 Found 177 hits with Last Name = 'ahlijanian' and Initial = 'mk'
Found 177 hits with Last Name = 'ahlijanian' and Initial = 'mk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50458163
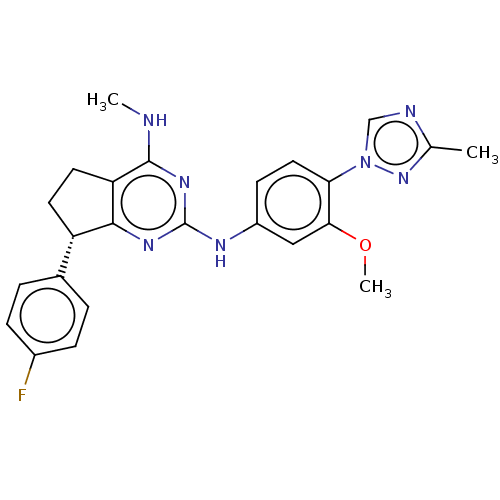
(CHEMBL4209316)Show SMILES CNc1nc(Nc2ccc(c(OC)c2)-n2cnc(C)n2)nc2[C@@H](CCc12)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H24FN7O/c1-14-27-13-32(31-14)20-11-8-17(12-21(20)33-3)28-24-29-22-18(15-4-6-16(25)7-5-15)9-10-19(22)23(26-2)30-24/h4-8,11-13,18H,9-10H2,1-3H3,(H2,26,28,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Time-dependent inhibition of CYP3A4 in human liver microsomes assessed as inhibition constant by Kitz-Wilson plot analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127530
BindingDB Entry DOI: 10.7270/Q24X5CFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50553647
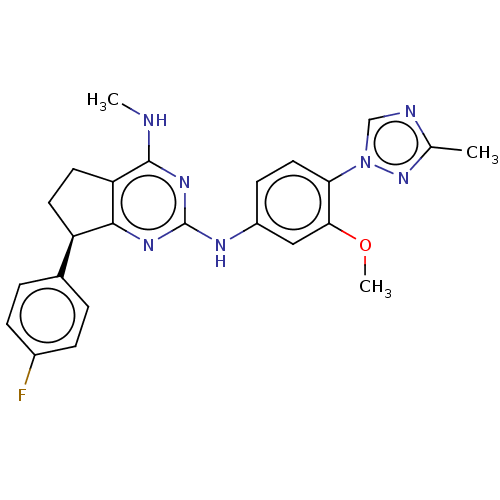
(CHEMBL4793756)Show SMILES CNc1nc(Nc2ccc(c(OC)c2)-n2cnc(C)n2)nc2[C@H](CCc12)c1ccc(F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Time-dependent inhibition of CYP3A4 in human liver microsomes assessed as inhibition constant by Kitz-Wilson plot analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127530
BindingDB Entry DOI: 10.7270/Q24X5CFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50458163

(CHEMBL4209316)Show SMILES CNc1nc(Nc2ccc(c(OC)c2)-n2cnc(C)n2)nc2[C@@H](CCc12)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H24FN7O/c1-14-27-13-32(31-14)20-11-8-17(12-21(20)33-3)28-24-29-22-18(15-4-6-16(25)7-5-15)9-10-19(22)23(26-2)30-24/h4-8,11-13,18H,9-10H2,1-3H3,(H2,26,28,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00541
BindingDB Entry DOI: 10.7270/Q24171P7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50169351
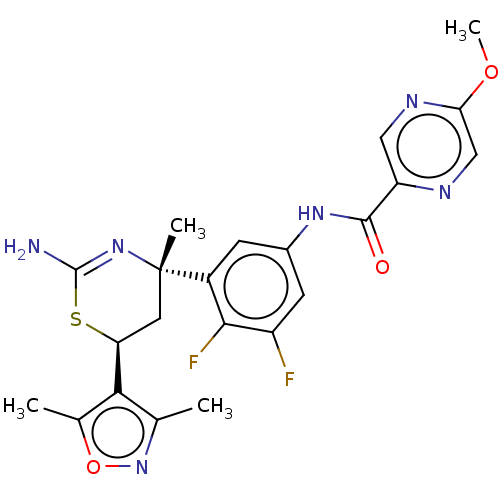
(CHEMBL3805852)Show SMILES COc1cnc(cn1)C(=O)Nc1cc(F)c(F)c(c1)[C@]1(C)C[C@H](SC(N)=N1)c1c(C)noc1C |r,c:27| Show InChI InChI=1S/C22H22F2N6O3S/c1-10-18(11(2)33-30-10)16-7-22(3,29-21(25)34-16)13-5-12(6-14(23)19(13)24)28-20(31)15-8-27-17(32-4)9-26-15/h5-6,8-9,16H,7H2,1-4H3,(H2,25,29)(H,28,31)/t16-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells expressing APP751 Swedish mutant assessed as inhibition of amyloid beta 40 or amyloid beta 42 production incuba... |
ACS Med Chem Lett 7: 271-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00432
BindingDB Entry DOI: 10.7270/Q2NS0WTZ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50169366
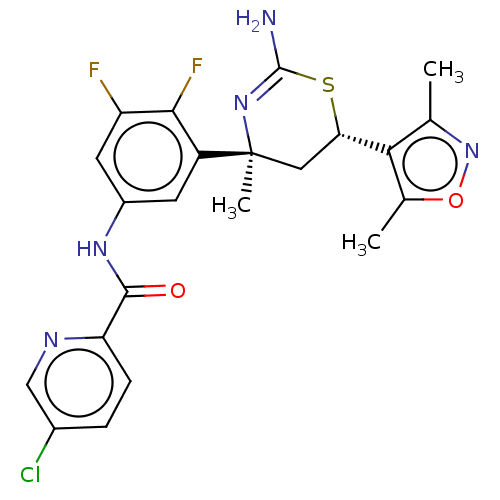
(CHEMBL3806322)Show SMILES Cc1noc(C)c1[C@@H]1C[C@](C)(N=C(N)S1)c1cc(NC(=O)c2ccc(Cl)cn2)cc(F)c1F |r,t:12| Show InChI InChI=1S/C22H20ClF2N5O2S/c1-10-18(11(2)32-30-10)17-8-22(3,29-21(26)33-17)14-6-13(7-15(24)19(14)25)28-20(31)16-5-4-12(23)9-27-16/h4-7,9,17H,8H2,1-3H3,(H2,26,29)(H,28,31)/t17-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells expressing APP751 Swedish mutant assessed as inhibition of amyloid beta 40 or amyloid beta 42 production incuba... |
ACS Med Chem Lett 7: 271-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00432
BindingDB Entry DOI: 10.7270/Q2NS0WTZ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50169365
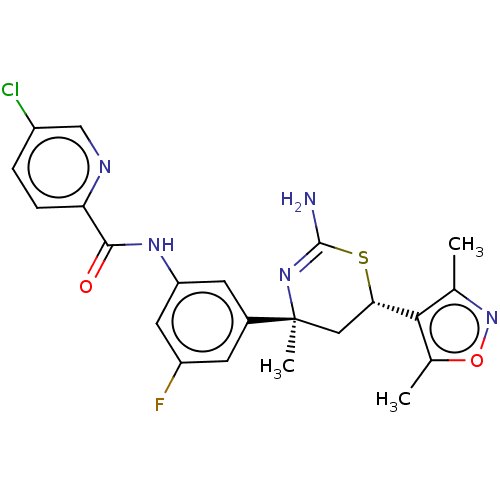
(CHEMBL3806215)Show SMILES Cc1noc(C)c1[C@@H]1C[C@](C)(N=C(N)S1)c1cc(F)cc(NC(=O)c2ccc(Cl)cn2)c1 |r,t:12| Show InChI InChI=1S/C22H21ClFN5O2S/c1-11-19(12(2)31-29-11)18-9-22(3,28-21(25)32-18)13-6-15(24)8-16(7-13)27-20(30)17-5-4-14(23)10-26-17/h4-8,10,18H,9H2,1-3H3,(H2,25,28)(H,27,30)/t18-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells expressing APP751 Swedish mutant assessed as inhibition of amyloid beta 40 or amyloid beta 42 production incuba... |
ACS Med Chem Lett 7: 271-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00432
BindingDB Entry DOI: 10.7270/Q2NS0WTZ |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50458163

(CHEMBL4209316)Show SMILES CNc1nc(Nc2ccc(c(OC)c2)-n2cnc(C)n2)nc2[C@@H](CCc12)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H24FN7O/c1-14-27-13-32(31-14)20-11-8-17(12-21(20)33-3)28-24-29-22-18(15-4-6-16(25)7-5-15)9-10-19(22)23(26-2)30-24/h4-8,11-13,18H,9-10H2,1-3H3,(H2,26,28,29,30)/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Modulation of gamma secretase (unknown origin) assessed as inhibition of amyloid beta (1 to 42 residues) production |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127530
BindingDB Entry DOI: 10.7270/Q24X5CFF |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50553650

(CHEMBL4757307)Show SMILES CNc1nc(Nc2ccc(c(OC)c2)-n2cnc(C)n2)nc2[C@@H](C\C(=N/O)c12)c1ccc(F)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Modulation of gamma secretase (unknown origin) assessed as inhibition of amyloid beta (1 to 42 residues) production |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127530
BindingDB Entry DOI: 10.7270/Q24X5CFF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155213
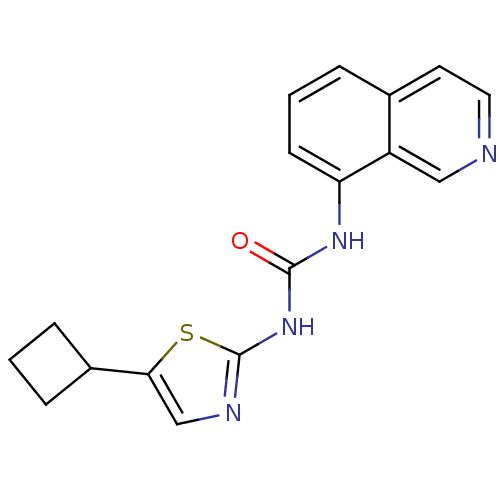
(1-(5-Cyclobutyl-thiazol-2-yl)-3-isoquinolin-8-yl-u...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)12-4-1-5-12)20-14-6-2-3-11-7-8-18-9-13(11)14/h2-3,6-10,12H,1,4-5H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50169368
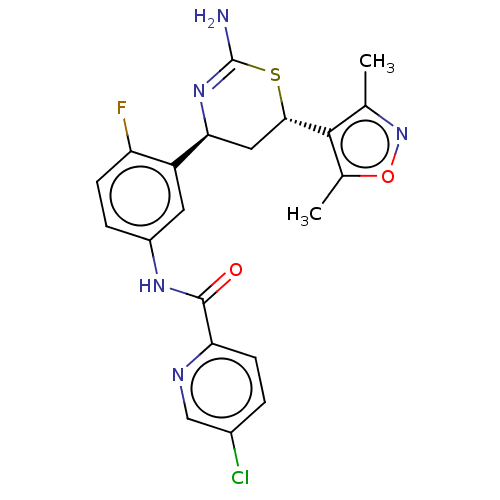
(CHEMBL3806050)Show SMILES Cc1noc(C)c1[C@@H]1C[C@H](N=C(N)S1)c1cc(NC(=O)c2ccc(Cl)cn2)ccc1F |r,t:11| Show InChI InChI=1S/C21H19ClFN5O2S/c1-10-19(11(2)30-28-10)18-8-17(27-21(24)31-18)14-7-13(4-5-15(14)23)26-20(29)16-6-3-12(22)9-25-16/h3-7,9,17-18H,8H2,1-2H3,(H2,24,27)(H,26,29)/t17-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells expressing APP751 Swedish mutant assessed as inhibition of amyloid beta 40 or amyloid beta 42 production incuba... |
ACS Med Chem Lett 7: 271-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00432
BindingDB Entry DOI: 10.7270/Q2NS0WTZ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415046

(CHEMBL583658)Show SMILES Cc1cccc(n1)C(=O)N[C@H]1C[C@H](C1)n1cnc(NC(=O)Cc2cccc3ccccc23)c1 |r,wU:10.10,12.15,(14.65,-10.99,;14.63,-9.45,;13.28,-8.7,;13.27,-7.16,;14.59,-6.38,;15.92,-7.14,;15.95,-8.67,;17.25,-6.35,;17.24,-4.81,;18.59,-7.11,;19.92,-6.33,;20.6,-4.95,;21.99,-5.63,;21.3,-7.01,;23.45,-5.14,;23.91,-3.67,;25.45,-3.66,;25.94,-5.12,;27.27,-5.89,;27.27,-7.43,;25.93,-8.2,;28.6,-8.21,;28.59,-9.75,;29.92,-10.51,;29.92,-12.05,;28.58,-12.82,;27.25,-12.05,;25.93,-12.81,;24.6,-12.05,;24.6,-10.51,;25.93,-9.74,;27.25,-10.51,;24.7,-6.03,)| Show InChI InChI=1S/C26H25N5O2/c1-17-6-4-11-23(28-17)26(33)29-20-13-21(14-20)31-15-24(27-16-31)30-25(32)12-19-9-5-8-18-7-2-3-10-22(18)19/h2-11,15-16,20-21H,12-14H2,1H3,(H,29,33)(H,30,32)/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415045

(CHEMBL571782)Show SMILES Clc1cccc(n1)C(=O)N[C@H]1C[C@H](C1)n1cnc(NC(=O)Cc2cccc3ccccc23)c1 |r,wU:10.10,12.15,(-7.08,-11.69,;-7.1,-10.15,;-8.45,-9.39,;-8.47,-7.85,;-7.14,-7.07,;-5.81,-7.83,;-5.78,-9.36,;-4.48,-7.05,;-4.5,-5.51,;-3.14,-7.8,;-1.81,-7.02,;-1.13,-5.64,;.26,-6.32,;-.44,-7.71,;1.72,-5.83,;2.18,-4.37,;3.72,-4.35,;4.21,-5.81,;5.54,-6.58,;5.54,-8.13,;4.2,-8.89,;6.87,-8.9,;6.86,-10.44,;8.19,-11.21,;8.19,-12.74,;6.85,-13.52,;5.52,-12.74,;4.19,-13.5,;2.87,-12.74,;2.87,-11.2,;4.2,-10.44,;5.52,-11.21,;2.97,-6.73,)| Show InChI InChI=1S/C25H22ClN5O2/c26-22-10-4-9-21(29-22)25(33)28-18-12-19(13-18)31-14-23(27-15-31)30-24(32)11-17-7-3-6-16-5-1-2-8-20(16)17/h1-10,14-15,18-19H,11-13H2,(H,28,33)(H,30,32)/t18-,19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155231
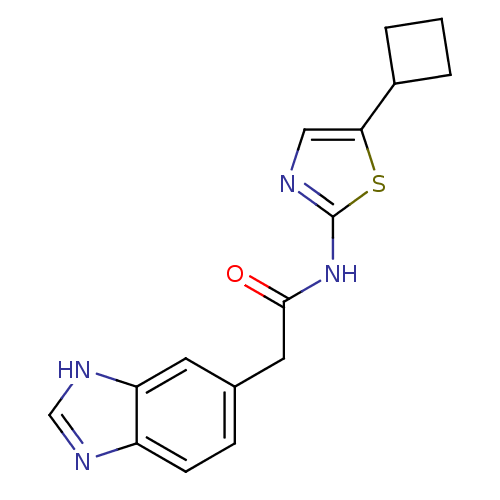
(2-(1H-Benzoimidazol-5-yl)-N-(5-cyclobutyl-thiazol-...)Show InChI InChI=1S/C16H16N4OS/c21-15(7-10-4-5-12-13(6-10)19-9-18-12)20-16-17-8-14(22-16)11-2-1-3-11/h4-6,8-9,11H,1-3,7H2,(H,18,19)(H,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155209

(1-(5-Cyclobutyl-thiazol-2-yl)-3-isoquinolin-5-yl-u...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)11-3-1-4-11)20-14-6-2-5-12-9-18-8-7-13(12)14/h2,5-11H,1,3-4H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50088174

(CHEMBL3427251)Show SMILES Cc1noc(C)c1[C@@H]1C[C@](C)(N=C(N)S1)c1cc(c(F)cc1F)-c1cncnc1 |r,t:12| Show InChI InChI=1S/C20H19F2N5OS/c1-10-18(11(2)28-27-10)17-6-20(3,26-19(23)29-17)14-4-13(15(21)5-16(14)22)12-7-24-9-25-8-12/h4-5,7-9,17H,6H2,1-3H3,(H2,23,26)/t17-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells expressing APP751 Swedish mutant assessed as inhibition of amyloid beta 40 or amyloid beta 42 production incuba... |
ACS Med Chem Lett 7: 271-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00432
BindingDB Entry DOI: 10.7270/Q2NS0WTZ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415039

(CHEMBL571780)Show SMILES CC(=O)N[C@H]1C[C@H](C1)n1cnc(NC(=O)Cc2cccc3ccccc23)c1 |r,wU:6.8,4.3,(6.38,-30.85,;7.71,-30.06,;9.05,-30.82,;7.69,-28.52,;9.02,-27.74,;9.7,-26.36,;11.09,-27.05,;10.4,-28.43,;12.55,-26.56,;13.01,-25.09,;14.55,-25.07,;15.04,-26.53,;16.37,-27.31,;16.37,-28.85,;15.03,-29.61,;17.7,-29.62,;17.69,-31.16,;19.02,-31.93,;19.02,-33.46,;17.68,-34.24,;16.35,-33.46,;15.03,-34.22,;13.7,-33.46,;13.7,-31.92,;15.03,-31.16,;16.35,-31.93,;13.81,-27.45,)| Show InChI InChI=1S/C21H22N4O2/c1-14(26)23-17-10-18(11-17)25-12-20(22-13-25)24-21(27)9-16-7-4-6-15-5-2-3-8-19(15)16/h2-8,12-13,17-18H,9-11H2,1H3,(H,23,26)(H,24,27)/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415037

(CHEMBL576502)Show SMILES COc1ccc(CC(=O)Nc2cn(cn2)[C@@H]2C[C@@H](C2)NC(C)=O)cc1 |r,wU:15.15,17.20,(2.53,9.19,;1,9.03,;.37,7.63,;-1.16,7.46,;-1.78,6.06,;-.88,4.81,;-1.51,3.41,;-.6,2.16,;.93,2.32,;-1.23,.75,;-.32,-.49,;-.8,-1.96,;.45,-2.86,;1.69,-1.96,;1.22,-.49,;.45,-4.4,;-.64,-5.49,;.45,-6.58,;1.54,-5.49,;.45,-8.12,;-.89,-8.89,;-.89,-10.43,;-2.22,-8.12,;.65,4.97,;1.28,6.38,)| Show InChI InChI=1S/C18H22N4O3/c1-12(23)20-14-8-15(9-14)22-10-17(19-11-22)21-18(24)7-13-3-5-16(25-2)6-4-13/h3-6,10-11,14-15H,7-9H2,1-2H3,(H,20,23)(H,21,24)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155236
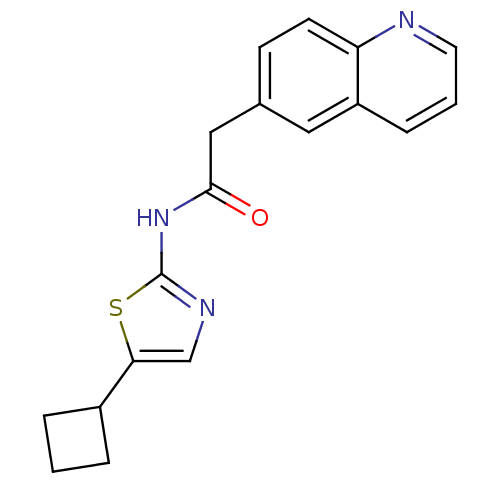
(CHEMBL363954 | N-(5-Cyclobutyl-thiazol-2-yl)-2-qui...)Show InChI InChI=1S/C18H17N3OS/c22-17(21-18-20-11-16(23-18)13-3-1-4-13)10-12-6-7-15-14(9-12)5-2-8-19-15/h2,5-9,11,13H,1,3-4,10H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50553649

(CHEMBL4753645)Show SMILES CNc1nc(Nc2ccc(c(OC)c2)-n2cnc(C)n2)nc2C(C\C(=N/O)c12)c1ccc(F)cc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Modulation of gamma secretase (unknown origin) assessed as inhibition of amyloid beta (1 to 42 residues) production |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127530
BindingDB Entry DOI: 10.7270/Q24X5CFF |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50012647
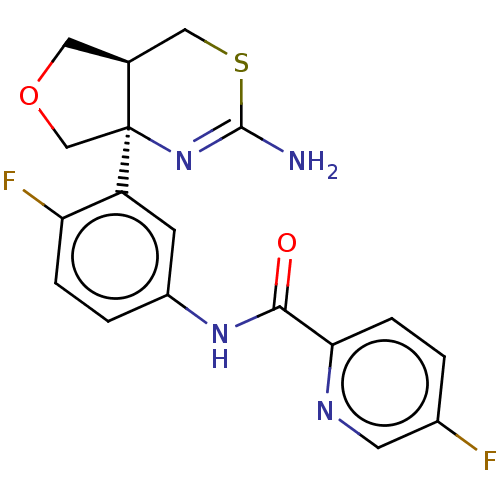
(CHEMBL2396989)Show SMILES [H][C@@]12COC[C@@]1(N=C(N)SC2)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:7| Show InChI InChI=1S/C18H16F2N4O2S/c19-11-1-4-15(22-6-11)16(25)23-12-2-3-14(20)13(5-12)18-9-26-7-10(18)8-27-17(21)24-18/h1-6,10H,7-9H2,(H2,21,24)(H,23,25)/t10-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Bioorg Med Chem Lett 26: 5729-5731 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.055
BindingDB Entry DOI: 10.7270/Q2W66NRR |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155235
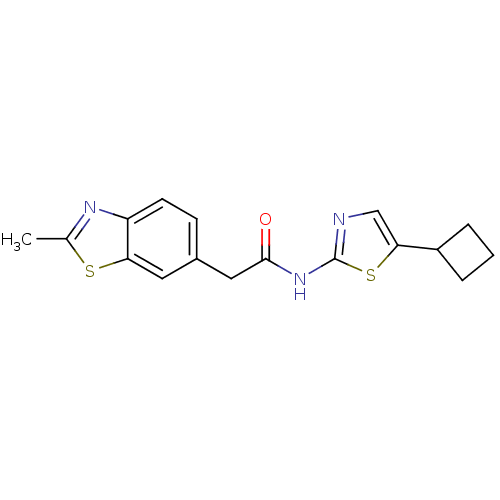
(CHEMBL186470 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(2-...)Show InChI InChI=1S/C17H17N3OS2/c1-10-19-13-6-5-11(7-14(13)22-10)8-16(21)20-17-18-9-15(23-17)12-3-2-4-12/h5-7,9,12H,2-4,8H2,1H3,(H,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155230
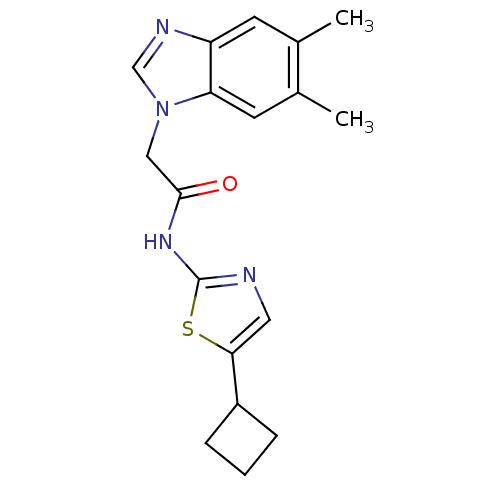
(CHEMBL187903 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(5,...)Show InChI InChI=1S/C18H20N4OS/c1-11-6-14-15(7-12(11)2)22(10-20-14)9-17(23)21-18-19-8-16(24-18)13-4-3-5-13/h6-8,10,13H,3-5,9H2,1-2H3,(H,19,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155207

(1-(5-Cyclobutyl-thiazol-2-yl)-3-quinolin-5-yl-urea...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)11-4-1-5-11)20-14-8-2-7-13-12(14)6-3-9-18-13/h2-3,6-11H,1,4-5H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155237

(CHEMBL184404 | N-(5-Cyclobutyl-thiazol-2-yl)-2-iso...)Show InChI InChI=1S/C18H17N3OS/c22-17(21-18-20-11-16(23-18)12-3-1-4-12)9-13-5-2-6-14-10-19-8-7-15(13)14/h2,5-8,10-12H,1,3-4,9H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50553648
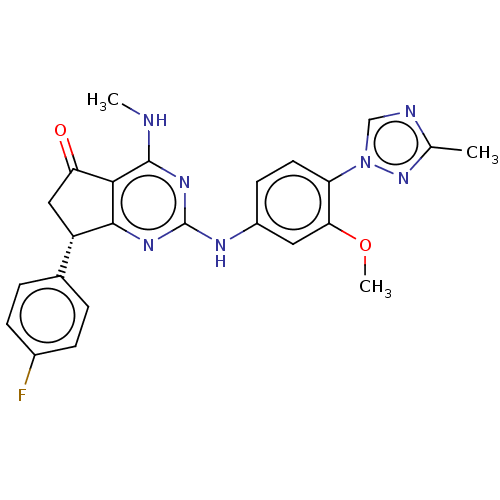
(CHEMBL4757003)Show SMILES CNc1nc(Nc2ccc(c(OC)c2)-n2cnc(C)n2)nc2[C@@H](CC(=O)c12)c1ccc(F)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Modulation of gamma secretase (unknown origin) assessed as inhibition of amyloid beta (1 to 42 residues) production |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127530
BindingDB Entry DOI: 10.7270/Q24X5CFF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415044

(CHEMBL569587)Show SMILES O=C(Cc1cccc2ccccc12)Nc1cn(cn1)[C@@H]1C[C@@H](C1)NC(=O)c1ccccn1 |r,wU:21.26,19.21,(28.29,2.47,;29.63,3.24,;30.96,2.46,;30.96,.92,;32.28,.16,;32.28,-1.38,;30.94,-2.15,;29.61,-1.38,;28.29,-2.14,;26.96,-1.38,;26.96,.16,;28.29,.93,;29.62,.16,;29.63,4.78,;28.3,5.55,;27.07,4.63,;25.81,5.53,;26.27,7,;27.81,7.01,;24.35,5.04,;22.96,5.72,;22.28,4.34,;23.66,3.66,;20.95,3.56,;19.61,4.32,;19.6,5.86,;18.29,3.53,;16.95,4.29,;15.63,3.51,;15.64,1.97,;16.99,1.21,;18.31,2,)| Show InChI InChI=1S/C25H23N5O2/c31-24(12-18-8-5-7-17-6-1-2-9-21(17)18)29-23-15-30(16-27-23)20-13-19(14-20)28-25(32)22-10-3-4-11-26-22/h1-11,15-16,19-20H,12-14H2,(H,28,32)(H,29,31)/t19-,20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155235

(CHEMBL186470 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(2-...)Show InChI InChI=1S/C17H17N3OS2/c1-10-19-13-6-5-11(7-14(13)22-10)8-16(21)20-17-18-9-15(23-17)12-3-2-4-12/h5-7,9,12H,2-4,8H2,1H3,(H,18,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155214
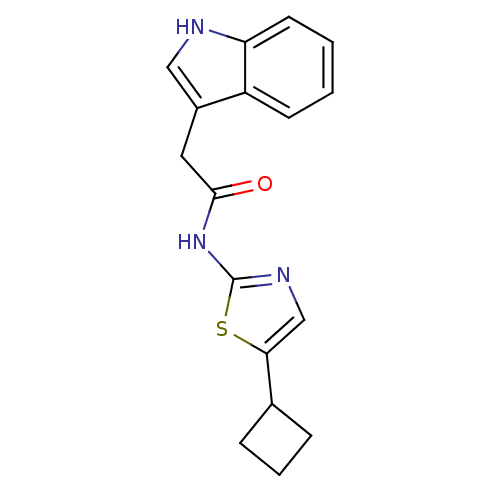
(CHEMBL186240 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(1H...)Show InChI InChI=1S/C17H17N3OS/c21-16(8-12-9-18-14-7-2-1-6-13(12)14)20-17-19-10-15(22-17)11-4-3-5-11/h1-2,6-7,9-11,18H,3-5,8H2,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50553651
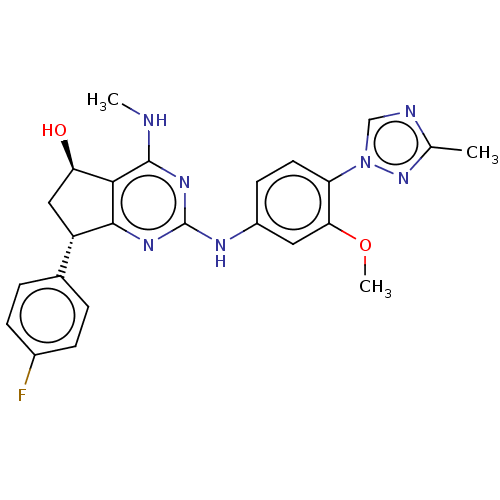
(CHEMBL4782161)Show SMILES CNc1nc(Nc2ccc(c(OC)c2)-n2cnc(C)n2)nc2[C@@H](C[C@@H](O)c12)c1ccc(F)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Modulation of gamma secretase (unknown origin) assessed as inhibition of amyloid beta (1 to 42 residues) production |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127530
BindingDB Entry DOI: 10.7270/Q24X5CFF |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50012647

(CHEMBL2396989)Show SMILES [H][C@@]12COC[C@@]1(N=C(N)SC2)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:7| Show InChI InChI=1S/C18H16F2N4O2S/c19-11-1-4-15(22-6-11)16(25)23-12-2-3-14(20)13(5-12)18-9-26-7-10(18)8-27-17(21)24-18/h1-6,10H,7-9H2,(H2,21,24)(H,23,25)/t10-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 by FRET assay |
Bioorg Med Chem Lett 26: 5729-5731 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.055
BindingDB Entry DOI: 10.7270/Q2W66NRR |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50203710

(CHEMBL3891534)Show SMILES [H][C@@]12CCO[C@@]1(N=C(N)SC2)c1cc(NC(=O)c2ccc(Cl)cn2)ccc1F |r,t:7| Show InChI InChI=1S/C18H16ClFN4O2S/c19-11-1-4-15(22-8-11)16(25)23-12-2-3-14(20)13(7-12)18-10(5-6-26-18)9-27-17(21)24-18/h1-4,7-8,10H,5-6,9H2,(H2,21,24)(H,23,25)/t10-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Bioorg Med Chem Lett 26: 5729-5731 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.055
BindingDB Entry DOI: 10.7270/Q2W66NRR |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155225
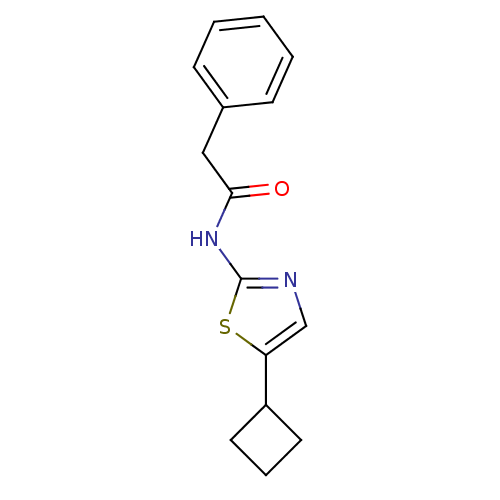
(CHEMBL365855 | N-(5-Cyclobutyl-thiazol-2-yl)-2-phe...)Show InChI InChI=1S/C15H16N2OS/c18-14(9-11-5-2-1-3-6-11)17-15-16-10-13(19-15)12-7-4-8-12/h1-3,5-6,10,12H,4,7-9H2,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155236

(CHEMBL363954 | N-(5-Cyclobutyl-thiazol-2-yl)-2-qui...)Show InChI InChI=1S/C18H17N3OS/c22-17(21-18-20-11-16(23-18)13-3-1-4-13)10-12-6-7-15-14(9-12)5-2-8-19-15/h2,5-9,11,13H,1,3-4,10H2,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155230

(CHEMBL187903 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(5,...)Show InChI InChI=1S/C18H20N4OS/c1-11-6-14-15(7-12(11)2)22(10-20-14)9-17(23)21-18-19-8-16(24-18)13-4-3-5-13/h6-8,10,13H,3-5,9H2,1-2H3,(H,19,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415042

(CHEMBL582813)Show SMILES O=C(Cc1cccc2ccccc12)Nc1cn(cn1)[C@@H]1C[C@@H](C1)NC(=O)c1ccncc1 |r,wU:21.26,19.21,(31.19,-43.99,;32.52,-43.23,;33.86,-44,;33.85,-45.54,;35.18,-46.31,;35.18,-47.84,;33.84,-48.62,;32.5,-47.84,;31.18,-48.6,;29.86,-47.84,;29.86,-46.3,;31.19,-45.54,;32.51,-46.31,;32.53,-41.68,;31.2,-40.91,;29.96,-41.83,;28.71,-40.93,;29.17,-39.47,;30.71,-39.45,;27.25,-41.42,;25.86,-40.74,;25.17,-42.12,;26.55,-42.81,;23.85,-42.9,;22.51,-42.15,;22.49,-40.61,;21.18,-42.93,;19.85,-42.17,;18.52,-42.95,;18.54,-44.49,;19.89,-45.25,;21.21,-44.46,)| Show InChI InChI=1S/C25H23N5O2/c31-24(12-19-6-3-5-17-4-1-2-7-22(17)19)29-23-15-30(16-27-23)21-13-20(14-21)28-25(32)18-8-10-26-11-9-18/h1-11,15-16,20-21H,12-14H2,(H,28,32)(H,29,31)/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50169367
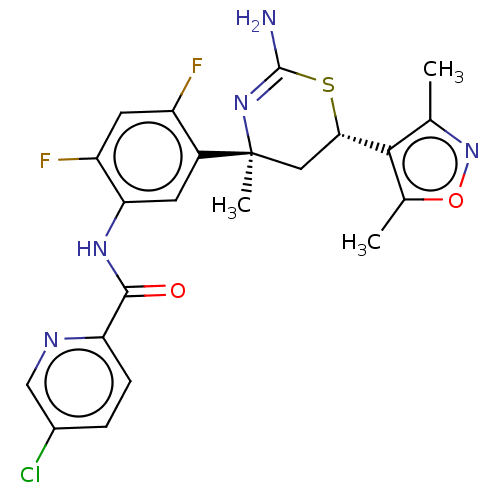
(CHEMBL3804970)Show SMILES Cc1noc(C)c1[C@@H]1C[C@](C)(N=C(N)S1)c1cc(NC(=O)c2ccc(Cl)cn2)c(F)cc1F |r,t:12| Show InChI InChI=1S/C22H20ClF2N5O2S/c1-10-19(11(2)32-30-10)18-8-22(3,29-21(26)33-18)13-6-17(15(25)7-14(13)24)28-20(31)16-5-4-12(23)9-27-16/h4-7,9,18H,8H2,1-3H3,(H2,26,29)(H,28,31)/t18-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells expressing APP751 Swedish mutant assessed as inhibition of amyloid beta 40 or amyloid beta 42 production incuba... |
ACS Med Chem Lett 7: 271-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00432
BindingDB Entry DOI: 10.7270/Q2NS0WTZ |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50553647

(CHEMBL4793756)Show SMILES CNc1nc(Nc2ccc(c(OC)c2)-n2cnc(C)n2)nc2[C@H](CCc12)c1ccc(F)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Modulation of gamma secretase (unknown origin) assessed as inhibition of amyloid beta (1 to 42 residues) production |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127530
BindingDB Entry DOI: 10.7270/Q24X5CFF |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50203709
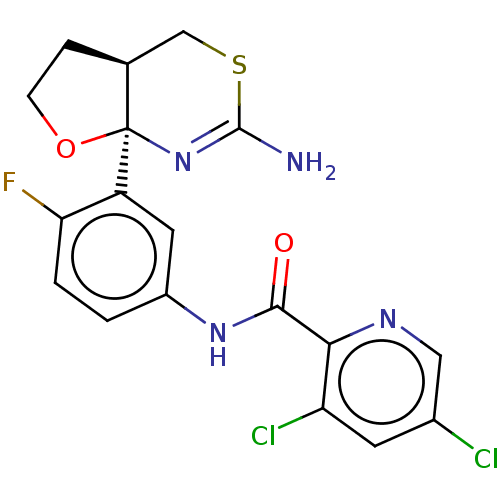
(CHEMBL3909581)Show SMILES [H][C@@]12CCO[C@@]1(N=C(N)SC2)c1cc(NC(=O)c2ncc(Cl)cc2Cl)ccc1F |r,t:7| Show InChI InChI=1S/C18H15Cl2FN4O2S/c19-10-5-13(20)15(23-7-10)16(26)24-11-1-2-14(21)12(6-11)18-9(3-4-27-18)8-28-17(22)25-18/h1-2,5-7,9H,3-4,8H2,(H2,22,25)(H,24,26)/t9-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Bioorg Med Chem Lett 26: 5729-5731 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.055
BindingDB Entry DOI: 10.7270/Q2W66NRR |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155231

(2-(1H-Benzoimidazol-5-yl)-N-(5-cyclobutyl-thiazol-...)Show InChI InChI=1S/C16H16N4OS/c21-15(7-10-4-5-12-13(6-10)19-9-18-12)20-16-17-8-14(22-16)11-2-1-3-11/h4-6,8-9,11H,1-3,7H2,(H,18,19)(H,17,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50203708
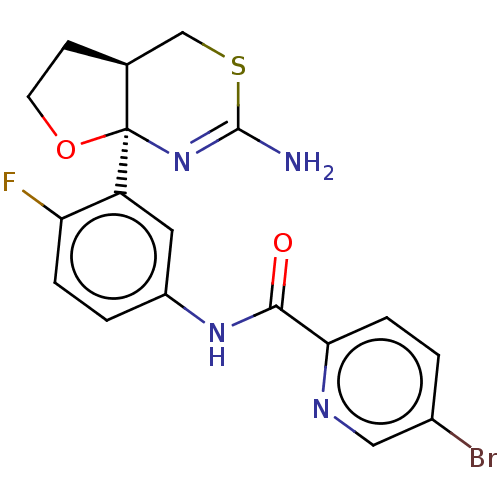
(CHEMBL3919597)Show SMILES [H][C@@]12CCO[C@@]1(N=C(N)SC2)c1cc(NC(=O)c2ccc(Br)cn2)ccc1F |r,t:7| Show InChI InChI=1S/C18H16BrFN4O2S/c19-11-1-4-15(22-8-11)16(25)23-12-2-3-14(20)13(7-12)18-10(5-6-26-18)9-27-17(21)24-18/h1-4,7-8,10H,5-6,9H2,(H2,21,24)(H,23,25)/t10-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Bioorg Med Chem Lett 26: 5729-5731 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.055
BindingDB Entry DOI: 10.7270/Q2W66NRR |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50203703

(CHEMBL3980733)Show SMILES [H][C@@]12CCO[C@@]1(N=C(N)SC2)c1cc(NC(=O)c2ccc(cn2)C#N)ccc1F |r,t:7| Show InChI InChI=1S/C19H16FN5O2S/c20-15-3-2-13(24-17(26)16-4-1-11(8-21)9-23-16)7-14(15)19-12(5-6-27-19)10-28-18(22)25-19/h1-4,7,9,12H,5-6,10H2,(H2,22,25)(H,24,26)/t12-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Bioorg Med Chem Lett 26: 5729-5731 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.055
BindingDB Entry DOI: 10.7270/Q2W66NRR |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155214

(CHEMBL186240 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(1H...)Show InChI InChI=1S/C17H17N3OS/c21-16(8-12-9-18-14-7-2-1-6-13(12)14)20-17-19-10-15(22-17)11-4-3-5-11/h1-2,6-7,9-11,18H,3-5,8H2,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155232
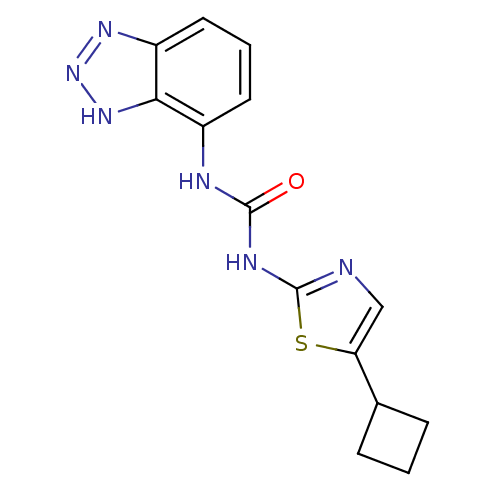
(1-(3H-Benzotriazol-4-yl)-3-(5-cyclobutyl-thiazol-2...)Show InChI InChI=1S/C14H14N6OS/c21-13(16-9-5-2-6-10-12(9)19-20-18-10)17-14-15-7-11(22-14)8-3-1-4-8/h2,5-8H,1,3-4H2,(H,18,19,20)(H2,15,16,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155207

(1-(5-Cyclobutyl-thiazol-2-yl)-3-quinolin-5-yl-urea...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)11-4-1-5-11)20-14-8-2-7-13-12(14)6-3-9-18-13/h2-3,6-11H,1,4-5H2,(H2,19,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155221
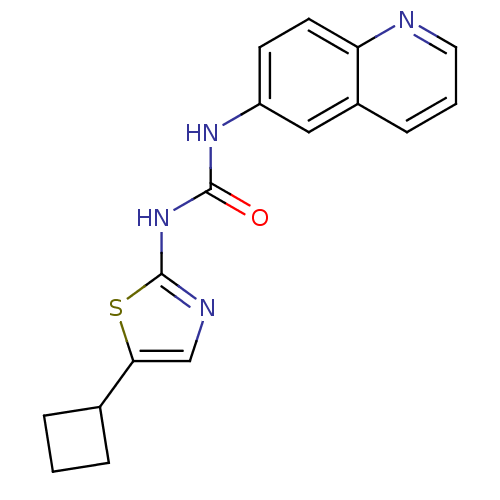
(1-(5-Cyclobutyl-thiazol-2-yl)-3-quinolin-6-yl-urea...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)11-3-1-4-11)20-13-6-7-14-12(9-13)5-2-8-18-14/h2,5-11H,1,3-4H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155222

(1-(5-Cyclobutyl-thiazol-2-yl)-3-isoquinolin-6-yl-u...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)11-2-1-3-11)20-14-5-4-13-9-18-7-6-12(13)8-14/h4-11H,1-3H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415026
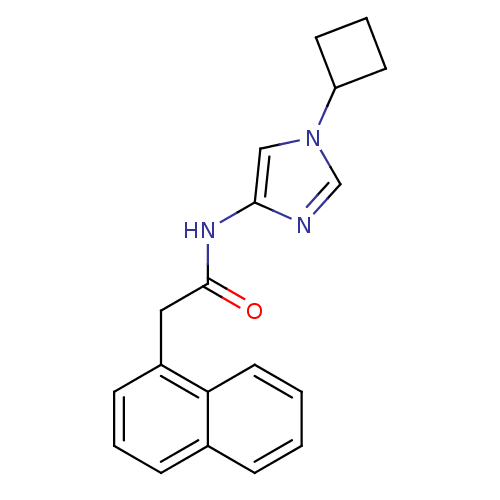
(CHEMBL571565)Show InChI InChI=1S/C19H19N3O/c23-19(21-18-12-22(13-20-18)16-8-4-9-16)11-15-7-3-6-14-5-1-2-10-17(14)15/h1-3,5-7,10,12-13,16H,4,8-9,11H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415043

(CHEMBL569586)Show SMILES O=C(Cc1cccc2ccccc12)Nc1cn(cn1)[C@@H]1C[C@@H](C1)NC(=O)c1cccnc1 |r,wU:21.26,19.21,(3.14,2.57,;4.48,3.33,;5.81,2.56,;5.8,1.02,;7.13,.25,;7.13,-1.29,;5.79,-2.06,;4.46,-1.28,;3.13,-2.05,;1.81,-1.28,;1.81,.26,;3.14,1.02,;4.46,.25,;4.48,4.87,;3.15,5.65,;1.91,4.73,;.66,5.62,;1.12,7.09,;2.66,7.11,;-.8,5.13,;-2.19,5.82,;-2.88,4.44,;-1.5,3.75,;-4.2,3.65,;-5.54,4.41,;-5.56,5.95,;-6.87,3.63,;-8.2,4.39,;-9.53,3.61,;-9.51,2.07,;-8.16,1.31,;-6.84,2.09,)| Show InChI InChI=1S/C25H23N5O2/c31-24(11-18-7-3-6-17-5-1-2-9-22(17)18)29-23-15-30(16-27-23)21-12-20(13-21)28-25(32)19-8-4-10-26-14-19/h1-10,14-16,20-21H,11-13H2,(H,28,32)(H,29,31)/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155220

(CHEMBL364338 | N-(5-Cyclobutyl-thiazol-2-yl)-2-ind...)Show InChI InChI=1S/C17H17N3OS/c21-16(11-20-9-8-12-4-1-2-7-14(12)20)19-17-18-10-15(22-17)13-5-3-6-13/h1-2,4,7-10,13H,3,5-6,11H2,(H,18,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155213

(1-(5-Cyclobutyl-thiazol-2-yl)-3-isoquinolin-8-yl-u...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)12-4-1-5-12)20-14-6-2-3-11-7-8-18-9-13(11)14/h2-3,6-10,12H,1,4-5H2,(H2,19,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data