

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50040108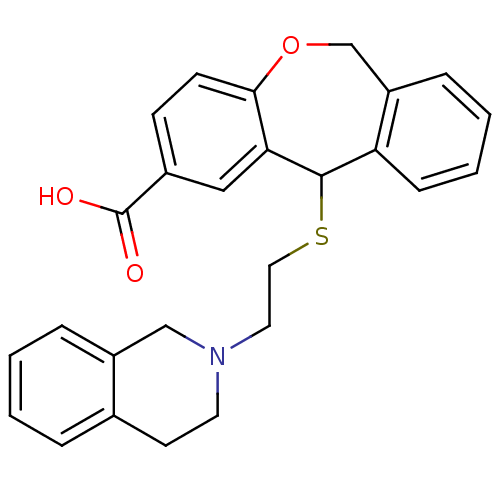 (11-[2-(3,4-Dihydro-1H-isoquinolin-2-yl)-ethylsulfa...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002084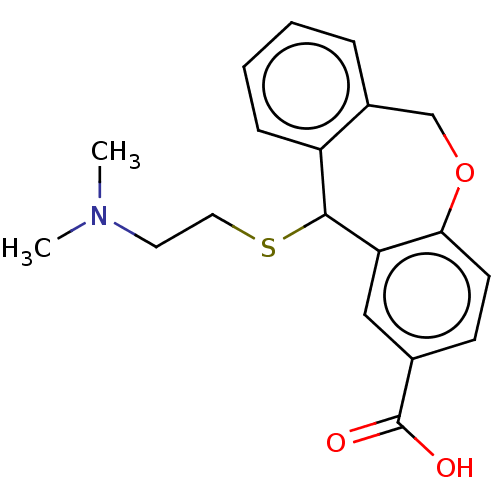 (11-(2-Dimethylamino-ethylsulfanyl)-6,11-dihydro-di...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50040114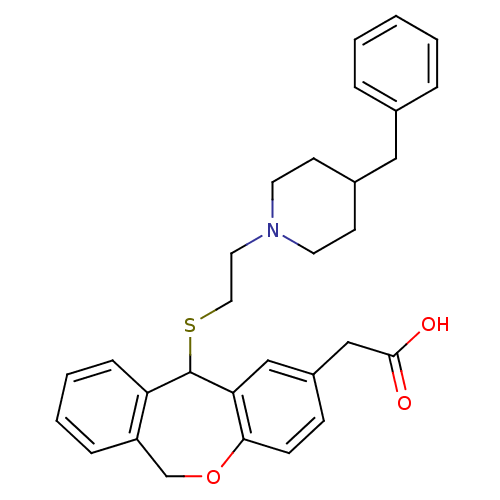 (CHEMBL336198 | {11-[2-(4-Benzyl-piperidin-1-yl)-et...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002088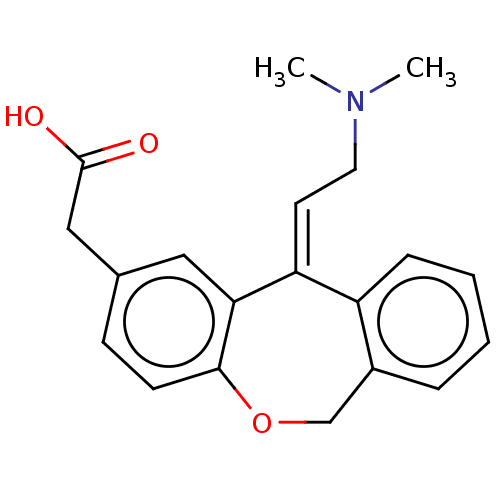 (CHEMBL302005 | [11-(2-Dimethylamino-ethylidene)-6,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50040109 (3-{11-[2-(4-Benzyl-piperidin-1-yl)-ethylsulfanyl]-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50040112 (11-[2-(4-Benzyl-piperazin-1-yl)-ethylsulfanyl]-6,1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50175516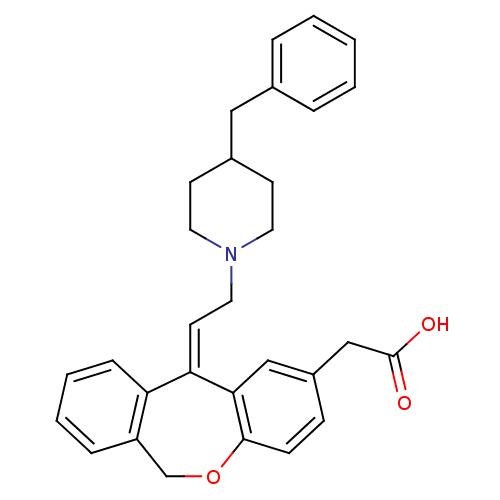 (CHEMBL372588 | {11-[2-(4-Benzyl-piperidin-1-yl)-et...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50040110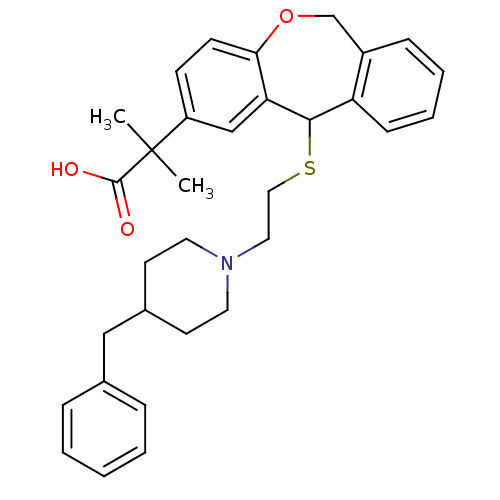 (2-{11-[2-(4-Benzyl-piperidin-1-yl)-ethylsulfanyl]-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50040111 (CHEMBL133080 | {11-[2-(4-Benzyl-piperidin-1-yl)-et...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50040121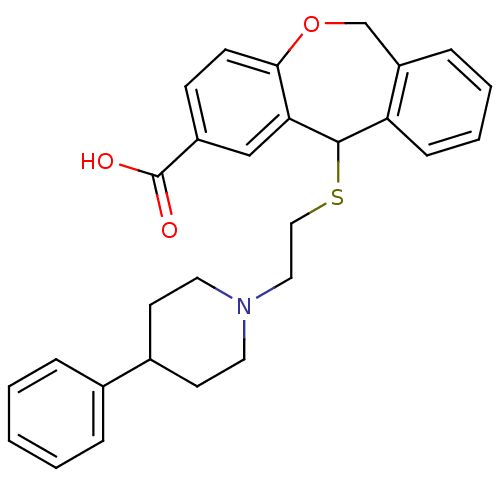 (11-[2-(4-Phenyl-piperidin-1-yl)-ethylsulfanyl]-6,1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50040113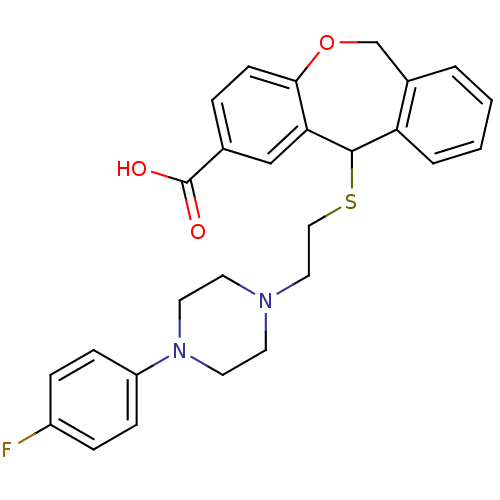 (11-{2-[4-(4-Fluoro-phenyl)-piperazin-1-yl]-ethylsu...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50040116 (11-(2-Morpholin-4-yl-ethylsulfanyl)-6,11-dihydro-d...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50040119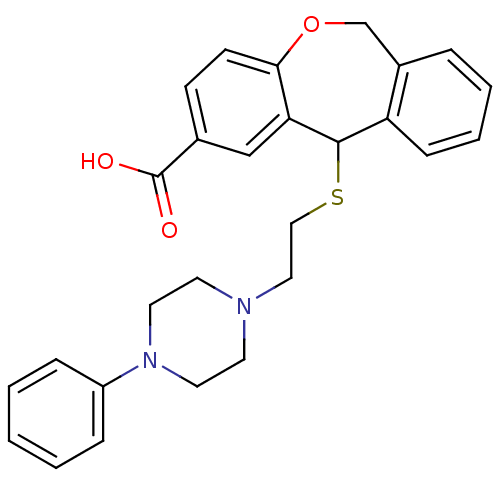 (11-[2-(4-Phenyl-piperazin-1-yl)-ethylsulfanyl]-6,1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50040117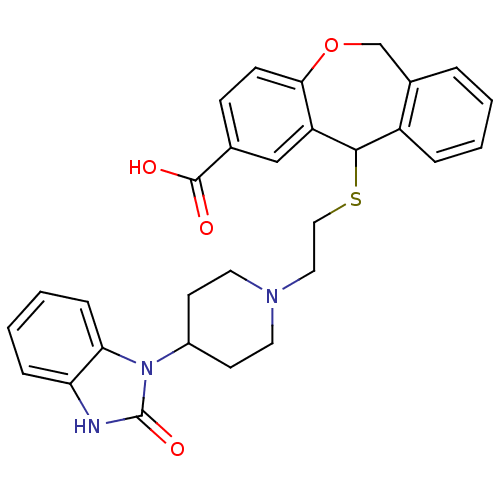 (11-{2-[4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-pi...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50040115 (CHEMBL133778 | Sodium; 11-[2-(4-benzyl-piperidin-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50227346 (CHEMBL319928) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity was measured for Muscarinic acetylcholine receptor | J Med Chem 31: 779-85 (1988) BindingDB Entry DOI: 10.7270/Q2FN18D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50227346 (CHEMBL319928) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity was measured for Muscarinic acetylcholine receptor | J Med Chem 31: 779-85 (1988) BindingDB Entry DOI: 10.7270/Q2FN18D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50227346 (CHEMBL319928) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity was measured for Muscarinic acetylcholine receptor | J Med Chem 31: 779-85 (1988) BindingDB Entry DOI: 10.7270/Q2FN18D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062251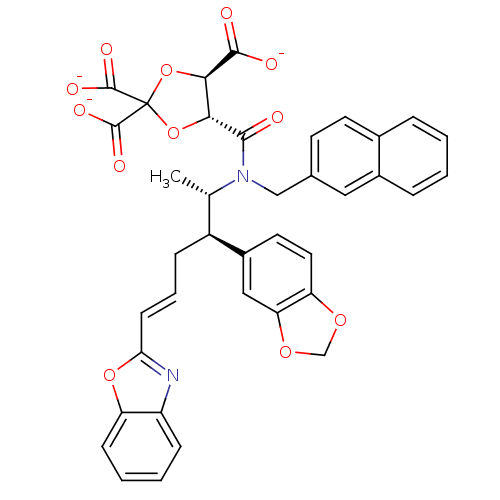 (CHEMBL285263 | trisodium 5-[2-benzo[d][1,3]dioxol-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062250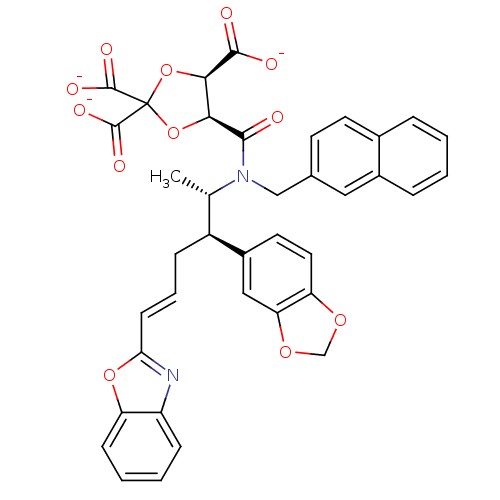 (CHEMBL36407 | trisodium 5-[2-benzo[d][1,3]dioxol-5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062249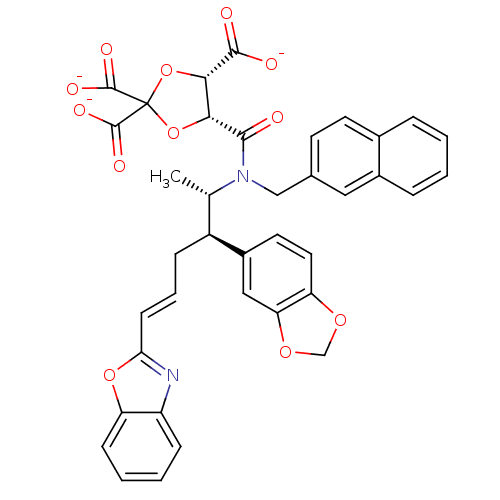 (CHEMBL285434 | trisodium 5-[2-benzo[d][1,3]dioxol-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062249 (CHEMBL285434 | trisodium 5-[2-benzo[d][1,3]dioxol-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase in competitive manner with respect to FPP (farnesyl diphosphate) at 0.6 microM FPP and 0.36 microM Ras peptide | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062252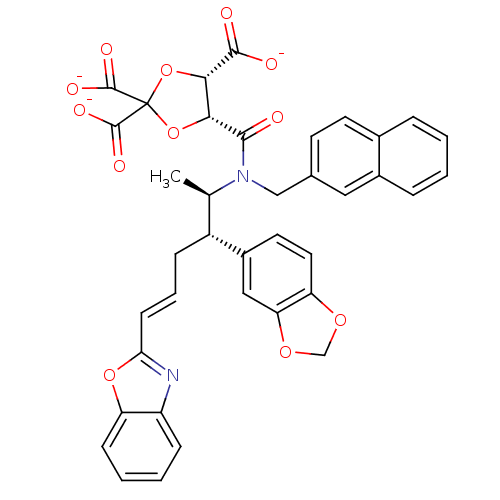 (CHEMBL36339 | trisodium 5-[2-benzo[d][1,3]dioxol-5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50040815 (2-Methylsulfanyl-6,11-dihydro-dibenzo[b,e]oxepine-...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase in liver microsomes isolated from cholesterol-fed rabbits | J Med Chem 37: 804-10 (1994) BindingDB Entry DOI: 10.7270/Q20C4TTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062248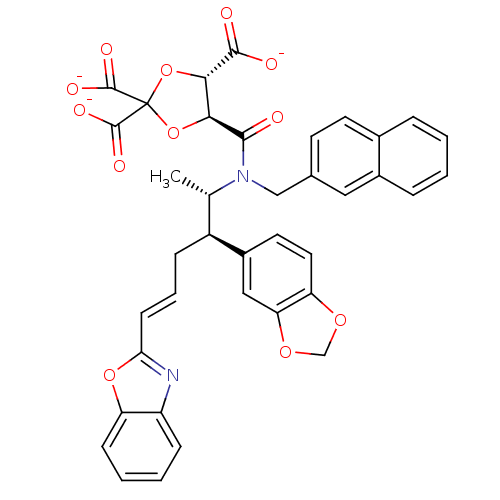 (CHEMBL36515 | trisodium 5-[2-benzo[d][1,3]dioxol-5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062249 (CHEMBL285434 | trisodium 5-[2-benzo[d][1,3]dioxol-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase in competitive manner with respect to FPP (farnesyl diphosphate) at 0.6 microM FPP and 3.6 microM Ras peptide. | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062256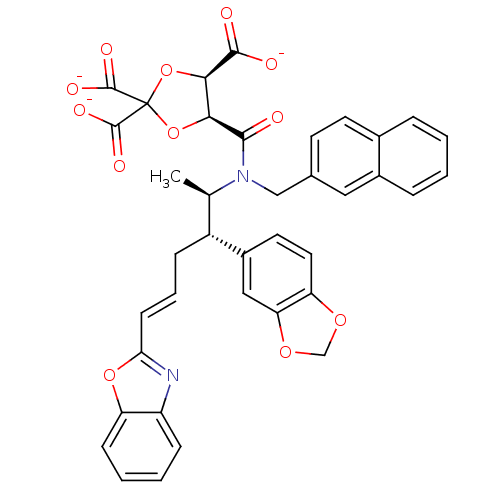 (CHEMBL284073 | trisodium 5-[2-benzo[d][1,3]dioxol-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50284967 (1-[1-(2,6-Dichloro-phenyl)-1H-benzoimidazol-2-yl]-...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity towards acyl CoA cholesterol acyltransferase (ACAT) of cholesterol-fed rabbits by the displacement of [14C]-oleolyl-CoA | Bioorg Med Chem Lett 5: 1829-1832 (1995) Article DOI: 10.1016/0960-894X(95)00296-6 BindingDB Entry DOI: 10.7270/Q2VD6ZDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50284976 (1-[1-(2-Chloro-phenyl)-1H-benzoimidazol-2-yl]-3-(2...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity towards acyl CoA cholesterol acyltransferase (ACAT) of cholesterol-fed rabbits by the displacement of [14C]-oleolyl-CoA | Bioorg Med Chem Lett 5: 1829-1832 (1995) Article DOI: 10.1016/0960-894X(95)00296-6 BindingDB Entry DOI: 10.7270/Q2VD6ZDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50284971 (1-(2,6-Diisopropyl-phenyl)-3-(1-o-tolyl-1H-benzoim...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity towards acyl CoA cholesterol acyltransferase (ACAT) of cholesterol-fed rabbits by the displacement of [14C]-oleolyl-CoA | Bioorg Med Chem Lett 5: 1829-1832 (1995) Article DOI: 10.1016/0960-894X(95)00296-6 BindingDB Entry DOI: 10.7270/Q2VD6ZDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50284972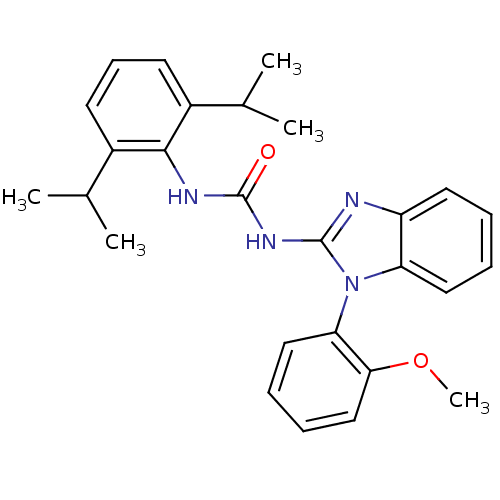 (1-(2,6-Diisopropyl-phenyl)-3-[1-(2-methoxy-phenyl)...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity towards acyl CoA cholesterol acyltransferase (ACAT) of cholesterol-fed rabbits by the displacement of [14C]-oleolyl-CoA | Bioorg Med Chem Lett 5: 1829-1832 (1995) Article DOI: 10.1016/0960-894X(95)00296-6 BindingDB Entry DOI: 10.7270/Q2VD6ZDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50284979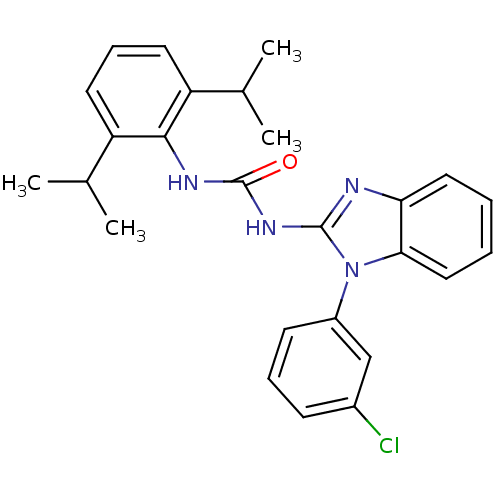 (1-[1-(3-Chloro-phenyl)-1H-benzoimidazol-2-yl]-3-(2...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity towards acyl CoA cholesterol acyltransferase (ACAT) of cholesterol-fed rabbits by the displacement of [14C]-oleolyl-CoA | Bioorg Med Chem Lett 5: 1829-1832 (1995) Article DOI: 10.1016/0960-894X(95)00296-6 BindingDB Entry DOI: 10.7270/Q2VD6ZDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50284973 (1-[1-(2-Bromo-phenyl)-1H-benzoimidazol-2-yl]-3-(2,...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity towards acyl CoA cholesterol acyltransferase (ACAT) of cholesterol-fed rabbits by the displacement of [14C]-oleolyl-CoA | Bioorg Med Chem Lett 5: 1829-1832 (1995) Article DOI: 10.1016/0960-894X(95)00296-6 BindingDB Entry DOI: 10.7270/Q2VD6ZDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50040830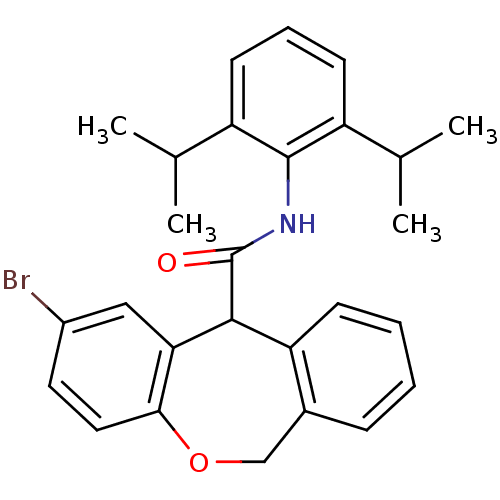 (2-Bromo-6,11-dihydro-dibenzo[b,e]oxepine-11-carbox...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase in liver microsomes isolated from cholesterol-fed rabbits | J Med Chem 37: 804-10 (1994) BindingDB Entry DOI: 10.7270/Q20C4TTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062258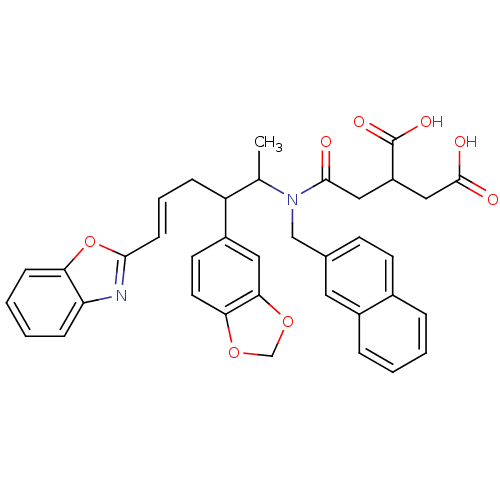 (2-{[((E)-2-Benzo[1,3]dioxol-5-yl-5-benzooxazol-2-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50040849 (5H-Dibenzo[a,d]cycloheptene-5-carboxylic acid (2,6...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase in liver microsomes isolated from cholesterol-fed rabbits | J Med Chem 37: 804-10 (1994) BindingDB Entry DOI: 10.7270/Q20C4TTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50040854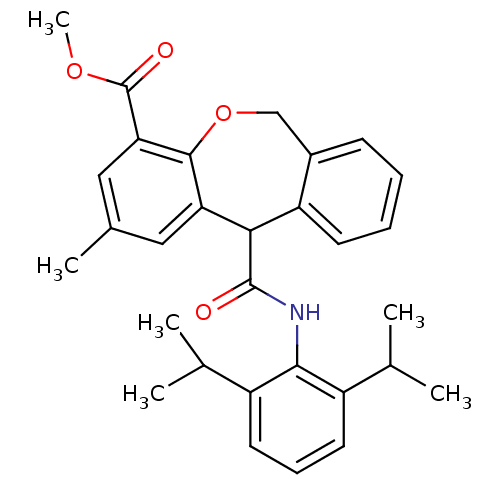 (11-(2,6-Diisopropyl-phenylcarbamoyl)-2-methyl-6,11...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase in liver microsomes isolated from cholesterol-fed rabbits | J Med Chem 37: 804-10 (1994) BindingDB Entry DOI: 10.7270/Q20C4TTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062257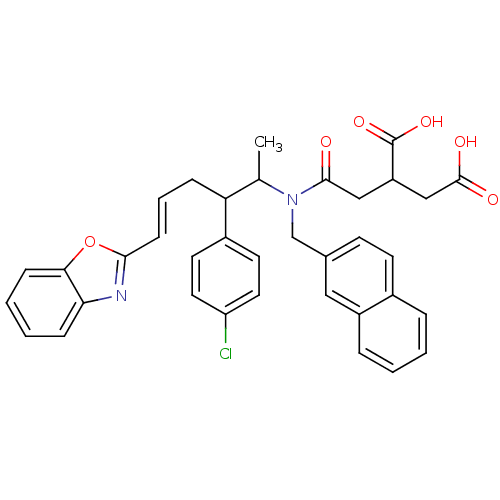 (2-({[(E)-5-Benzooxazol-2-yl-2-(4-chloro-phenyl)-1-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50284965 (1-[3-(2-Chloro-phenyl)-3H-imidazo[4,5-b]pyridin-2-...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity towards acyl CoA cholesterol acyltransferase (ACAT) of cholesterol-fed rabbits by the displacement of [14C]-oleolyl-CoA | Bioorg Med Chem Lett 5: 1829-1832 (1995) Article DOI: 10.1016/0960-894X(95)00296-6 BindingDB Entry DOI: 10.7270/Q2VD6ZDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50040828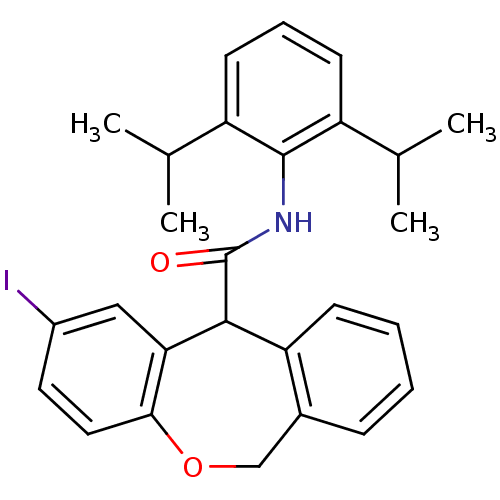 (2-Iodo-6,11-dihydro-dibenzo[b,e]oxepine-11-carboxy...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase in liver microsomes isolated from cholesterol-fed rabbits | J Med Chem 37: 804-10 (1994) BindingDB Entry DOI: 10.7270/Q20C4TTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50040831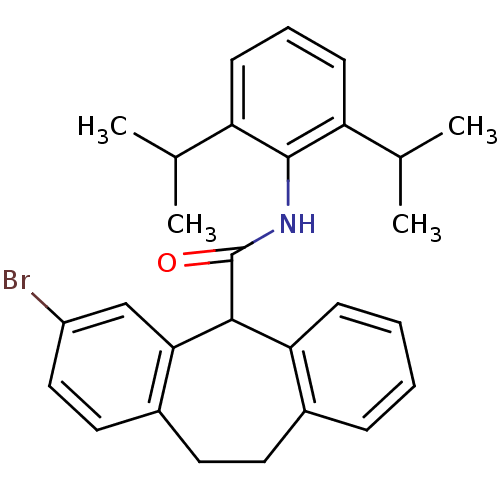 (3-Bromo-10,11-dihydro-5H-dibenzo[a,d]cycloheptene-...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase in liver microsomes isolated from cholesterol-fed rabbits | J Med Chem 37: 804-10 (1994) BindingDB Entry DOI: 10.7270/Q20C4TTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50040826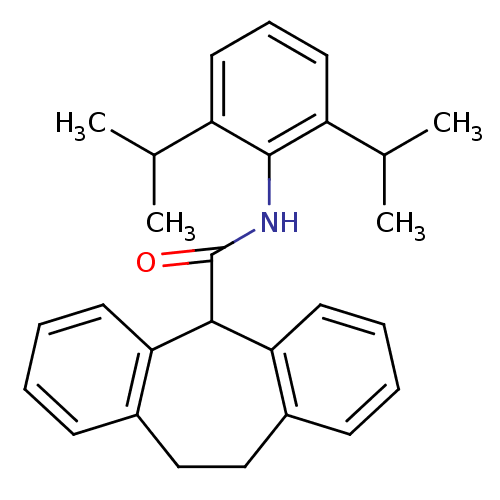 (10,11-Dihydro-5H-dibenzo[a,d]cycloheptene-5-carbox...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase in liver microsomes isolated from cholesterol-fed rabbits | J Med Chem 37: 804-10 (1994) BindingDB Entry DOI: 10.7270/Q20C4TTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50284978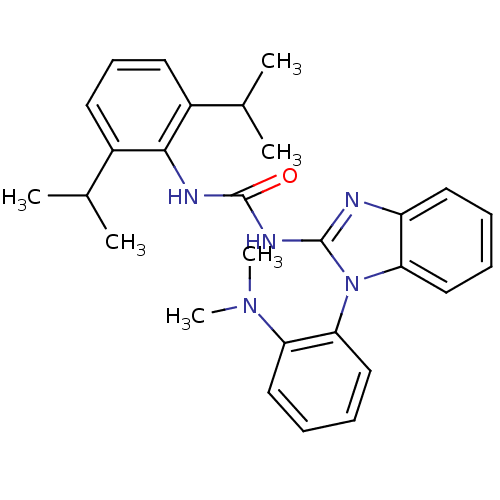 (1-(2,6-Diisopropyl-phenyl)-3-[1-(2-dimethylamino-p...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity towards acyl CoA cholesterol acyltransferase (ACAT) of cholesterol-fed rabbits by the displacement of [14C]-oleolyl-CoA | Bioorg Med Chem Lett 5: 1829-1832 (1995) Article DOI: 10.1016/0960-894X(95)00296-6 BindingDB Entry DOI: 10.7270/Q2VD6ZDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062249 (CHEMBL285434 | trisodium 5-[2-benzo[d][1,3]dioxol-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of FTase in competitive manner with respect to FPP (farnesyl diphosphate) at 6.0 microM FPP and 0.36 microM Ras peptide. | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50040855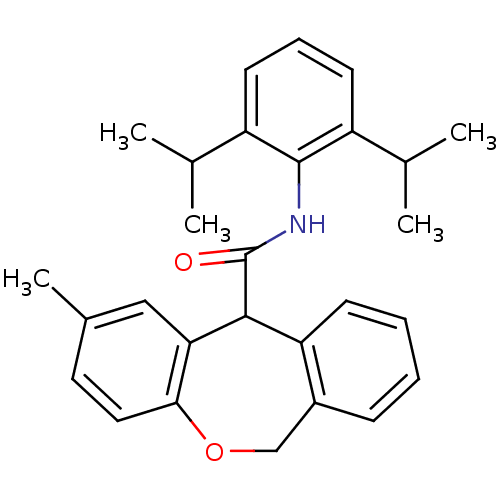 (2-Methyl-6,11-dihydro-dibenzo[b,e]oxepine-11-carbo...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase in liver microsomes isolated from cholesterol-fed rabbits | J Med Chem 37: 804-10 (1994) BindingDB Entry DOI: 10.7270/Q20C4TTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062253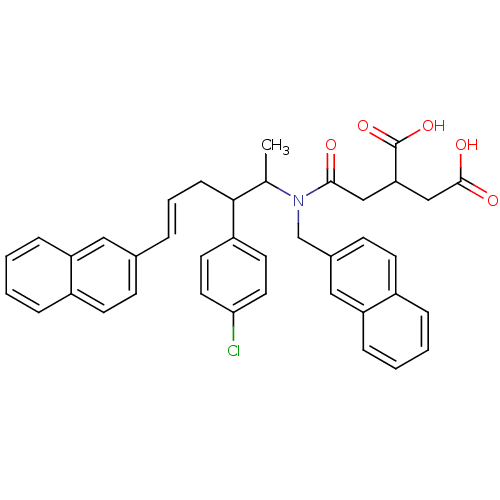 (2-({[(E)-2-(4-Chloro-phenyl)-1-methyl-5-naphthalen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50040851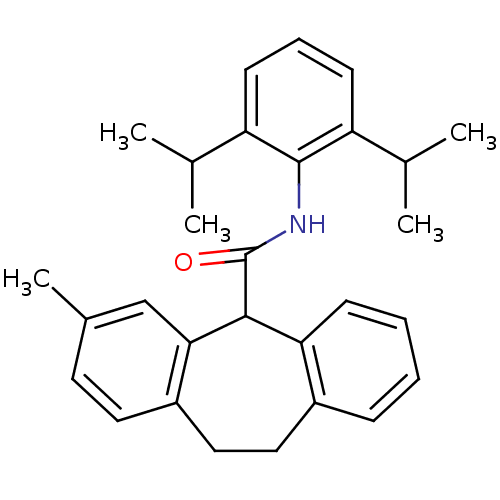 (3-Methyl-10,11-dihydro-5H-dibenzo[a,d]cycloheptene...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase in liver microsomes isolated from cholesterol-fed rabbits | J Med Chem 37: 804-10 (1994) BindingDB Entry DOI: 10.7270/Q20C4TTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50040841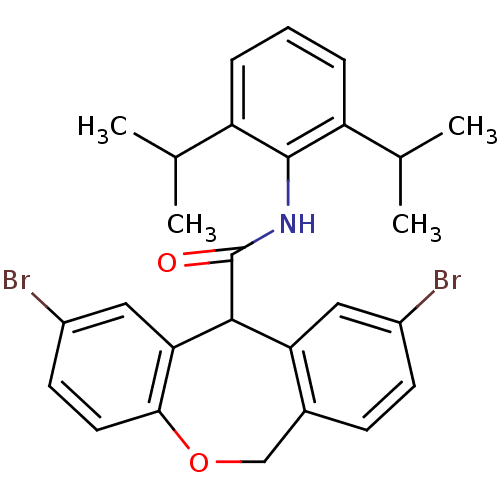 (2,9-Dibromo-6,11-dihydro-dibenzo[b,e]oxepine-11-ca...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase in liver microsomes isolated from cholesterol-fed rabbits | J Med Chem 37: 804-10 (1994) BindingDB Entry DOI: 10.7270/Q20C4TTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50040848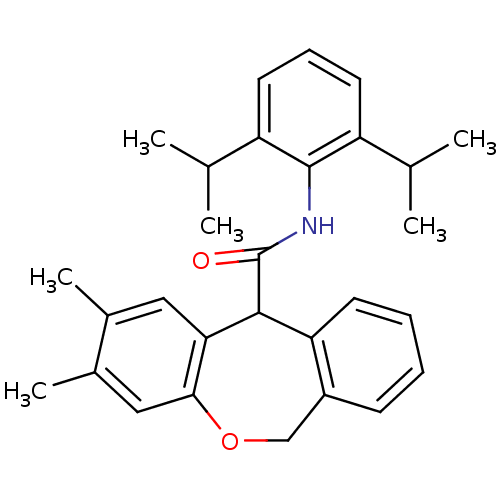 (2,3-Dimethyl-6,11-dihydro-dibenzo[b,e]oxepine-11-c...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase in liver microsomes isolated from cholesterol-fed rabbits | J Med Chem 37: 804-10 (1994) BindingDB Entry DOI: 10.7270/Q20C4TTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50284970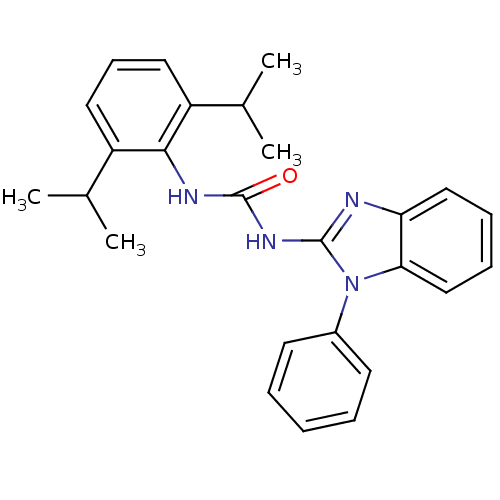 (1-(2,6-Diisopropyl-phenyl)-3-(1-phenyl-1H-benzoimi...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity towards acyl CoA cholesterol acyltransferase (ACAT) of cholesterol-fed rabbits by the displacement of [14C]-oleolyl-CoA | Bioorg Med Chem Lett 5: 1829-1832 (1995) Article DOI: 10.1016/0960-894X(95)00296-6 BindingDB Entry DOI: 10.7270/Q2VD6ZDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 320 total ) | Next | Last >> |