

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50290071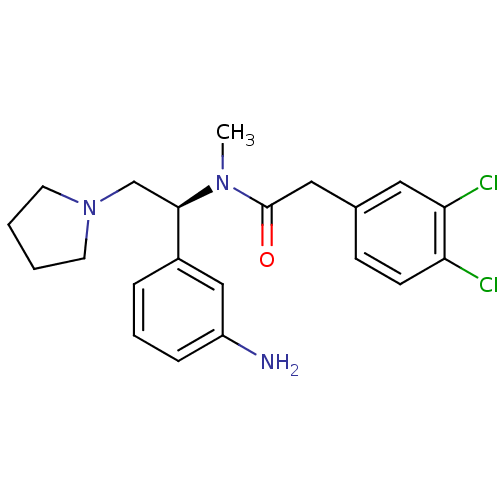 (CHEMBL542149 | N-[(S)-1-(3-Amino-phenyl)-2-pyrroli...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0592 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor kappa1 binding to guinea pig brain membranes, using 1 nM [3H]- U69,593 as radioligand | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50290076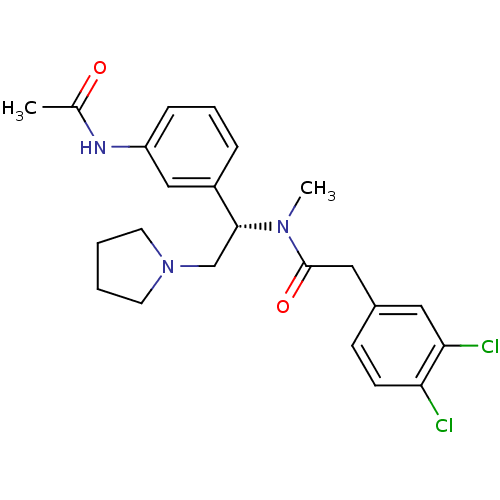 (CHEMBL558596 | N-[(S)-1-(3-Acetylamino-phenyl)-2-p...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0855 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor kappa1 binding to guinea pig brain membranes, using 1 nM [3H]- U69,593 as radioligand | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM85393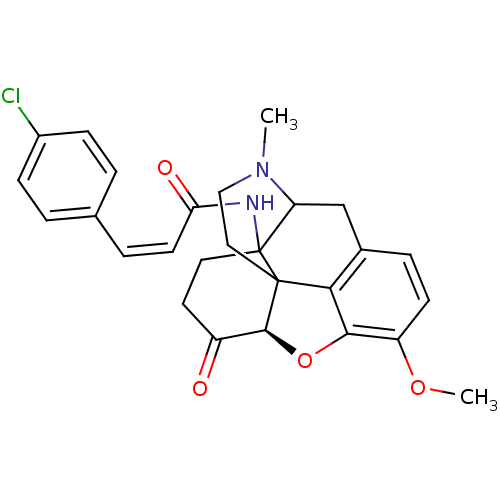 (14β-(p-chlorocinnamoylamino)-7,8-dihydrocodei...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM85396 (CACO) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor delta 1 (Bos taurus) | BDBM85393 (14β-(p-chlorocinnamoylamino)-7,8-dihydrocodei...) | PDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50001707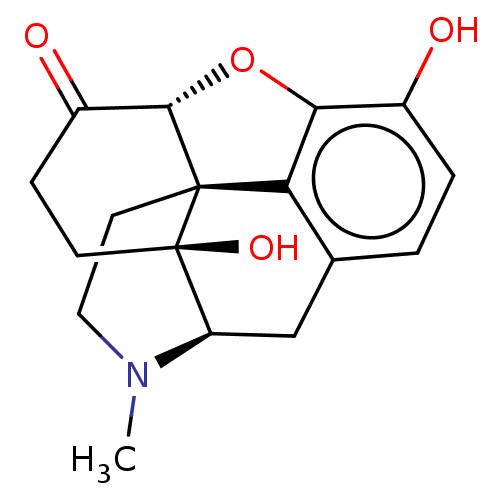 (10,17-dihydroxy-4-methyl-(13R,17S)-12-oxa-4-azapen...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding constant in guinea pig brain homogenate was reported at mu site at a temperature 25 degree Centigrade labeled with [3H](D-Ala2-MeP... | J Med Chem 28: 974-6 (1985) BindingDB Entry DOI: 10.7270/Q27W6CSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50290072 (5-{3-[3-((S)-1-{[2-(3,4-Dichloro-phenyl)-acetyl]-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.898 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor delta 1 binding to guinea pig brain membranes, using 0.2 nM [3H]-naltrindole as radiolig... | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding constant in guinea pig brain homogenate was reported at mu site at a temperature 25 degree Centigrade labeled with [3H](D-Ala2-MeP... | J Med Chem 28: 974-6 (1985) BindingDB Entry DOI: 10.7270/Q27W6CSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM85394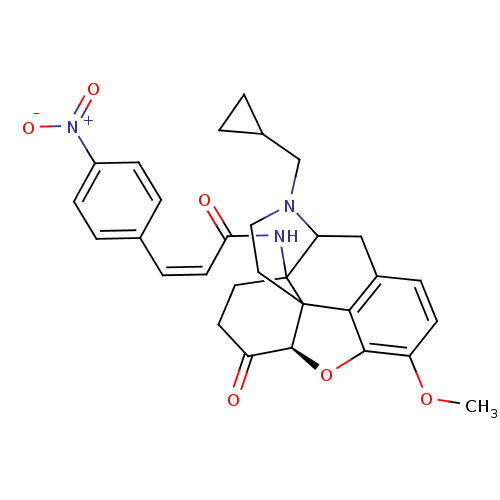 (N-CPM-CACO) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (CALF) | BDBM85393 (14β-(p-chlorocinnamoylamino)-7,8-dihydrocodei...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM85395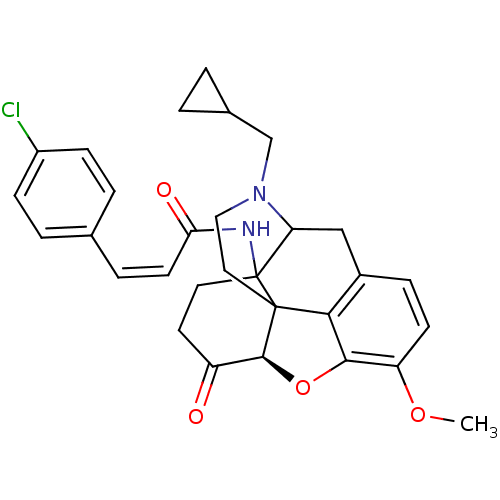 (MC-CAM) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50290072 (5-{3-[3-((S)-1-{[2-(3,4-Dichloro-phenyl)-acetyl]-m...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor kappa1 binding to guinea pig brain membranes, using 1 nM [3H]- U69,593 as radioligand | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50290070 (5-{3-[3-(1-{[2-(3,4-Dichloro-phenyl)-acetyl]-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor delta 1 binding to guinea pig brain membranes, using 0.2 nM [3H]-naltrindole as radiolig... | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding constant in guinea pig brain homogenate was reported at Opioid receptor delta 1 at a a temperature 25 degree Celsius labeled with ... | J Med Chem 28: 974-6 (1985) BindingDB Entry DOI: 10.7270/Q27W6CSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50290071 (CHEMBL542149 | N-[(S)-1-(3-Amino-phenyl)-2-pyrroli...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor mu 1 binding to guinea pig brain membranes, using [3H]- DAMGO as radioligand | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50290071 (CHEMBL542149 | N-[(S)-1-(3-Amino-phenyl)-2-pyrroli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor delta 1 binding to guinea pig brain membranes, using 0.2 nM [3H]-naltrindole as radiolig... | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50290070 (5-{3-[3-(1-{[2-(3,4-Dichloro-phenyl)-acetyl]-methy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor kappa1 binding to guinea pig brain membranes, using 1 nM [3H]- U69,593 as radioligand | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50290076 (CHEMBL558596 | N-[(S)-1-(3-Acetylamino-phenyl)-2-p...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor mu 1 binding to guinea pig brain membranes, using [3H]- DAMGO as radioligand | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding constant in guinea pig brain homogenate was reported at Opioid receptor kappa 1 at temperature 25 degree Celsius labeled with (-)-... | J Med Chem 28: 974-6 (1985) BindingDB Entry DOI: 10.7270/Q27W6CSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (CALF) | BDBM85394 (N-CPM-CACO) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor delta 1 (Bos taurus) | BDBM85395 (MC-CAM) | PDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50290076 (CHEMBL558596 | N-[(S)-1-(3-Acetylamino-phenyl)-2-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor delta 1 binding to guinea pig brain membranes, using 0.2 nM [3H]-naltrindole as radiolig... | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (CALF) | BDBM85395 (MC-CAM) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50290074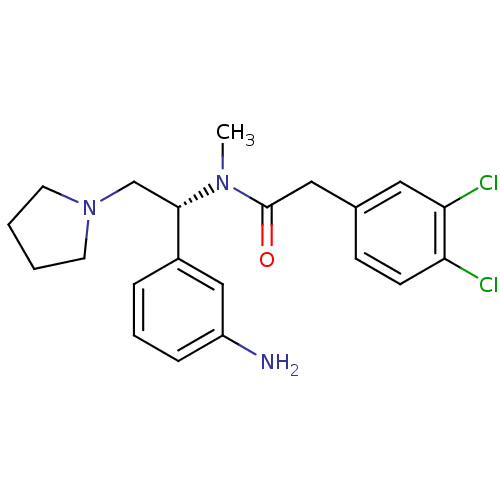 (CHEMBL558597 | N-[(R)-1-(3-Amino-phenyl)-2-pyrroli...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor kappa1 binding to guinea pig brain membranes, using 1 nM [3H]- U69,593 as radioligand | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor delta 1 (Bos taurus) | BDBM85394 (N-CPM-CACO) | PDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50290075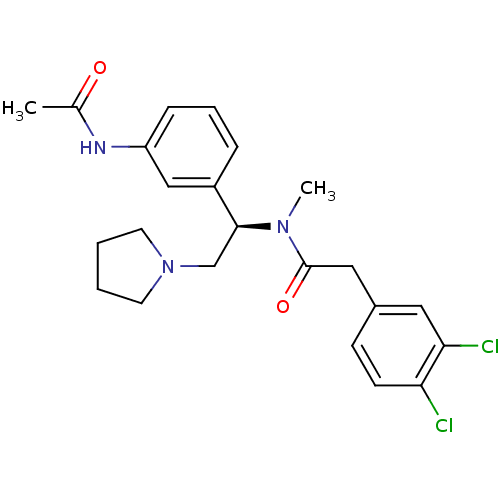 (CHEMBL542403 | N-[(R)-1-(3-Acetylamino-phenyl)-2-p...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor kappa1 binding to guinea pig brain membranes, using 1 nM [3H]- U69,593 as radioligand | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (CALF) | BDBM85396 (CACO) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50016086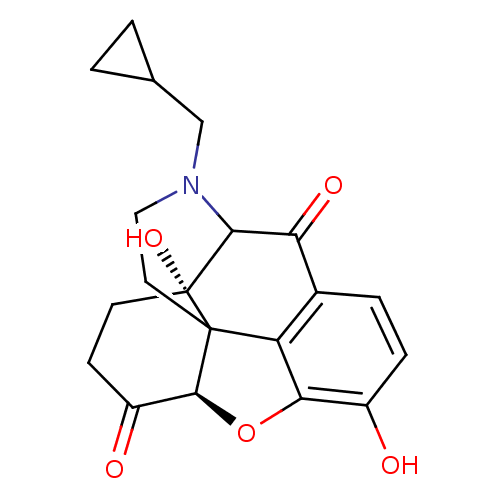 (4-cyclopropylmethyl-10,17-dihydroxy-(13R,17S)-12-o...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding constant in guinea pig brain homogenate was reported at mu site at a temperature 25 degree Centigrade labeled with [3H](D-Ala2-MeP... | J Med Chem 28: 974-6 (1985) BindingDB Entry DOI: 10.7270/Q27W6CSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50001707 (10,17-dihydroxy-4-methyl-(13R,17S)-12-oxa-4-azapen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding constant in guinea pig brain homogenate was reported at Opioid receptor delta 1 at a a temperature 25 degree Celsius labeled with ... | J Med Chem 28: 974-6 (1985) BindingDB Entry DOI: 10.7270/Q27W6CSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50290073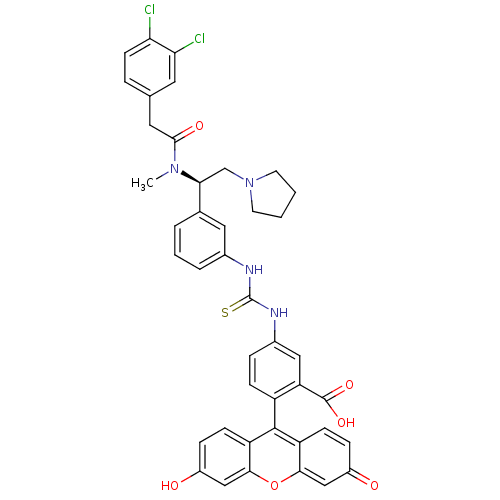 (5-{3-[3-((R)-1-{[2-(3,4-Dichloro-phenyl)-acetyl]-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor delta 1 binding to guinea pig brain membranes, using 0.2 nM [3H]-naltrindole as radiolig... | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50290072 (5-{3-[3-((S)-1-{[2-(3,4-Dichloro-phenyl)-acetyl]-m...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor mu 1 binding to guinea pig brain membranes, using [3H]- DAMGO as radioligand | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50016086 (4-cyclopropylmethyl-10,17-dihydroxy-(13R,17S)-12-o...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding constant in guinea pig brain homogenate was reported at Opioid receptor kappa 1 at temperature 25 degree Celsius labeled with (-)-... | J Med Chem 28: 974-6 (1985) BindingDB Entry DOI: 10.7270/Q27W6CSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001707 (10,17-dihydroxy-4-methyl-(13R,17S)-12-oxa-4-azapen...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding constant in guinea pig brain homogenate was reported at Opioid receptor kappa 1 at temperature 25 degree Celsius labeled with (-)-... | J Med Chem 28: 974-6 (1985) BindingDB Entry DOI: 10.7270/Q27W6CSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50290073 (5-{3-[3-((R)-1-{[2-(3,4-Dichloro-phenyl)-acetyl]-m...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor kappa1 binding to guinea pig brain membranes, using 1 nM [3H]- U69,593 as radioligand | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50290070 (5-{3-[3-(1-{[2-(3,4-Dichloro-phenyl)-acetyl]-methy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor mu 1 binding to guinea pig brain membranes, using [3H]- DAMGO as radioligand | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50016086 (4-cyclopropylmethyl-10,17-dihydroxy-(13R,17S)-12-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding constant in guinea pig brain homogenate was reported at Opioid receptor delta 1 at a temperature 25 degree Celsius labeled with [3... | J Med Chem 28: 974-6 (1985) BindingDB Entry DOI: 10.7270/Q27W6CSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50290075 (CHEMBL542403 | N-[(R)-1-(3-Acetylamino-phenyl)-2-p...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor mu 1 binding to guinea pig brain membranes, using [3H]- DAMGO as radioligand | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50290073 (5-{3-[3-((R)-1-{[2-(3,4-Dichloro-phenyl)-acetyl]-m...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor mu 1 binding to guinea pig brain membranes, using [3H]- DAMGO as radioligand | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50290074 (CHEMBL558597 | N-[(R)-1-(3-Amino-phenyl)-2-pyrroli...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor mu 1 binding to guinea pig brain membranes, using [3H]- DAMGO as radioligand | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50290074 (CHEMBL558597 | N-[(R)-1-(3-Amino-phenyl)-2-pyrroli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor delta 1 binding to guinea pig brain membranes, using 0.2 nM [3H]-naltrindole as radiolig... | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50290075 (CHEMBL542403 | N-[(R)-1-(3-Acetylamino-phenyl)-2-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Opioid receptor delta 1 binding to guinea pig brain membranes, using 0.2 nM [3H]-naltrindole as radiolig... | Bioorg Med Chem Lett 7: 2271-2276 (1997) Article DOI: 10.1016/S0960-894X(97)00406-X BindingDB Entry DOI: 10.7270/Q2GB242Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50287332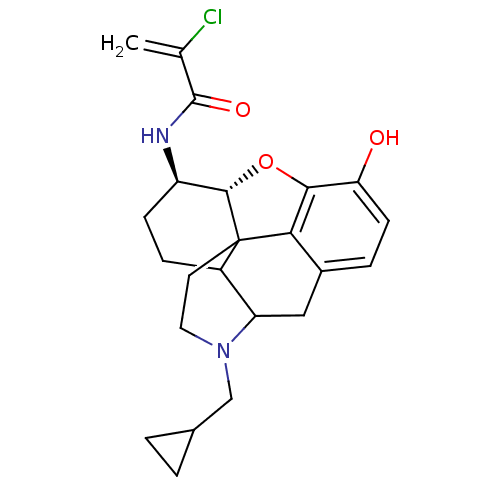 (1N-[4-cyclopropylmethyl-10-hydroxy-(13R,14R)-12-ox...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for Inhibition of [3H]-DAMGO (0.25 nM) binding to mu receptor from bovine striatal membranes | Bioorg Med Chem Lett 6: 1563-1566 (1996) Article DOI: 10.1016/S0960-894X(96)00274-0 BindingDB Entry DOI: 10.7270/Q28P60GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50456193 (CHEMBL607068) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity against mu opioid receptor from calf frontal cortex. | J Med Chem 36: 3154-60 (1993) BindingDB Entry DOI: 10.7270/Q2K64H31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50033645 (1N-[10-hydroxy-4,13-dimethyl-14-oxo-(13R,17S)-12-o...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity against mu opioid receptor from calf frontal cortex. | J Med Chem 36: 3154-60 (1993) BindingDB Entry DOI: 10.7270/Q2K64H31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50030143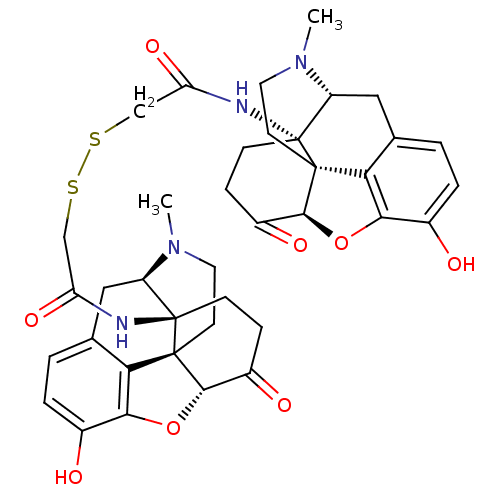 (CHEMBL41098 | bis-(1N-[10-hydroxy-4-methyl-14-oxo-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Evaluation for the inhibition of mu opioid binding to bovine striatal membranes by the affinity ligand 0.25 nM [3H]DAMGO radiolabeled opioid | J Med Chem 37: 1578-85 (1994) BindingDB Entry DOI: 10.7270/Q29024FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50030143 (CHEMBL41098 | bis-(1N-[10-hydroxy-4-methyl-14-oxo-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Opioid receptor mu 1 binding to bovine striatal membrane | Bioorg Med Chem Lett 5: 1695-1700 (1995) Article DOI: 10.1016/0960-894X(95)00287-4 BindingDB Entry DOI: 10.7270/Q2125SNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50033654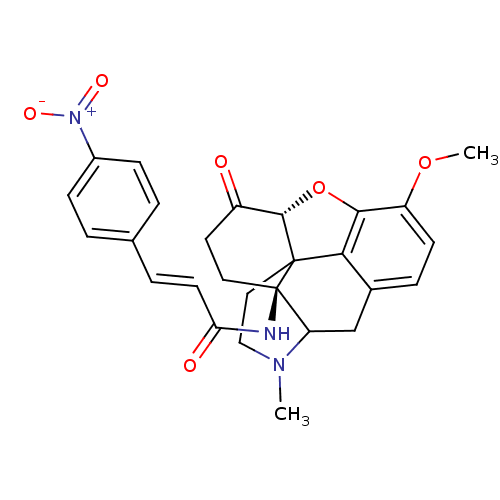 (1N-[10-methoxy-4-methyl-14-oxo-(13R,17S)-12-oxa-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity against mu opioid receptor from calf frontal cortex. | J Med Chem 36: 3154-60 (1993) BindingDB Entry DOI: 10.7270/Q2K64H31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50033647 (1N-[4-cyclopropylmethyl-10-hydroxy-13-methyl-14-ox...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity against mu opioid receptor from calf frontal cortex. | J Med Chem 36: 3154-60 (1993) BindingDB Entry DOI: 10.7270/Q2K64H31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50287332 (1N-[4-cyclopropylmethyl-10-hydroxy-(13R,14R)-12-ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for Inhibition of 9 (1nM) binding to Opioid receptor delta 1 from bovine striatal Membranes | Bioorg Med Chem Lett 6: 1563-1566 (1996) Article DOI: 10.1016/S0960-894X(96)00274-0 BindingDB Entry DOI: 10.7270/Q28P60GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50287331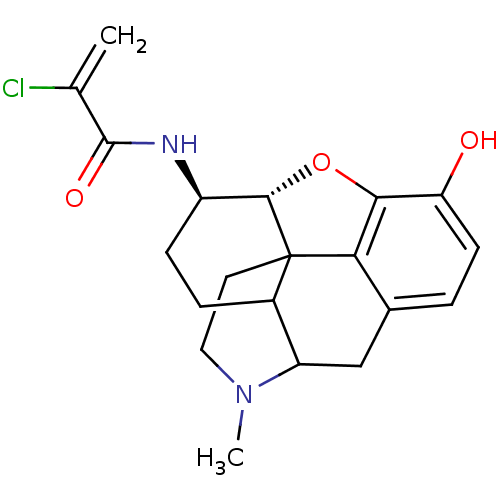 (1N-[10-hydroxy-4-methyl-(13R,14R)-12-oxa-4-azapent...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for Inhibition of [3H]-DAMGO (0.25 nM) binding to mu receptor from bovine striatal membranes | Bioorg Med Chem Lett 6: 1563-1566 (1996) Article DOI: 10.1016/S0960-894X(96)00274-0 BindingDB Entry DOI: 10.7270/Q28P60GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 180 total ) | Next | Last >> |