

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50046213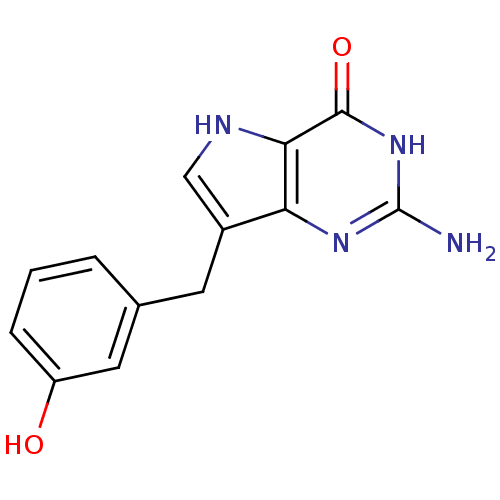 (2-Amino-7-(3-hydroxy-benzyl)-3,5-dihydro-pyrrolo[3...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50118337 (1-(benzo[b]thiophen-3-yl)-3-(4-(3,4-dihydro-2H-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50415830 (CHEMBL1095256) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50118336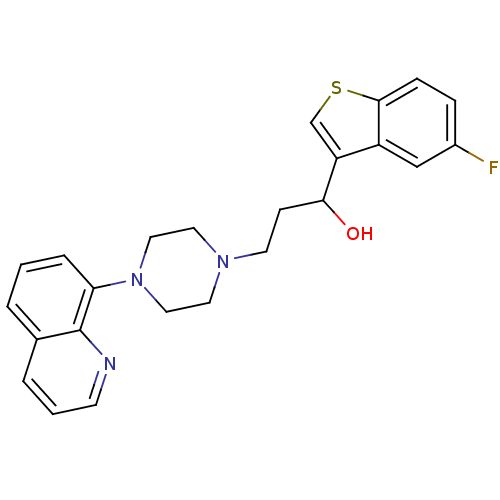 (1-(5-Fluoro-benzo[b]thiophen-3-yl)-3-(4-quinolin-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50118327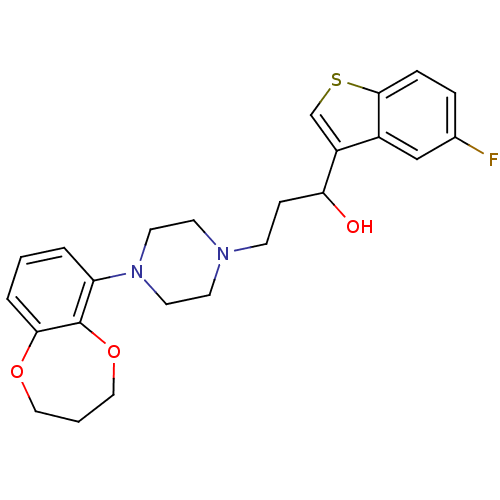 (3-(4-(3,4-dihydro-2H-benzo[b][1,4]dioxepin-6-yl)pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50039542 (2-Amino-7-pyridin-3-ylmethyl-3,5-dihydro-pyrrolo[3...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50048046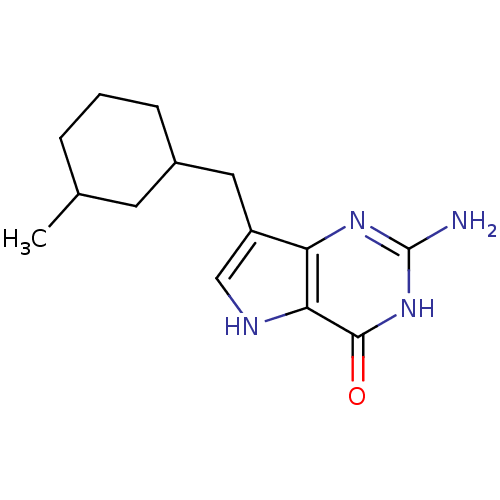 (2-Amino-7-(3-methyl-cyclohexylmethyl)-3,5-dihydro-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50039544 (2-Amino-7-thiophen-3-ylmethyl-3,5-dihydro-pyrrolo[...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50039547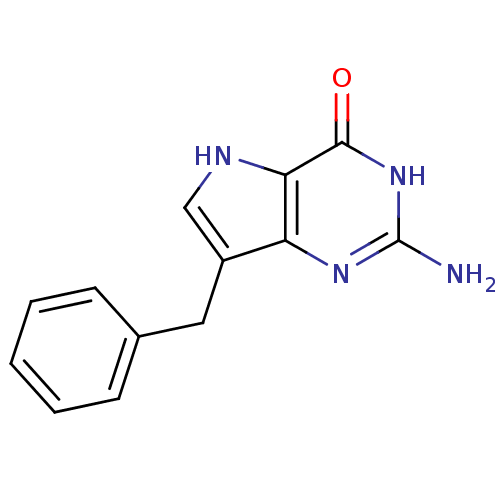 (2-Amino-7-benzyl-3,5-dihydro-pyrrolo[3,2-d]pyrimid...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50048053 (2-Amino-7-(3-trifluoromethyl-cyclohexylmethyl)-3,5...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50046219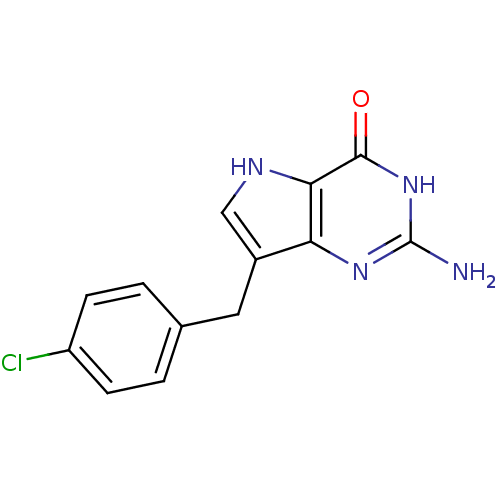 (2-Amino-7-(4-chloro-benzyl)-3,5-dihydro-pyrrolo[3,...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity of the compound against calf spleen Purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50078452 ((S)-3-(2-Amino-4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using inosine as substrate at a concentration of 10 uM. | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50042804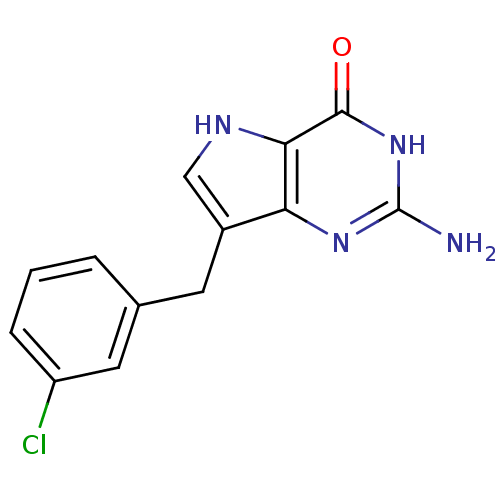 (2-Amino-7-(3-chloro-benzyl)-3,5-dihydro-pyrrolo[3,...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50039549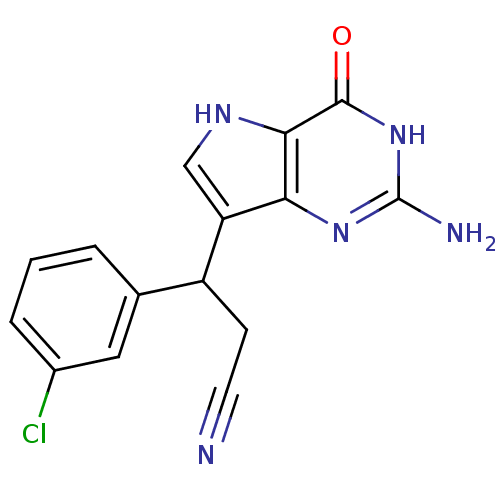 (3-(2-Amino-4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]pyri...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50039548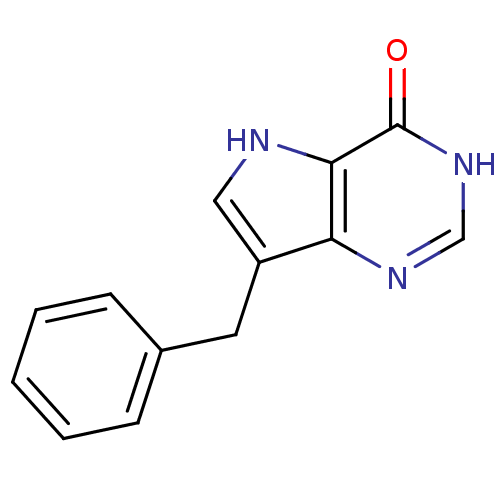 (7-Benzyl-3,5-dihydro-pyrrolo[3,2-d]pyrimidin-4-one...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50048044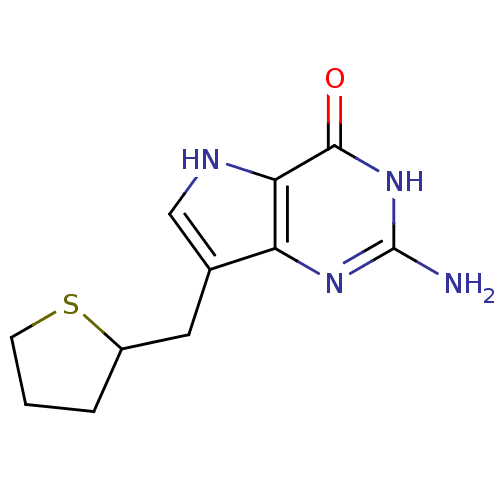 (2-Amino-7-(tetrahydro-thiophen-2-ylmethyl)-3,5-dih...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50118335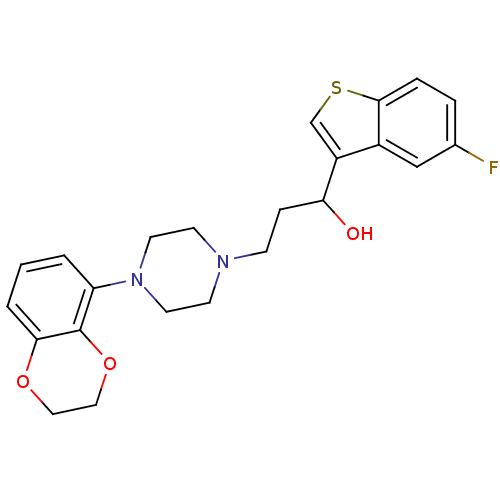 (3-(4-(2,3-dihydrobenzo[b][1,4]dioxin-5-yl)piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50039562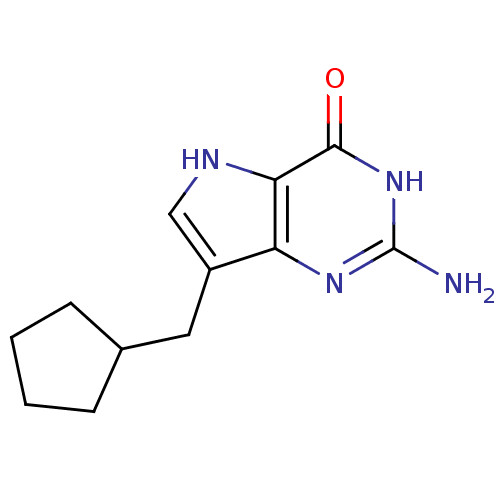 (2-Amino-7-cyclopentylmethyl-3,5-dihydro-pyrrolo[3,...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50042800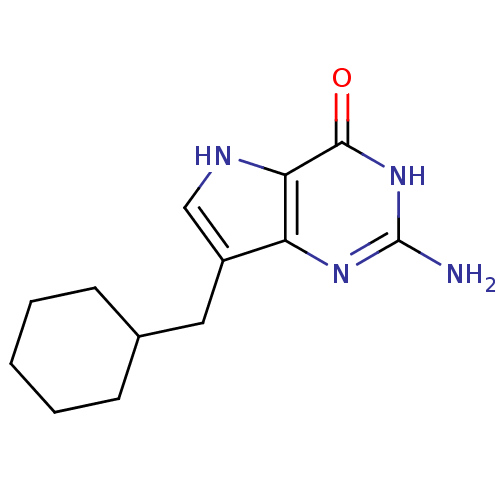 (2-Amino-7-cyclohexylmethyl-3,5-dihydro-pyrrolo[3,2...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102377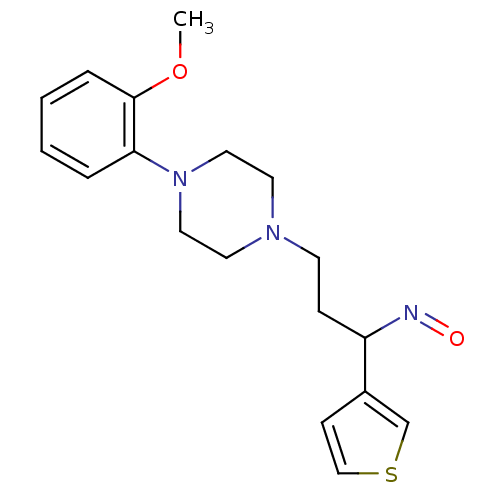 (3-(4-(2-methoxyphenyl)piperazin-1-yl)-1-(thiophen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102352 (1-(2,5-Dimethyl-thiophen-3-yl)-3-[4-(2-hydroxy-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50048044 (2-Amino-7-(tetrahydro-thiophen-2-ylmethyl)-3,5-dih...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using inosine as substrate at a concentration of 10 uM. | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50042799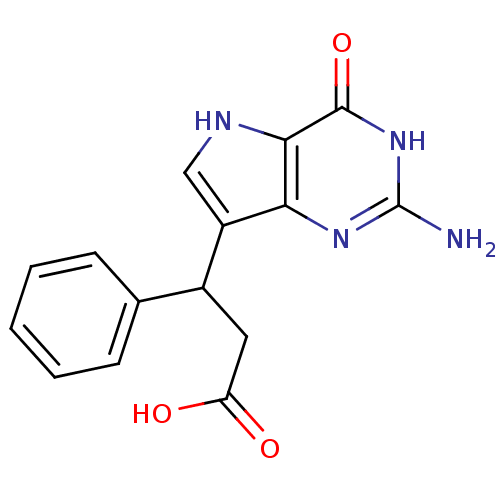 (3-(2-Amino-4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]pyri...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using inosine as substrate at a concentration of 10 uM. | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50039562 (2-Amino-7-cyclopentylmethyl-3,5-dihydro-pyrrolo[3,...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using inosine as substrate at a concentration of 10 uM. | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50415828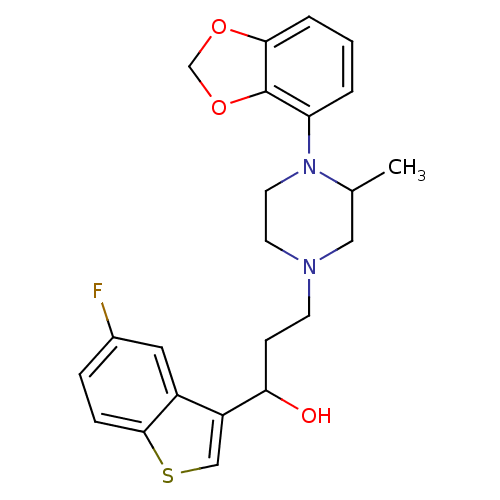 (CHEMBL1096903) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50048053 (2-Amino-7-(3-trifluoromethyl-cyclohexylmethyl)-3,5...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using inosine as substrate at a concentration of 10 uM. | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102371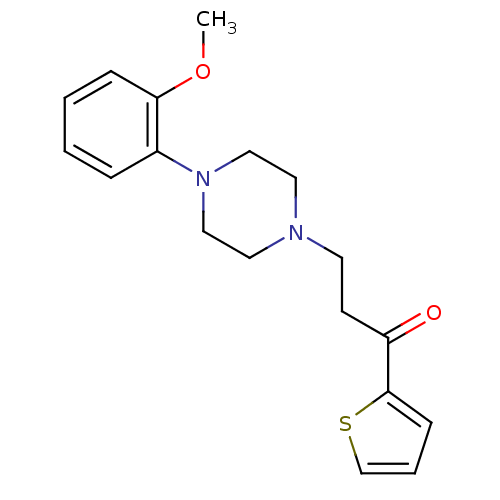 (3-(4-(2-methoxyphenyl)piperazin-1-yl)-1-(thiophen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102333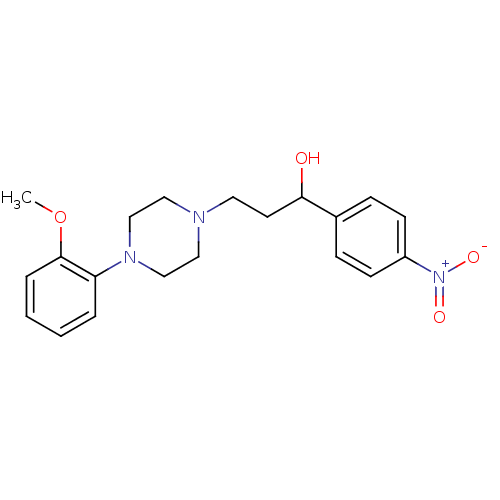 (3-(4-(2-methoxyphenyl)piperazin-1-yl)-1-(4-nitroph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50415829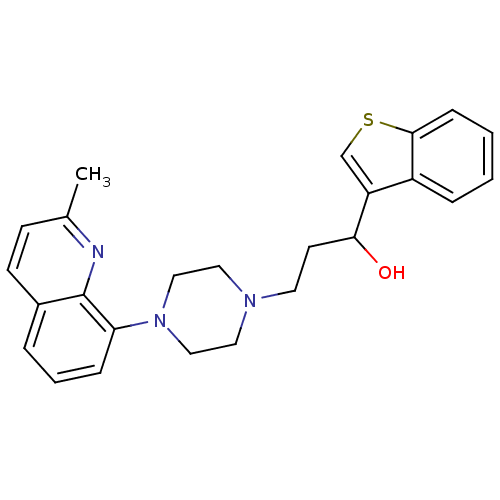 (CHEMBL1099255) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50046219 (2-Amino-7-(4-chloro-benzyl)-3,5-dihydro-pyrrolo[3,...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using inosine as substrate at a concentration of 10 uM. | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50039549 (3-(2-Amino-4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]pyri...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using inosine as substrate at a concentration of 10 uM. | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50039547 (2-Amino-7-benzyl-3,5-dihydro-pyrrolo[3,2-d]pyrimid...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using inosine as substrate at a concentration of 10 uM. | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50048046 (2-Amino-7-(3-methyl-cyclohexylmethyl)-3,5-dihydro-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using inosine as substrate at a concentration of 10 uM. | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102412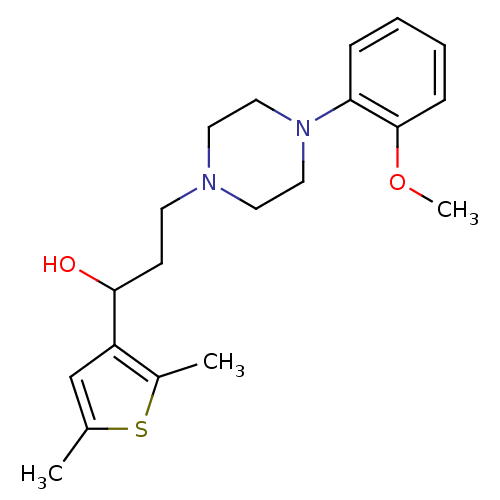 (1-(2,5-Dimethyl-thiophen-3-yl)-3-[4-(2-methoxy-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102386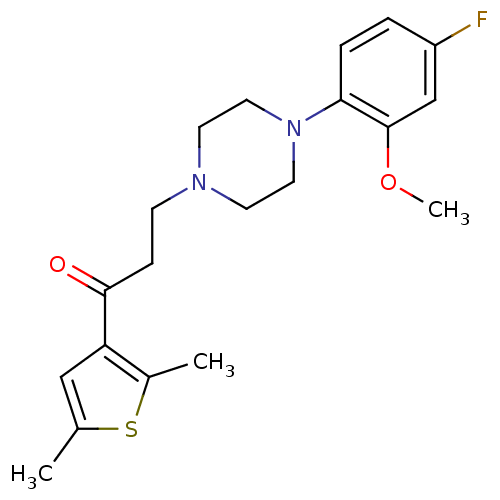 (1-(2,5-Dimethyl-thiophen-3-yl)-3-[4-(4-fluoro-2-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50042800 (2-Amino-7-cyclohexylmethyl-3,5-dihydro-pyrrolo[3,2...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using inosine as substrate at a concentration of 10 uM. | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50039542 (2-Amino-7-pyridin-3-ylmethyl-3,5-dihydro-pyrrolo[3...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using inosine as substrate at a concentration of 10 uM. | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50046253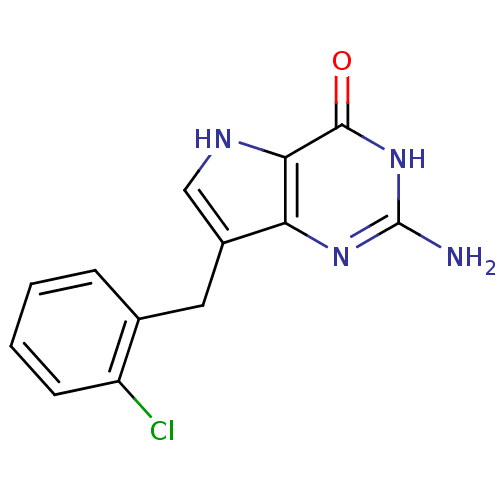 (2-Amino-7-(2-chloro-benzyl)-3,5-dihydro-pyrrolo[3,...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50039544 (2-Amino-7-thiophen-3-ylmethyl-3,5-dihydro-pyrrolo[...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using inosine as substrate at a concentration of 10 uM. | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50046213 (2-Amino-7-(3-hydroxy-benzyl)-3,5-dihydro-pyrrolo[3...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using inosine as substrate at a concentration of 10 uM. | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50078453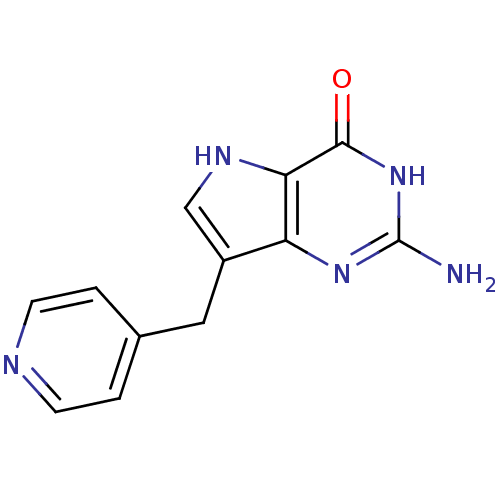 (2-Amino-7-pyridin-4-ylmethyl-3,5-dihydro-pyrrolo[3...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102374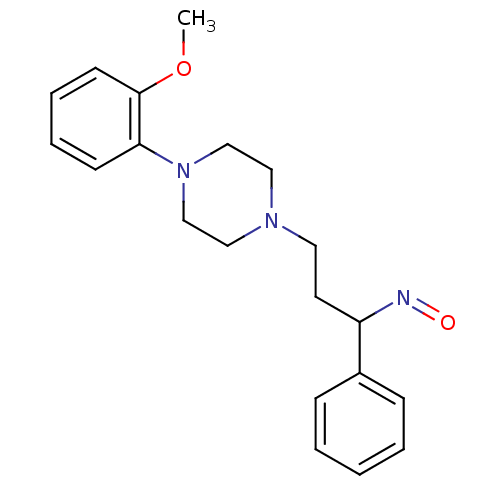 (3-(4-(2-methoxyphenyl)piperazin-1-yl)-1-phenylprop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102350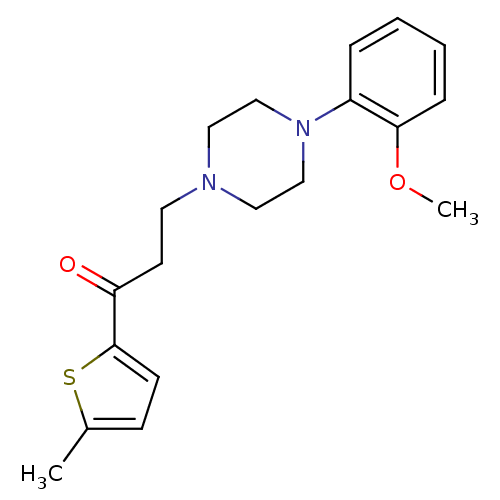 (3-(4-(2-methoxyphenyl)piperazin-1-yl)-1-(5-methylt...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102330 (2-(4-(3-(benzo[b]thiophen-3-yl)-3-hydroxypropyl)pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50039548 (7-Benzyl-3,5-dihydro-pyrrolo[3,2-d]pyrimidin-4-one...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using inosine as substrate at a concentration of 10 uM. | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50042804 (2-Amino-7-(3-chloro-benzyl)-3,5-dihydro-pyrrolo[3,...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using inosine as substrate at a concentration of 10 uM. | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102355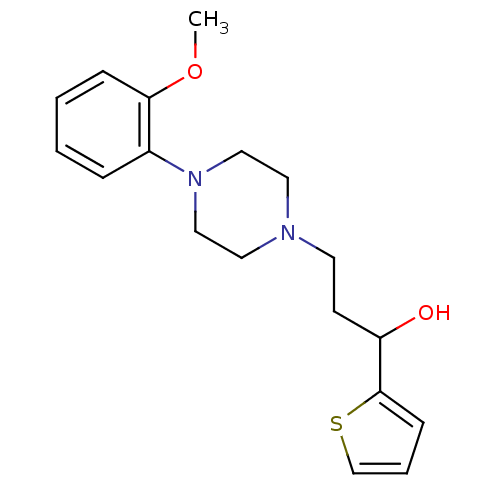 (3-(4-(2-methoxyphenyl)piperazin-1-yl)-1-(thiophen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102380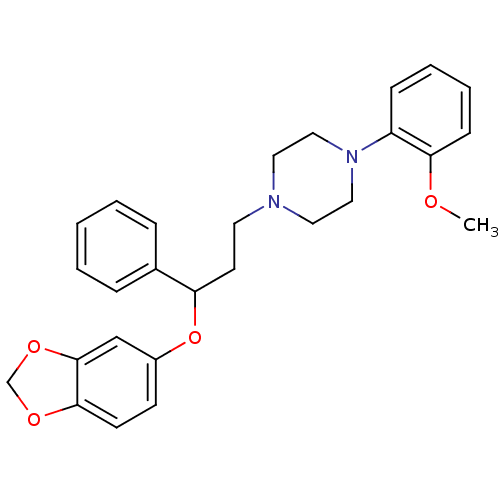 (1-(3-(benzo[d][1,3]dioxol-5-yloxy)-3-phenylpropyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102359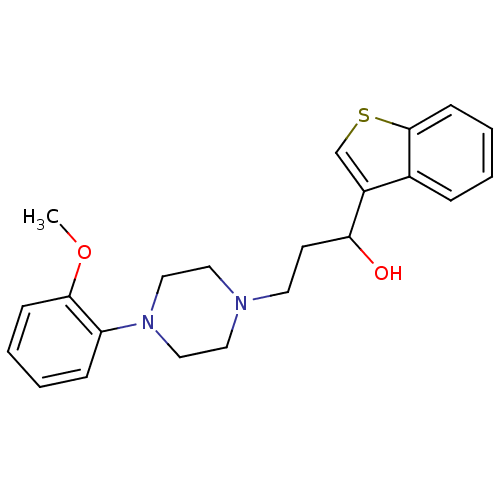 (1-(benzo[b]thiophen-3-yl)-3-(4-(2-methoxyphenyl)pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50078455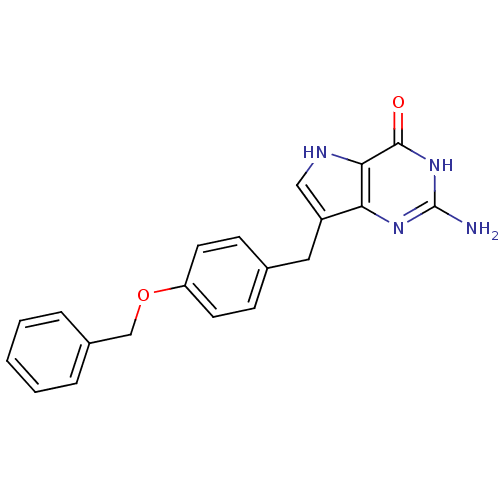 (2-Amino-7-(4-benzyloxy-benzyl)-3,5-dihydro-pyrrolo...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 662 total ) | Next | Last >> |