17-2)14-8(11)12-6(13-14)5(9)10/h2-4,7,15-16H,1H3,(H3,9,10)(H2,11,12,13)/t2-,3-,4-,7?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nucleic Acid Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against purine nucleoside phosphorylase (PNPase ) |
J Med Chem 31: 330-5 (1988)
BindingDB Entry DOI: 10.7270/Q25H7GV0 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50225889

(CHEMBL3144008)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)C1=NNC2C1=NC=NC2=O |r,c:17,t:10,15| Show InChI InChI=1S/C10H12N4O5/c15-1-3-7(16)8(17)9(19-3)5-4-6(14-13-5)10(18)12-2-11-4/h2-3,6-9,14-17H,1H2/t3-,6?,7-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nucleic Acid Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against purine nucleoside phosphorylase (PNPase ) |
J Med Chem 31: 330-5 (1988)
BindingDB Entry DOI: 10.7270/Q25H7GV0 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125265
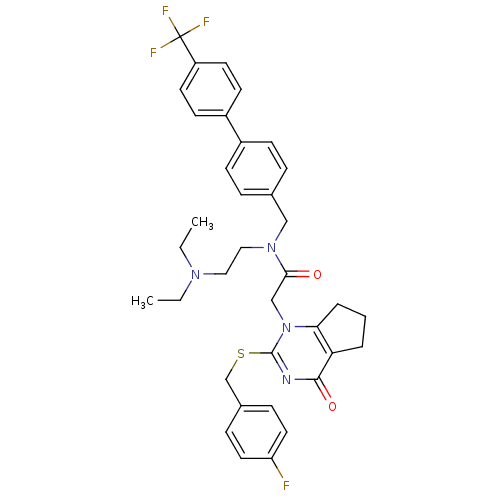
(CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H38F4N4O2S/c1-3-42(4-2)20-21-43(22-25-8-12-27(13-9-25)28-14-16-29(17-15-28)36(38,39)40)33(45)23-44-32-7-5-6-31(32)34(46)41-35(44)47-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Lp-PLA2 in whole human plasma pre-incubated for 15 mins before 2-thio-PAF substrate addition |
J Med Chem 59: 10738-10749 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01427
BindingDB Entry DOI: 10.7270/Q27946ND |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125265

(CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H38F4N4O2S/c1-3-42(4-2)20-21-43(22-25-8-12-27(13-9-25)28-14-16-29(17-15-28)36(38,39)40)33(45)23-44-32-7-5-6-31(32)34(46)41-35(44)47-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Lp-PLA2 pre-incubated for 30 mins before PED6 fluorogenic substrate |
J Med Chem 59: 10738-10749 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01427
BindingDB Entry DOI: 10.7270/Q27946ND |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125265

(CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H38F4N4O2S/c1-3-42(4-2)20-21-43(22-25-8-12-27(13-9-25)28-14-16-29(17-15-28)36(38,39)40)33(45)23-44-32-7-5-6-31(32)34(46)41-35(44)47-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Lp-PLA2 incubated for 20 mins by Thio-PAF assay |
J Med Chem 59: 10738-10749 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01427
BindingDB Entry DOI: 10.7270/Q27946ND |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503744
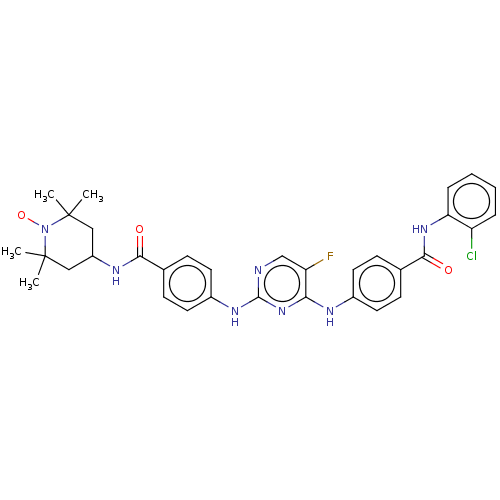
(CHEMBL4563026)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(F)c(-[#7]-c3ccc(cc3)-[#6](=O)-[#7]-c3ccccc3Cl)n2)cc1 |^1:10| Show InChI InChI=1S/C33H34ClFN7O3/c1-32(2)17-24(18-33(3,4)42(32)45)38-29(43)20-11-15-23(16-12-20)39-31-36-19-26(35)28(41-31)37-22-13-9-21(10-14-22)30(44)40-27-8-6-5-7-25(27)34/h5-16,19,24H,17-18H2,1-4H3,(H,38,43)(H,40,44)(H2,36,37,39,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503745
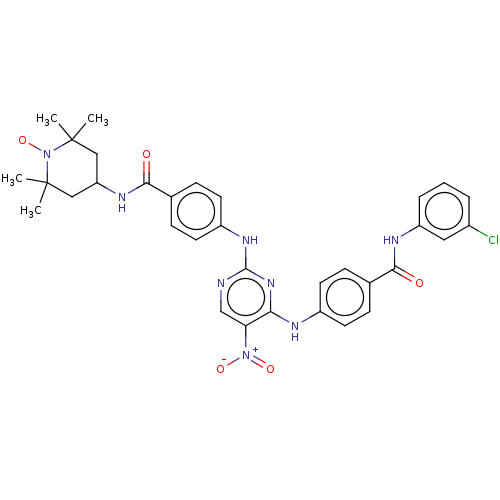
(CHEMBL4460717)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(c(-[#7]-c3ccc(cc3)-[#6](=O)-[#7]-c3cccc(Cl)c3)n2)-[#7+](-[#8-])=O)cc1 |^1:10| Show InChI InChI=1S/C33H34ClN8O5/c1-32(2)17-26(18-33(3,4)42(32)47)38-30(44)21-10-14-24(15-11-21)39-31-35-19-27(41(45)46)28(40-31)36-23-12-8-20(9-13-23)29(43)37-25-7-5-6-22(34)16-25/h5-16,19,26H,17-18H2,1-4H3,(H,37,43)(H,38,44)(H2,35,36,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503748
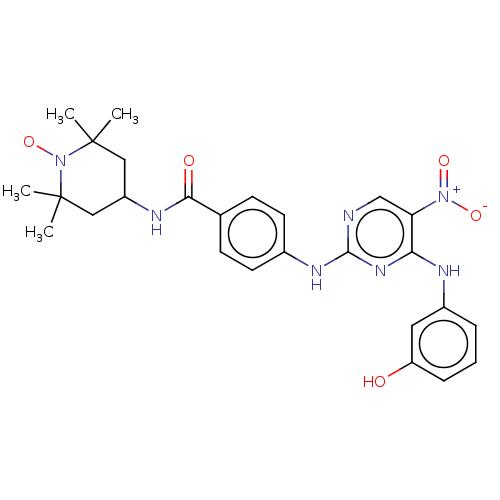
(CHEMBL4440617)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(c(-[#7]-c3cccc(-[#8])c3)n2)-[#7+](-[#8-])=O)cc1 |^1:10| Show InChI InChI=1S/C26H30N7O5/c1-25(2)13-19(14-26(3,4)33(25)38)29-23(35)16-8-10-17(11-9-16)30-24-27-15-21(32(36)37)22(31-24)28-18-6-5-7-20(34)12-18/h5-12,15,19,34H,13-14H2,1-4H3,(H,29,35)(H2,27,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50558869

(CHEMBL4747186)Show SMILES CCOc1nc(Nc2ccc(cc2OC(F)F)C(=O)N2CCN(C)CC2)nc2[nH]ccc12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant His6-Tev-LRRK2 (1326 to 2527 residues) (unknown origin) using LRRKtide as substrate preincubated for 30 mins followed by su... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2018.03.045
BindingDB Entry DOI: 10.7270/Q25142XZ |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503740
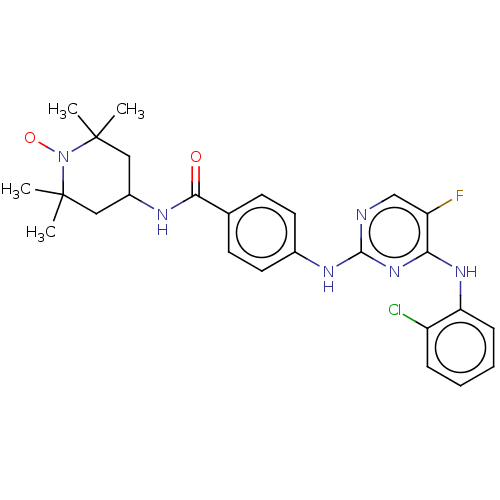
(CHEMBL4451940)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(F)c(-[#7]-c3ccccc3Cl)n2)cc1 |^1:10| Show InChI InChI=1S/C26H29ClFN6O2/c1-25(2)13-18(14-26(3,4)34(25)36)30-23(35)16-9-11-17(12-10-16)31-24-29-15-20(28)22(33-24)32-21-8-6-5-7-19(21)27/h5-12,15,18H,13-14H2,1-4H3,(H,30,35)(H2,29,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503749
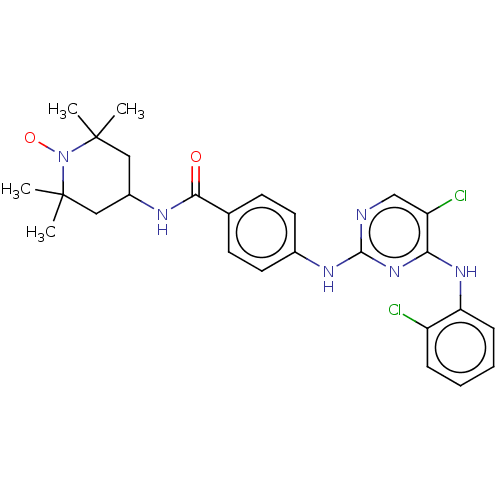
(CHEMBL4455202)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(Cl)c(-[#7]-c3ccccc3Cl)n2)cc1 |^1:10| Show InChI InChI=1S/C26H29Cl2N6O2/c1-25(2)13-18(14-26(3,4)34(25)36)30-23(35)16-9-11-17(12-10-16)31-24-29-15-20(28)22(33-24)32-21-8-6-5-7-19(21)27/h5-12,15,18H,13-14H2,1-4H3,(H,30,35)(H2,29,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50503749

(CHEMBL4455202)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(Cl)c(-[#7]-c3ccccc3Cl)n2)cc1 |^1:10| Show InChI InChI=1S/C26H29Cl2N6O2/c1-25(2)13-18(14-26(3,4)34(25)36)30-23(35)16-9-11-17(12-10-16)31-24-29-15-20(28)22(33-24)32-21-8-6-5-7-19(21)27/h5-12,15,18H,13-14H2,1-4H3,(H,30,35)(H2,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50503741
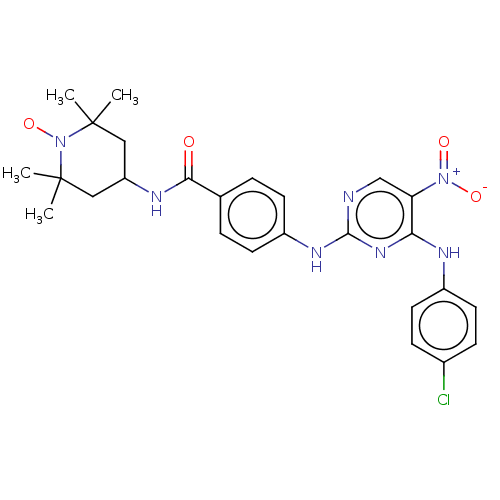
(CHEMBL4443125)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(c(-[#7]-c3ccc(Cl)cc3)n2)-[#7+](-[#8-])=O)cc1 |^1:10| Show InChI InChI=1S/C26H29ClN7O4/c1-25(2)13-20(14-26(3,4)34(25)38)30-23(35)16-5-9-19(10-6-16)31-24-28-15-21(33(36)37)22(32-24)29-18-11-7-17(27)8-12-18/h5-12,15,20H,13-14H2,1-4H3,(H,30,35)(H2,28,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50503737
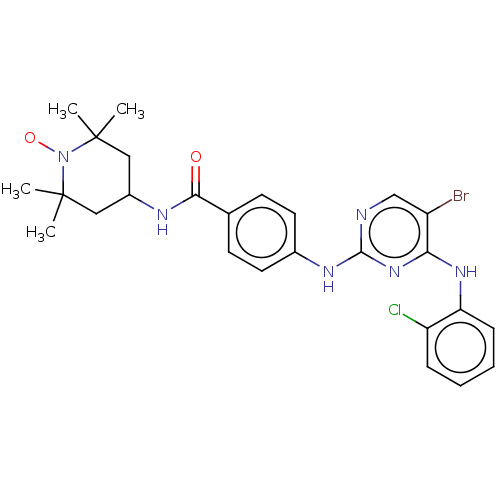
(CHEMBL4471448)Show SMILES [#6]C1([#6])[#6]-[#6](-[#6]C([#6])([#6])[#7]1-[#8;v1])-[#7]-[#6](=O)-c1ccc(-[#7]-c2ncc(Br)c(-[#7]-c3ccccc3Cl)n2)cc1 |^1:10| Show InChI InChI=1S/C26H29BrClN6O2/c1-25(2)13-18(14-26(3,4)34(25)36)30-23(35)16-9-11-17(12-10-16)31-24-29-15-19(27)22(33-24)32-21-8-6-5-7-20(21)28/h5-12,15,18H,13-14H2,1-4H3,(H,30,35)(H2,29,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) using kemptide acetate salt as substrate after 30 mins by kinase-Glo luminescence method |
Bioorg Med Chem 27: 65-78 (2019)
Article DOI: 10.1016/j.bmc.2018.11.006
BindingDB Entry DOI: 10.7270/Q2PN98WR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360084

(CHEMBL1928725)Show SMILES CN(\N=C\c1cnn2ccc(cc12)C#N)S(=O)(=O)c1cc(ccc1Cl)[N+]([O-])=O Show InChI InChI=1S/C16H11ClN6O4S/c1-21(28(26,27)16-7-13(23(24)25)2-3-14(16)17)19-9-12-10-20-22-5-4-11(8-18)6-15(12)22/h2-7,9-10H,1H3/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 58-68 (2011)
Article DOI: 10.1016/j.bmc.2011.11.031
BindingDB Entry DOI: 10.7270/Q21C1X99 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data