

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyamine oxidase 1 (Zea mays) | BDBM50294105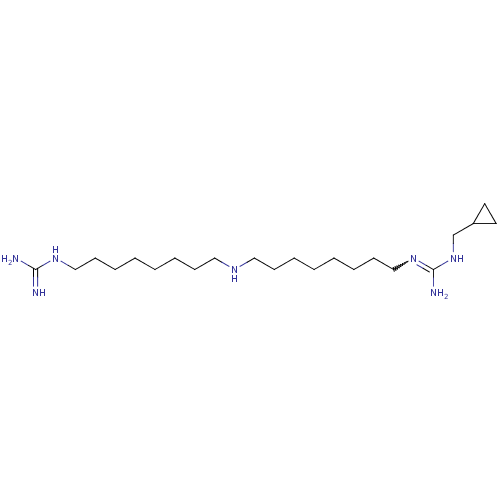 (1-(Guanidino)-17-(N1-(methylcyclopropyl)guanidino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294107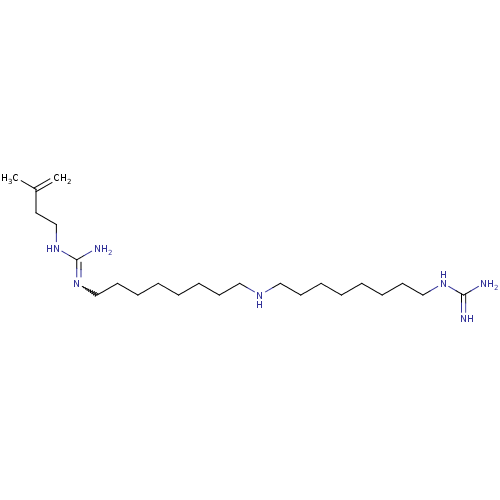 (1-(Guanidino)-17-(N1-(beta-methylallyl)guanidino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294109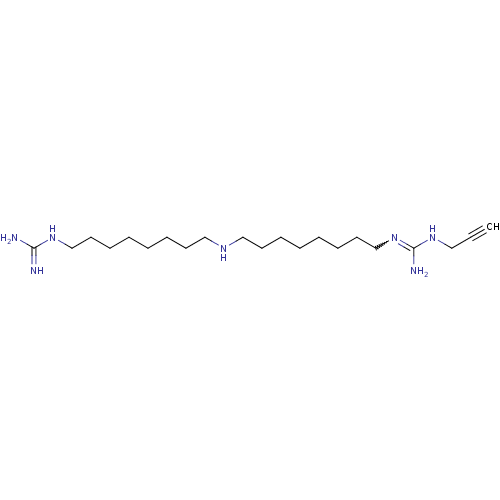 (1-(Guanidino)-17-(N1-(propargyl)guanidino)-9-azahe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294108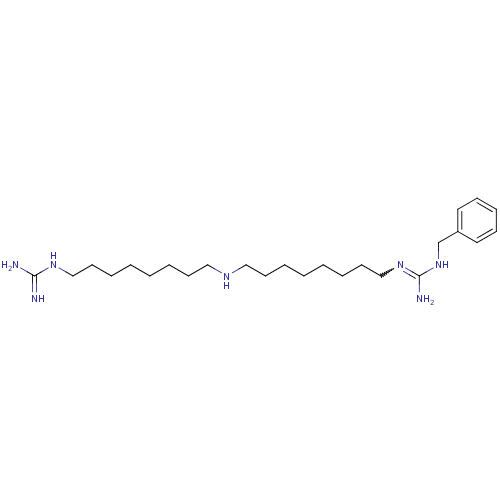 (1-(Guanidino)-17-(N1-(benzyl)guanidino)-9-azahepta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294106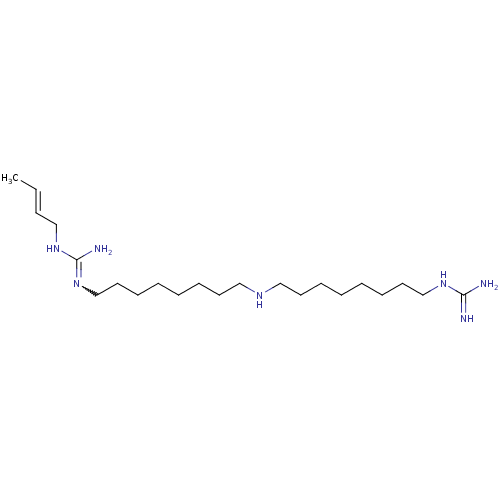 (1-(Guanidino)-17-(N1-(gamma-methylallyl)guanidino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294110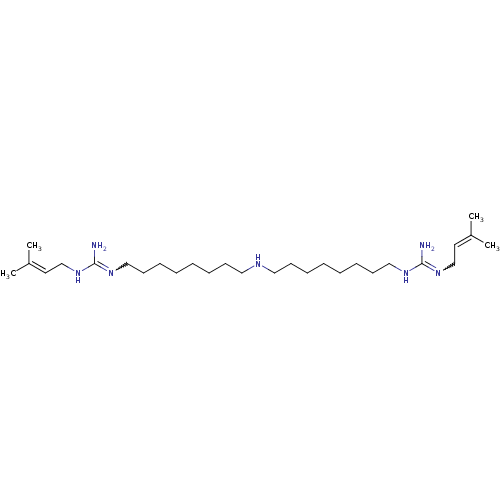 (1,1'-(8,8'-azanediylbis(octane-8,1-diyl))bis(3-(3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294104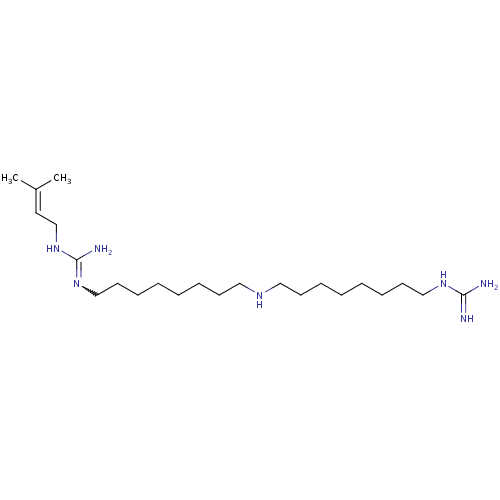 (1-(Guanidino)-17-(N1-(gamma,gamma-dimethylallyl)gu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA-dNTP ternary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50032500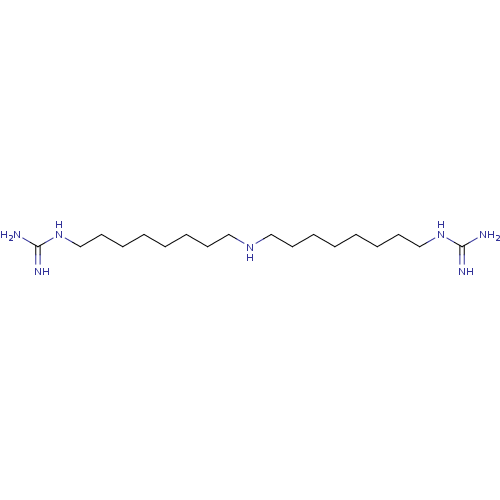 (1,1'-(8,8'-azanediylbis(octane-8,1-diyl))diguanidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479681 (CHEMBL478258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA binary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479681 (CHEMBL478258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA-dNTP ternary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294112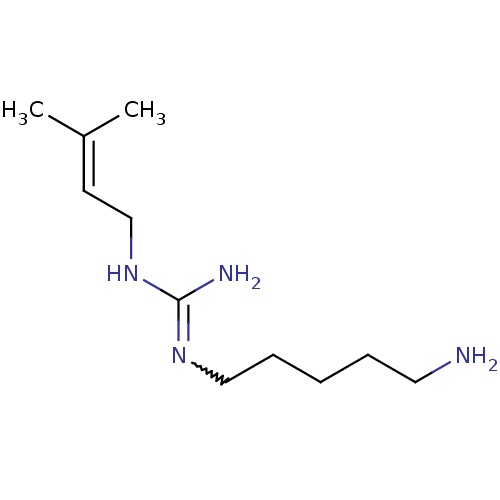 (1-(5-aminopentyl)-3-(3-methylbut-2-enyl)guanidine ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479681 (CHEMBL478258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 free reverse transcriptase | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294111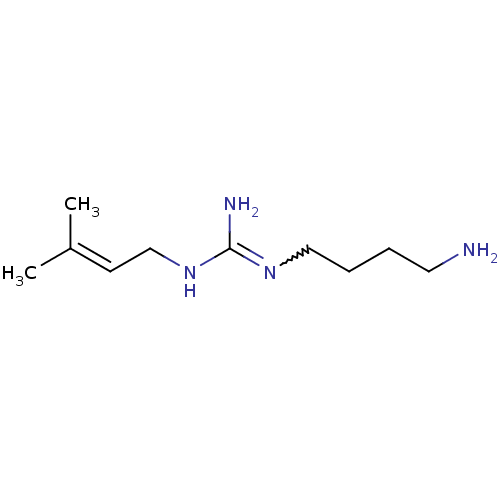 (1-(4-aminobutyl)-3-(3-methylbut-2-enyl)guanidine |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294113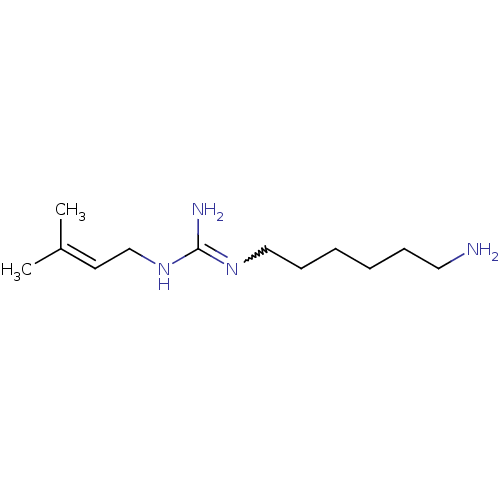 (1-(6-aminohexyl)-3-(3-methylbut-2-enyl)guanidine |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479681 (CHEMBL478258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA-dNTP ternary complex expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 free reverse transcriptase | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479680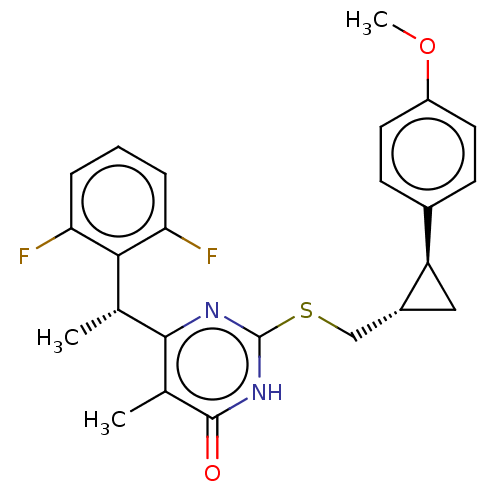 (CHEMBL459082) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA binary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA binary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479680 (CHEMBL459082) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA-dNTP ternary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479680 (CHEMBL459082) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 free reverse transcriptase | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479681 (CHEMBL478258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA binary complex expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479681 (CHEMBL478258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant free reverse transcriptase K103N mutant expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294114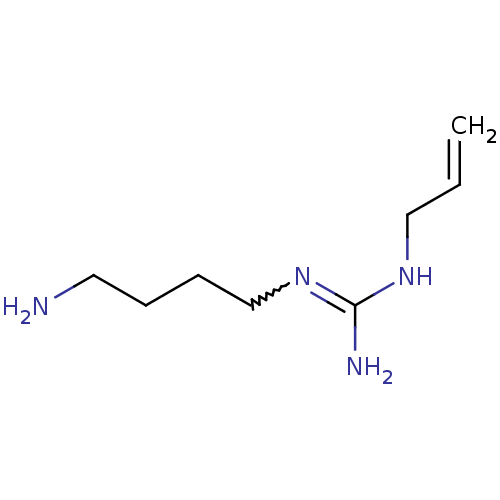 (1-allyl-3-(4-aminobutyl)guanidine | CHEMBL541766) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA-dNTP ternary complex expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294095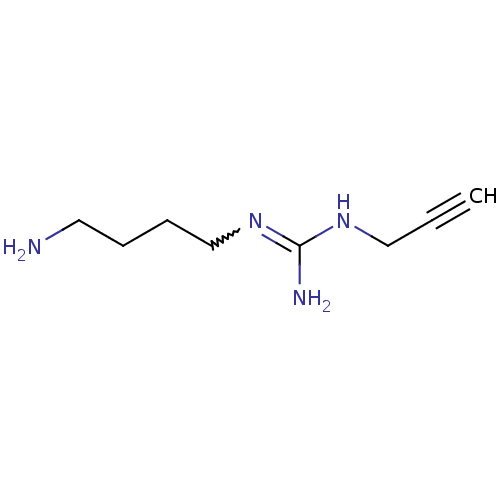 (1-(4-aminobutyl)-3-(prop-2-ynyl)guanidine | CHEMBL...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479680 (CHEMBL459082) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA-dNTP ternary complex expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50129716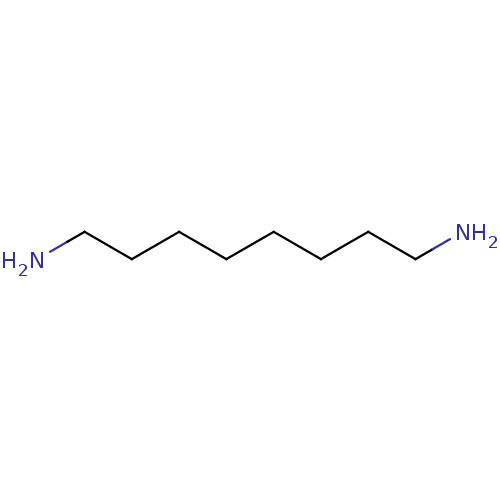 (1,8-diaminooctane | CHEMBL29392 | OCTANE 1,8-DIAMI...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479680 (CHEMBL459082) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA binary complex expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 free reverse transcriptase | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA-dNTP ternary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA binary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479680 (CHEMBL459082) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant free reverse transcriptase K103N mutant expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294094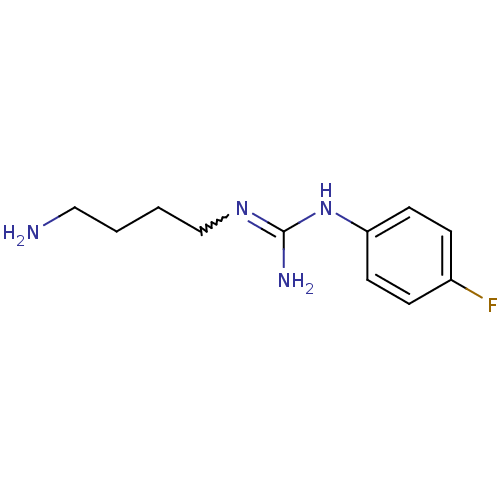 (1-(4-aminobutyl)-3-(4-fluorophenyl)guanidine | CHE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294103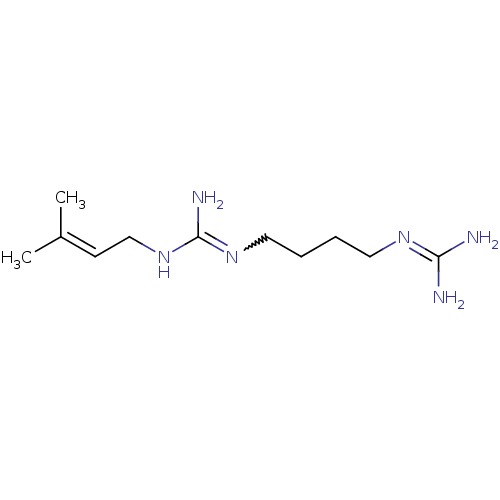 (4-[N3-gamma,gamma-Dimethyallyl)guanidino]-1-guanid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294100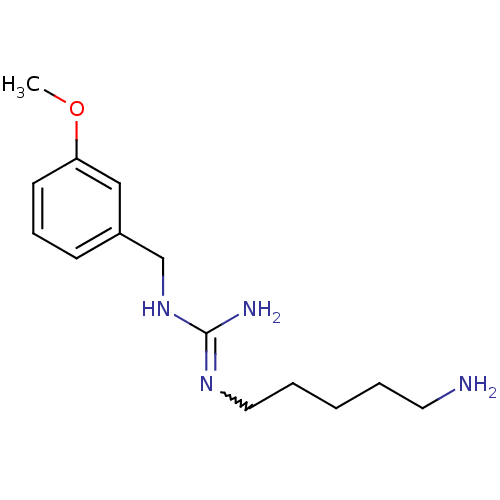 (CHEMBL550832 | N1-(5-Aminopentyl)-N3-(3-methoxyben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294102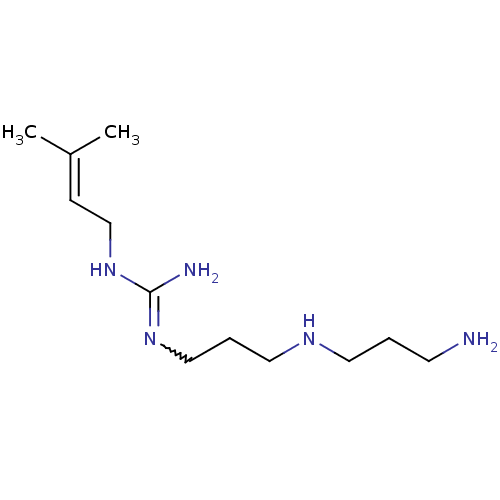 (CHEMBL553638 | N1-[3'-Aminopropyl)-3-aminopropyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant free reverse transcriptase K103N mutant expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294096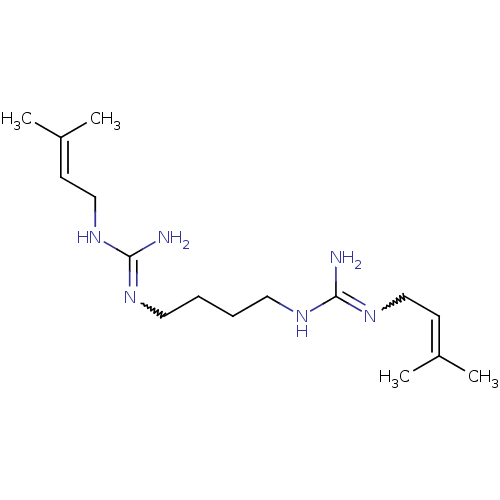 (CHEMBL561237 | N-(3-Methyl-but-2-enyl)-N'-{4-[N'-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294099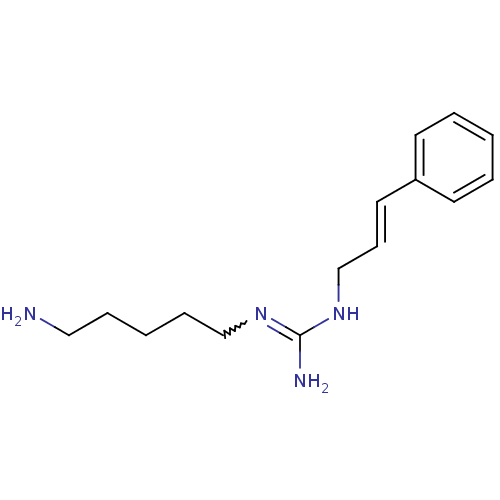 (CHEMBL561962 | N1-(5-Aminopentyl)-N3[(E)-3-phenyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA binary complex expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294097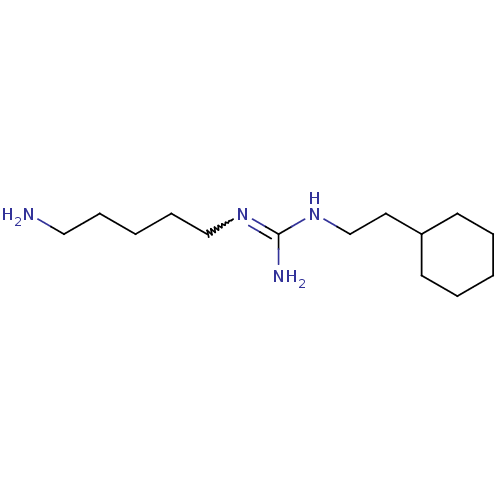 (CHEMBL550629 | N1-(5-Aminopentyl)-N3-(cyclohexylet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294098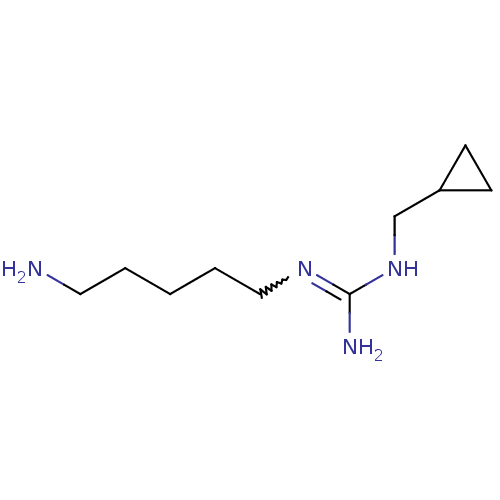 (CHEMBL552343 | N1-(5-Aminopentyl)-N3-(cyclopropylm...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM85213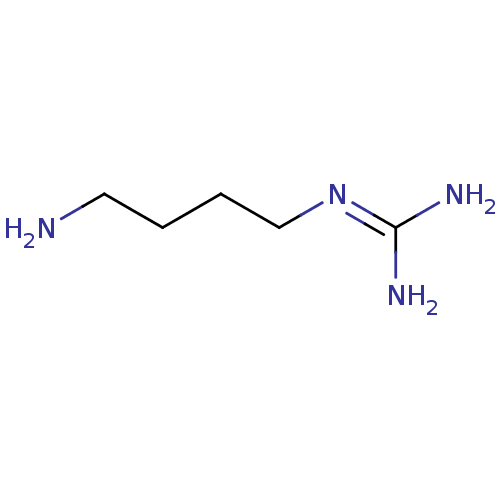 (Agmatine | CAS_306-60-5 | CHEMBL58343 | NSC_199 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA binary complex expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant free reverse transcriptase K103N mutant expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N mutant-DNA-dNTP ternary complex expressed in Escherichia coli BL21 | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine oxidase 1 (Zea mays) | BDBM50294101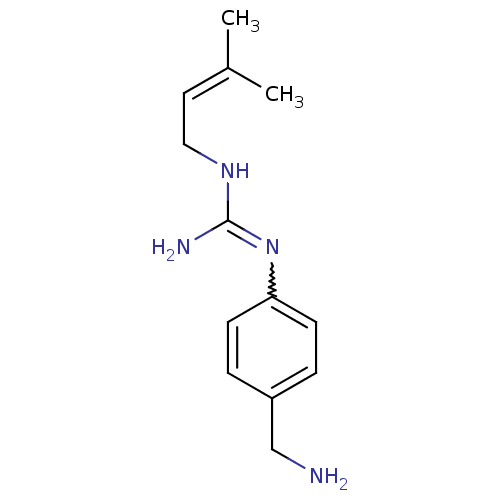 (CHEMBL558092 | N1-Benzylamine-N3-(gamma,gamma-dime...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method | J Med Chem 52: 4774-85 (2009) Article DOI: 10.1021/jm900371z BindingDB Entry DOI: 10.7270/Q2TB16W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50427868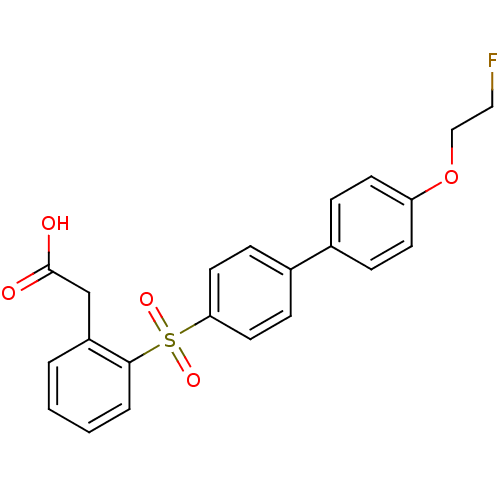 (CHEMBL2326366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale"Amedeo Avogadro" Curated by ChEMBL | Assay Description Inhibition of MMP-2 (unknown origin) using Mca-Lys-Pro-Leu-Gly-Leu-Dap(Dnp)-Ala-Arg-NH2 as substrate after 4 hrs by fluorometric assay | J Med Chem 56: 2676-89 (2013) Article DOI: 10.1021/jm4001743 BindingDB Entry DOI: 10.7270/Q2028SWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50427868 (CHEMBL2326366) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale"Amedeo Avogadro" Curated by ChEMBL | Assay Description Inhibition of MMP-13 (unknown origin) using Mca-Lys-Pro-Leu-Gly-Leu-Dap(Dnp)-Ala-Arg-NH2 as substrate after 4 hrs by fluorometric assay | J Med Chem 56: 2676-89 (2013) Article DOI: 10.1021/jm4001743 BindingDB Entry DOI: 10.7270/Q2028SWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 128 total ) | Next | Last >> |