

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50276262 (CHEMBL4129609) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay | Bioorg Med Chem Lett 28: 2408-2412 (2018) Article DOI: 10.1016/j.bmcl.2018.06.022 BindingDB Entry DOI: 10.7270/Q2T72KZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50276262 (CHEMBL4129609) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Binding affinity to EP4 receptor (unknown origin) | Bioorg Med Chem Lett 28: 2408-2412 (2018) Article DOI: 10.1016/j.bmcl.2018.06.022 BindingDB Entry DOI: 10.7270/Q2T72KZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50276262 (CHEMBL4129609) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Binding affinity to EP3 receptor (unknown origin) | Bioorg Med Chem Lett 28: 2408-2412 (2018) Article DOI: 10.1016/j.bmcl.2018.06.022 BindingDB Entry DOI: 10.7270/Q2T72KZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50276262 (CHEMBL4129609) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Binding affinity to EP2 receptor (unknown origin) | Bioorg Med Chem Lett 28: 2408-2412 (2018) Article DOI: 10.1016/j.bmcl.2018.06.022 BindingDB Entry DOI: 10.7270/Q2T72KZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50276262 (CHEMBL4129609) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay | Bioorg Med Chem Lett 28: 2408-2412 (2018) Article DOI: 10.1016/j.bmcl.2018.06.022 BindingDB Entry DOI: 10.7270/Q2T72KZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038898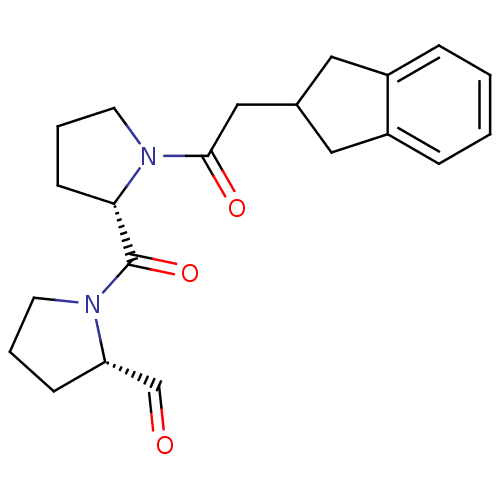 ((S)-1-((S)-1-(2-(2,3-dihydro-1H-inden-2-yl)acetyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038897 ((S)-1-[(S)-1-((S)-2-1,2,3,4-Tetrahydro-naphthalen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038899 ((S)-1-[(R)-3-((S)-2-1,2,3,4-Tetrahydro-naphthalen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038891 ((S)-1-[(S)-1-((S)-2-1,2,3,4-Tetrahydro-naphthalen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038893 ((S)-1-[(R)-3-((S)-2-1,2,3,4-Tetrahydro-naphthalen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50449137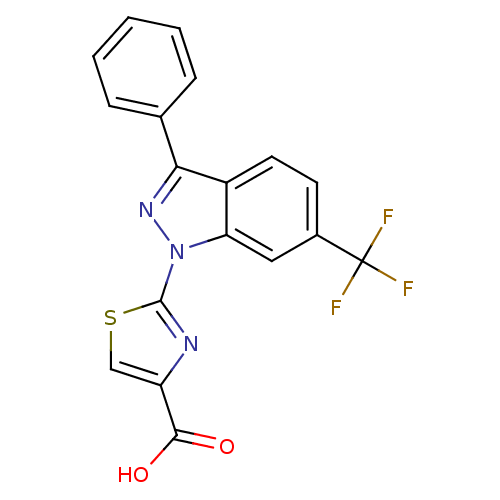 (CHEMBL3127163) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay | Bioorg Med Chem Lett 28: 2408-2412 (2018) Article DOI: 10.1016/j.bmcl.2018.06.022 BindingDB Entry DOI: 10.7270/Q2T72KZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50276250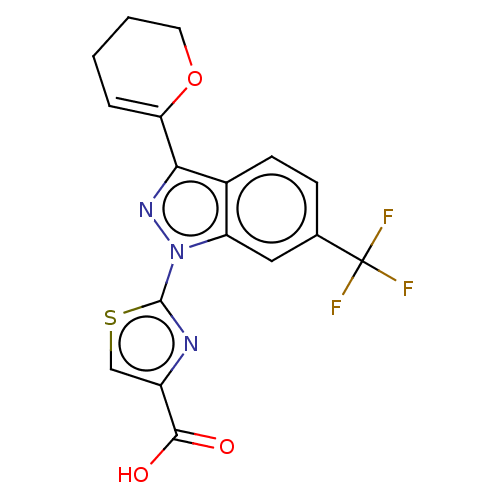 (CHEMBL4126319) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay | Bioorg Med Chem Lett 28: 2408-2412 (2018) Article DOI: 10.1016/j.bmcl.2018.06.022 BindingDB Entry DOI: 10.7270/Q2T72KZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038868 ((R)-3-[(R)-3-((S)-2-1,2,3,4-Tetrahydro-naphthalen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038876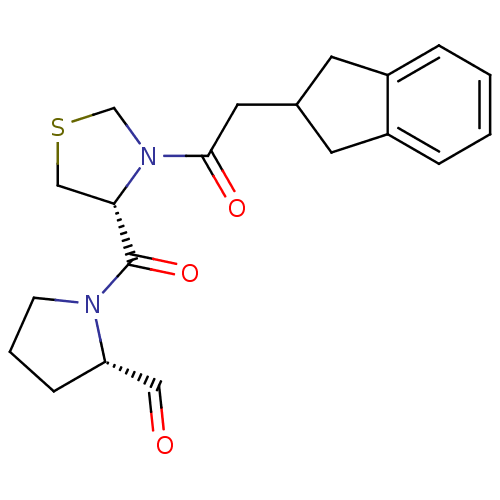 ((S)-1-[(R)-3-(2-Indan-2-yl-acetyl)-thiazolidine-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038879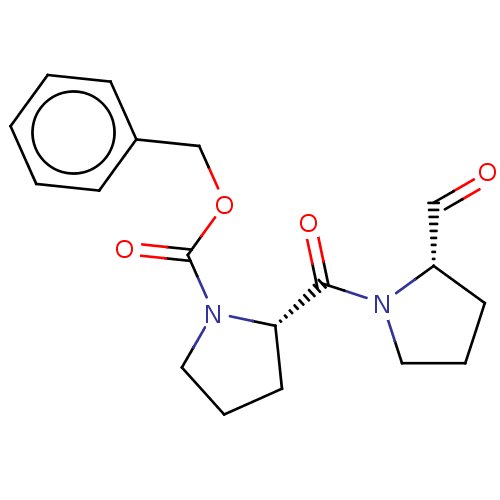 ((S)-2-(2-Formyl-pyrrolidine-1-carbonyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038885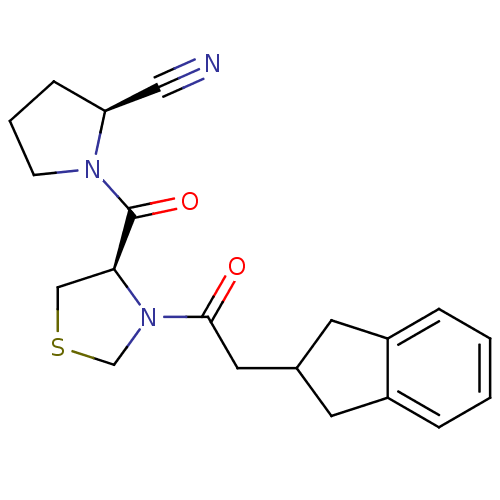 ((S)-1-[(R)-3-(2-Indan-2-yl-acetyl)-thiazolidine-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038889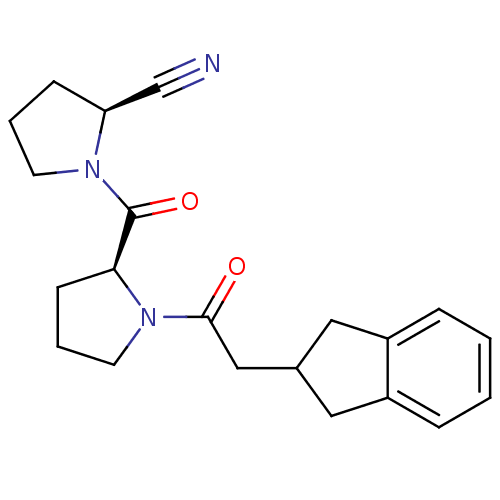 ((S)-1-((S)-1-(2-(2,3-dihydro-1H-inden-2-yl)acetyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038864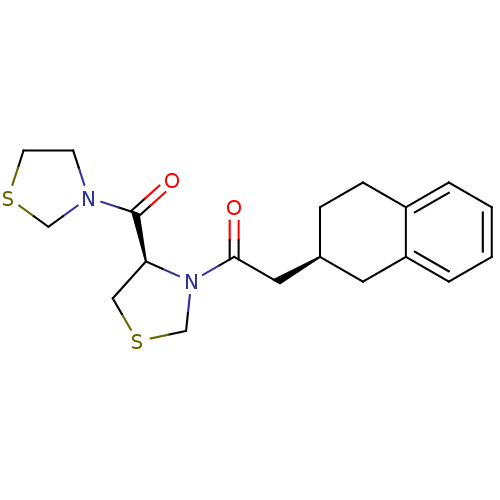 (2-(S)-1,2,3,4-Tetrahydro-naphthalen-2-yl-1-[(R)-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038887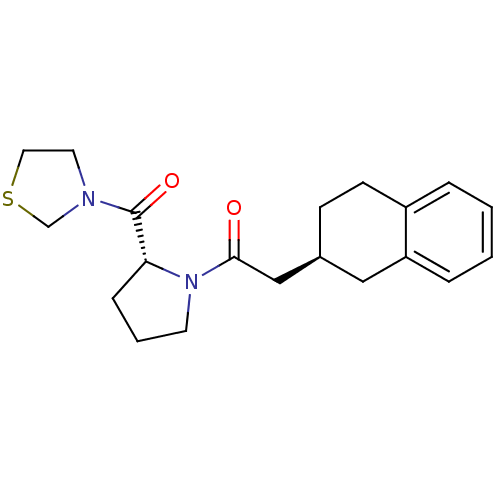 (2-(S)-1,2,3,4-Tetrahydro-naphthalen-2-yl-1-[(R)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038895 (1-[(R)-4-(Pyrrolidine-1-carbonyl)-thiazolidin-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50275347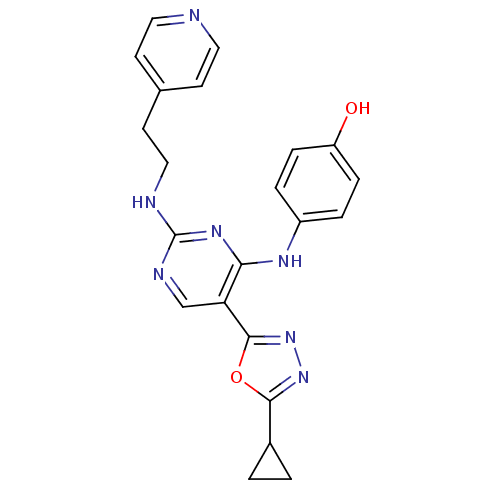 (4-(5-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)-2-(2-(py...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged FLT3 (unknown origin) by ELISA | Bioorg Med Chem Lett 18: 5472-7 (2008) Article DOI: 10.1016/j.bmcl.2008.09.031 BindingDB Entry DOI: 10.7270/Q2WD40DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50276003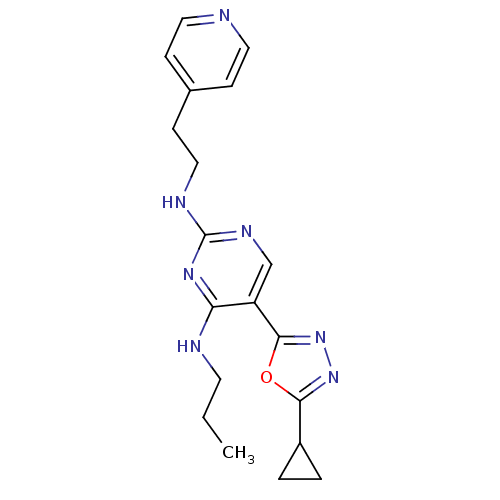 (5-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)-N4-propyl-N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged FLT3 (unknown origin) by ELISA | Bioorg Med Chem Lett 18: 5472-7 (2008) Article DOI: 10.1016/j.bmcl.2008.09.031 BindingDB Entry DOI: 10.7270/Q2WD40DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038883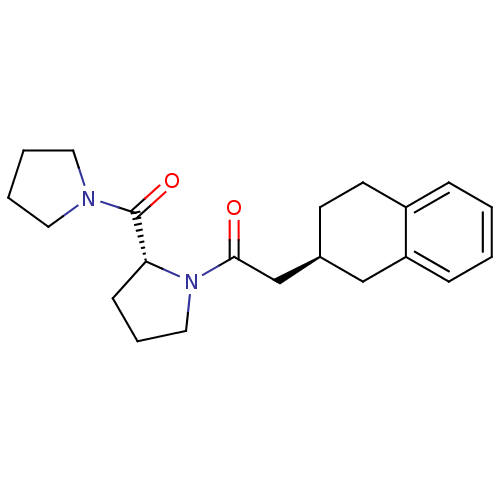 (1-[(R)-2-(Pyrrolidine-1-carbonyl)-pyrrolidin-1-yl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038888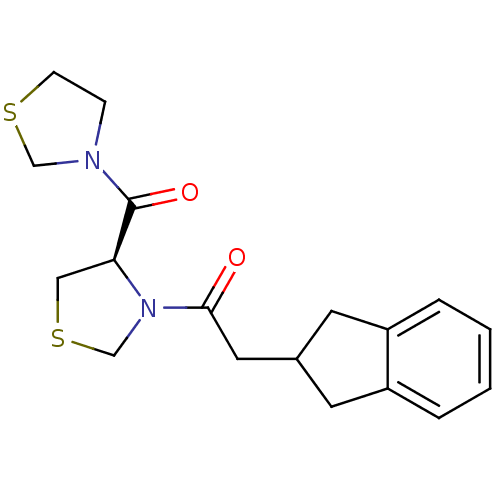 (2-Indan-2-yl-1-[(R)-4-(thiazolidine-3-carbonyl)-th...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038866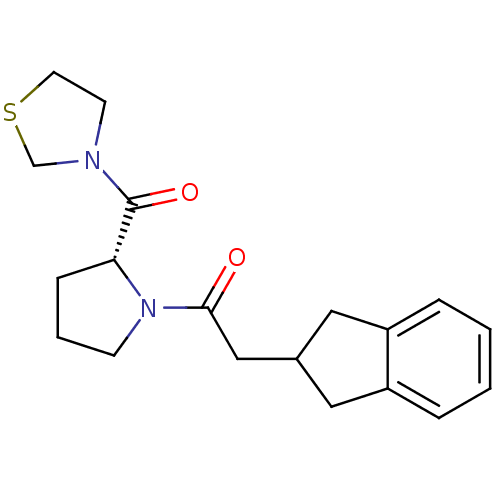 (2-Indan-2-yl-1-[(R)-2-(thiazolidine-3-carbonyl)-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038884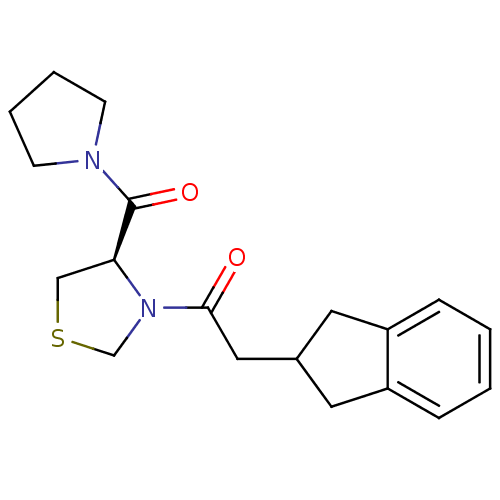 (2-Indan-2-yl-1-[(R)-4-(pyrrolidine-1-carbonyl)-thi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482699 (CHEMBL1241174) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity expressed in Escherichia coli after 60 mins | Bioorg Med Chem 18: 6771-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.050 BindingDB Entry DOI: 10.7270/Q23N2661 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50276264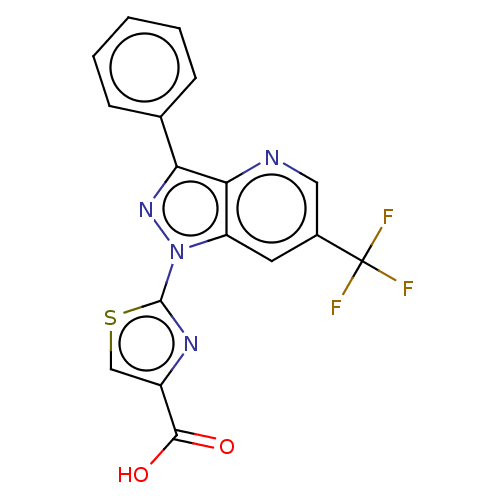 (CHEMBL4128163) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay | Bioorg Med Chem Lett 28: 2408-2412 (2018) Article DOI: 10.1016/j.bmcl.2018.06.022 BindingDB Entry DOI: 10.7270/Q2T72KZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50276265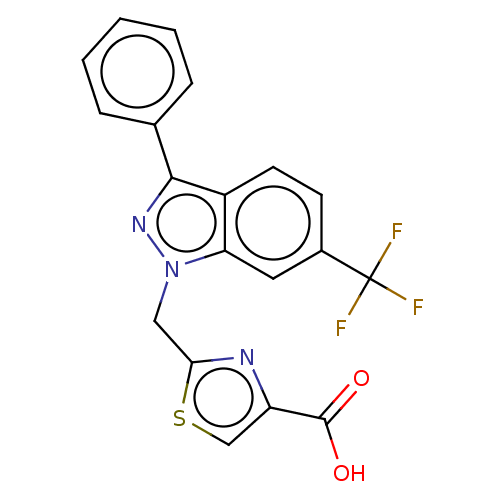 (CHEMBL4129545) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay | Bioorg Med Chem Lett 28: 2408-2412 (2018) Article DOI: 10.1016/j.bmcl.2018.06.022 BindingDB Entry DOI: 10.7270/Q2T72KZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50276263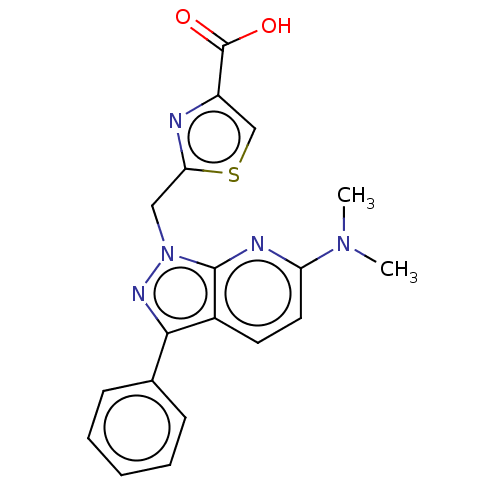 (CHEMBL4129401) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay | Bioorg Med Chem Lett 28: 2408-2412 (2018) Article DOI: 10.1016/j.bmcl.2018.06.022 BindingDB Entry DOI: 10.7270/Q2T72KZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50276249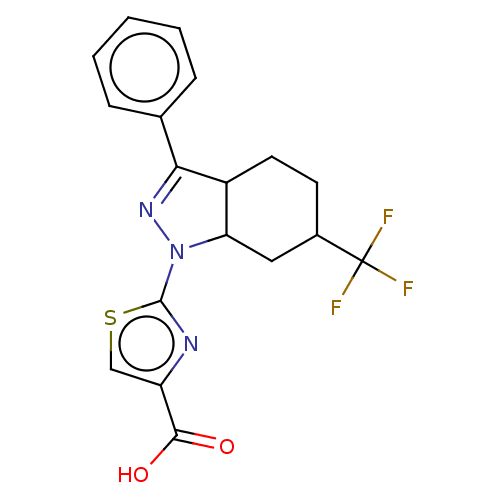 (CHEMBL4126167) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay | Bioorg Med Chem Lett 28: 2408-2412 (2018) Article DOI: 10.1016/j.bmcl.2018.06.022 BindingDB Entry DOI: 10.7270/Q2T72KZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038894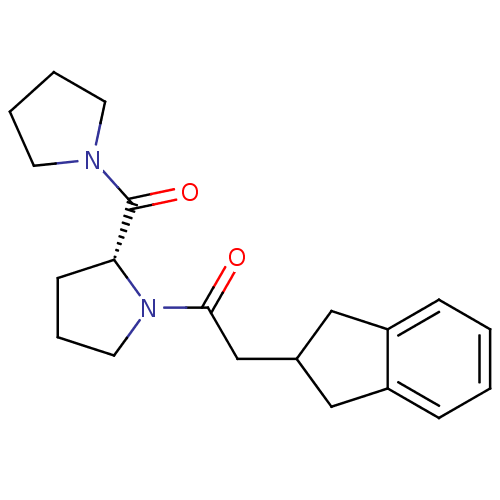 (2-Indan-2-yl-1-[(R)-2-(pyrrolidine-1-carbonyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038896 (1-[(R)-4-(Pyrrolidine-1-carbonyl)-thiazolidin-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038886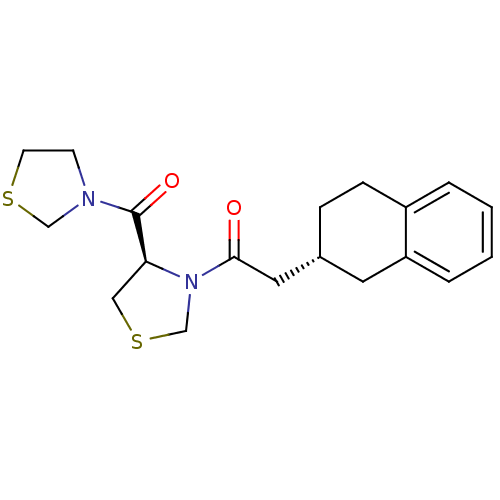 (2-(R)-1,2,3,4-Tetrahydro-naphthalen-2-yl-1-[(R)-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482700 (CHEMBL1241178) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity expressed in Escherichia coli after 60 mins | Bioorg Med Chem 18: 6771-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.050 BindingDB Entry DOI: 10.7270/Q23N2661 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50275348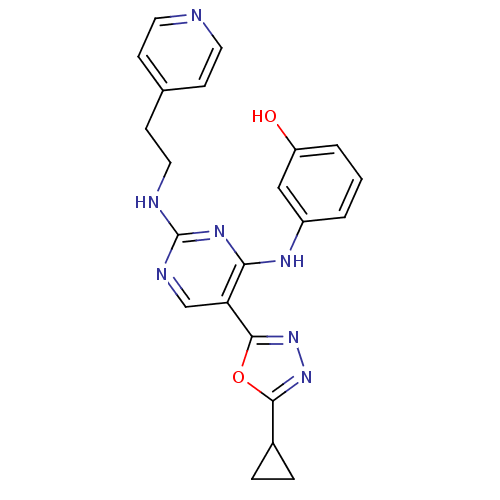 (3-(5-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)-2-(2-(py...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged FLT3 (unknown origin) by ELISA | Bioorg Med Chem Lett 18: 5472-7 (2008) Article DOI: 10.1016/j.bmcl.2008.09.031 BindingDB Entry DOI: 10.7270/Q2WD40DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50276053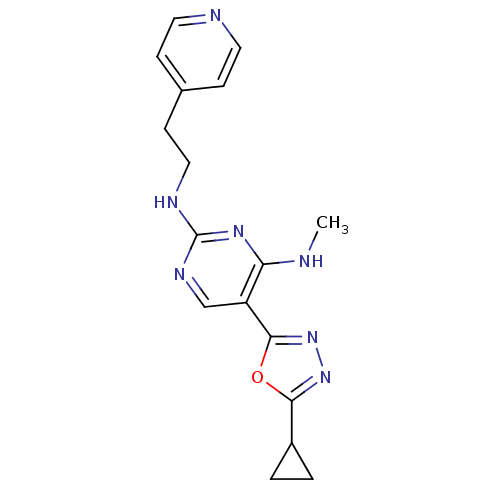 (5-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)-N4-methyl-N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged FLT3 (unknown origin) by ELISA | Bioorg Med Chem Lett 18: 5472-7 (2008) Article DOI: 10.1016/j.bmcl.2008.09.031 BindingDB Entry DOI: 10.7270/Q2WD40DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038880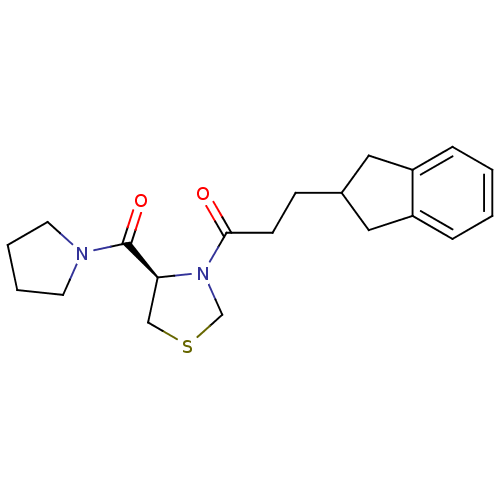 (3-Indan-2-yl-1-[(R)-4-(pyrrolidine-1-carbonyl)-thi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038890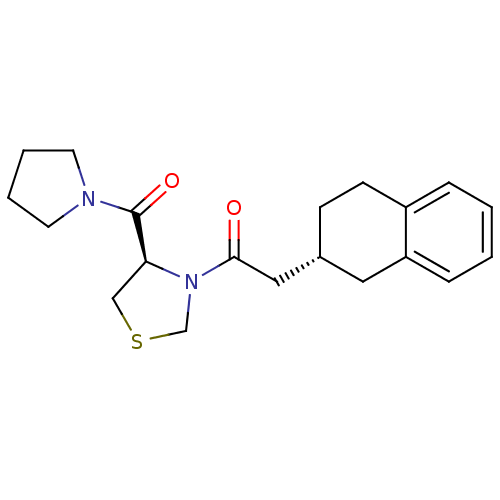 (1-[(R)-4-(Pyrrolidine-1-carbonyl)-thiazolidin-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482160 (CHEMBL1082257) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity expressed in Escherichia coli after 60 mins | Bioorg Med Chem 18: 6771-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.050 BindingDB Entry DOI: 10.7270/Q23N2661 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50276266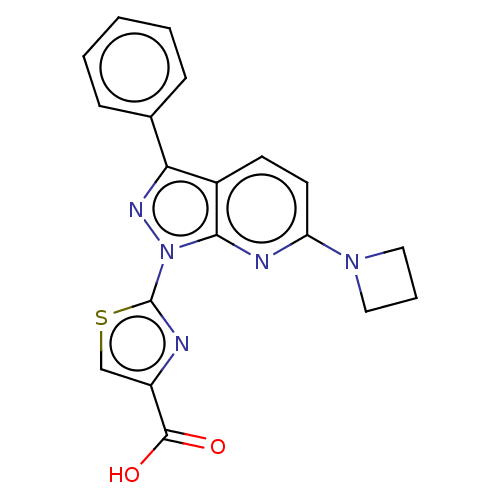 (CHEMBL4126096) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay | Bioorg Med Chem Lett 28: 2408-2412 (2018) Article DOI: 10.1016/j.bmcl.2018.06.022 BindingDB Entry DOI: 10.7270/Q2T72KZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038873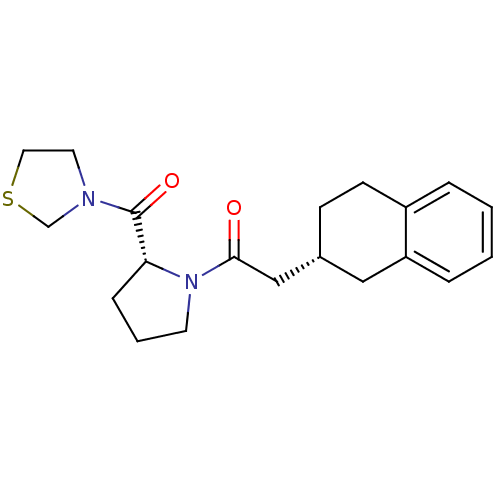 (2-(R)-1,2,3,4-Tetrahydro-naphthalen-2-yl-1-[(R)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482700 (CHEMBL1241178) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-processing activity expressed in Escherichia coli after 60 mins | Bioorg Med Chem 18: 6771-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.050 BindingDB Entry DOI: 10.7270/Q23N2661 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482714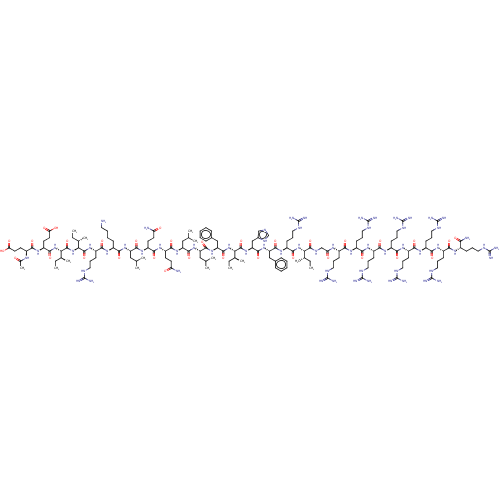 (CHEMBL1241175) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity expressed in Escherichia coli after 60 mins | Bioorg Med Chem 18: 6771-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.050 BindingDB Entry DOI: 10.7270/Q23N2661 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50276056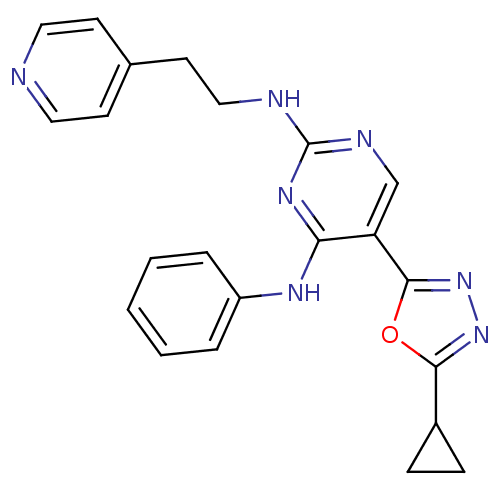 (5-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)-N4-phenyl-N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged FLT3 (unknown origin) by ELISA | Bioorg Med Chem Lett 18: 5472-7 (2008) Article DOI: 10.1016/j.bmcl.2008.09.031 BindingDB Entry DOI: 10.7270/Q2WD40DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038871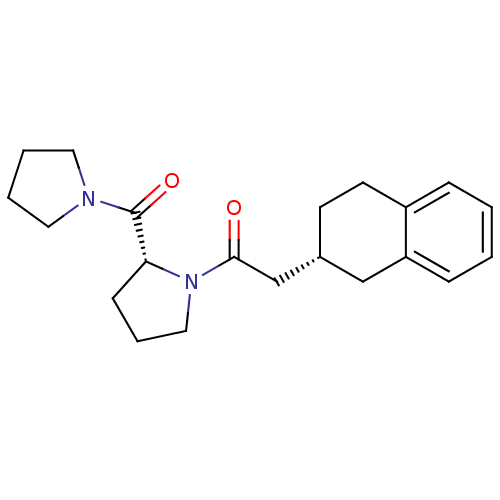 (1-[(R)-2-(Pyrrolidine-1-carbonyl)-pyrrolidin-1-yl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50051539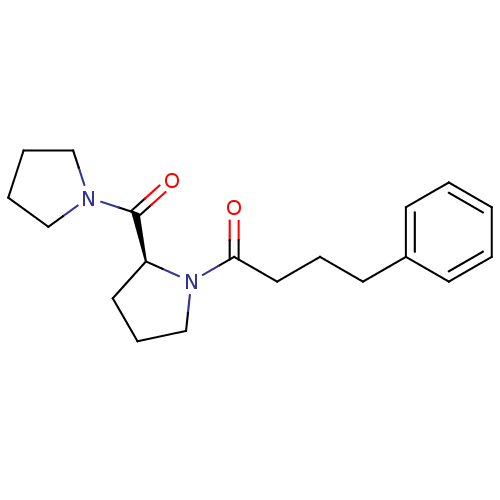 ((S)-4-phenyl-1-(2-(pyrrolidine-1-carbonyl)pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482698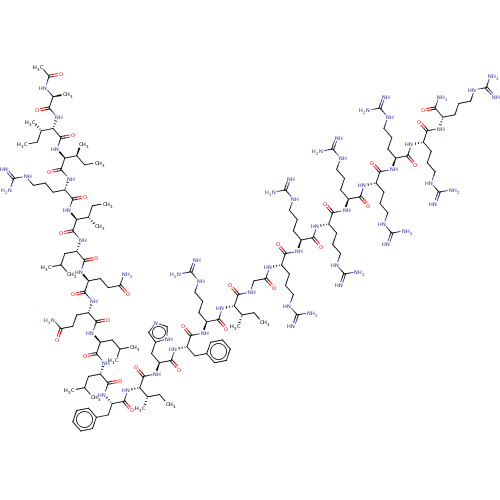 (CHEMBL1241173) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity expressed in Escherichia coli after 60 mins | Bioorg Med Chem 18: 6771-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.050 BindingDB Entry DOI: 10.7270/Q23N2661 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482710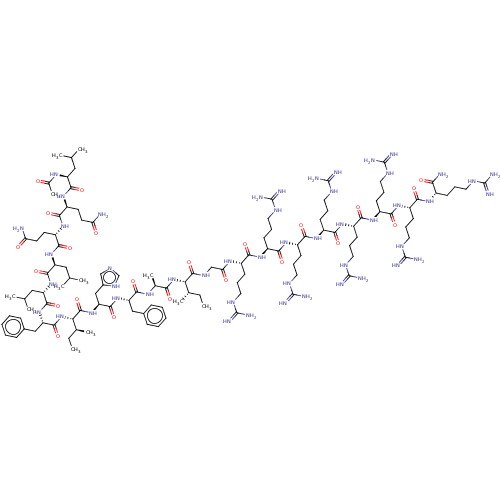 (CHEMBL1241189) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity expressed in Escherichia coli after 60 mins | Bioorg Med Chem 18: 6771-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.050 BindingDB Entry DOI: 10.7270/Q23N2661 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482699 (CHEMBL1241174) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-processing activity expressed in Escherichia coli after 60 mins | Bioorg Med Chem 18: 6771-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.050 BindingDB Entry DOI: 10.7270/Q23N2661 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 154 total ) | Next | Last >> |