

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50560609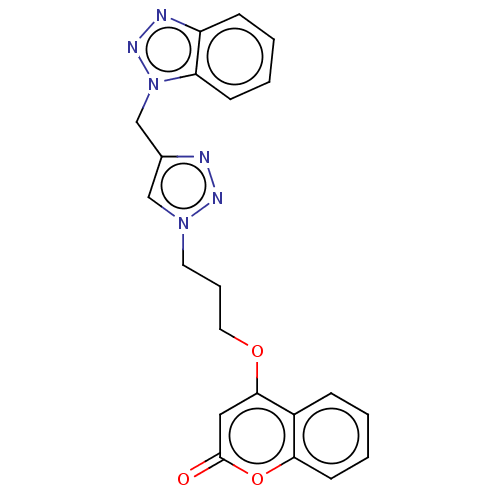 (CHEMBL4749763) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of electric eel AChE assessed as inhibition constant using varying levels of acetylthiocholine as substrate by reciprocal Linew... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127477 BindingDB Entry DOI: 10.7270/Q2W099MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50190362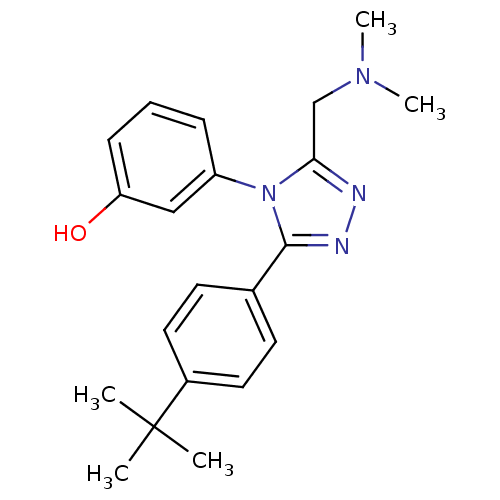 (1-{3-[4-(tert-butyl) phenyl]-5-N,N-dimethylaminome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse delta opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50190374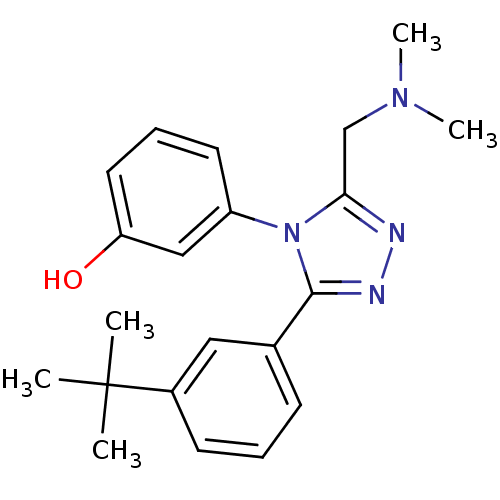 (1-{3-[3-(tert-butyl) phenyl]-5-N,N-dimethylaminome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse delta opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyruvate kinase PKM (Homo sapiens (Human)) | BDBM50140257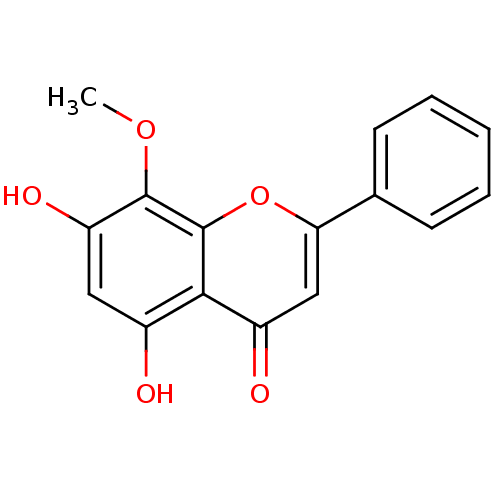 (5,7-Dihydroxy-8-methoxy-2-phenyl-chromen-4-one | 5...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00981 BindingDB Entry DOI: 10.7270/Q2M330SP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyruvate kinase PKM (Homo sapiens (Human)) | BDBM50187668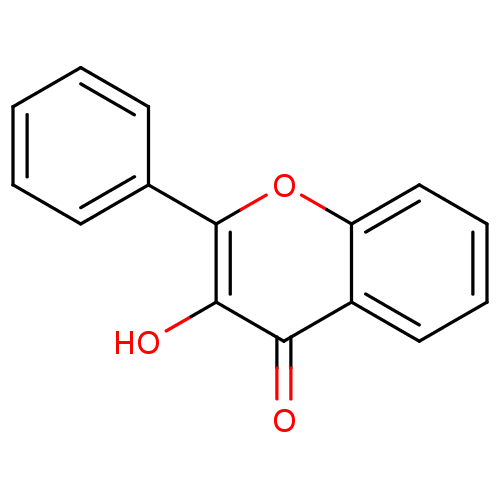 (3-Hydroxyflavone | 3-Hydroxyflavone (12) | 3-hydro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00981 BindingDB Entry DOI: 10.7270/Q2M330SP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyruvate kinase PKM (Homo sapiens (Human)) | BDBM26664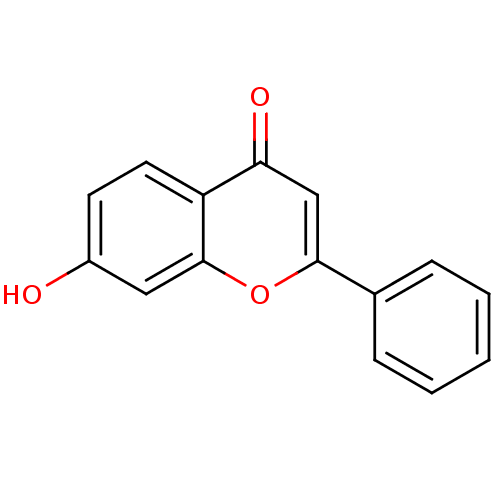 (7-Hydroxy-flavone, 5a | 7-Hydroxyflavone, 11 | 7-h...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00981 BindingDB Entry DOI: 10.7270/Q2M330SP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyruvate kinase PKM (Homo sapiens (Human)) | BDBM50081950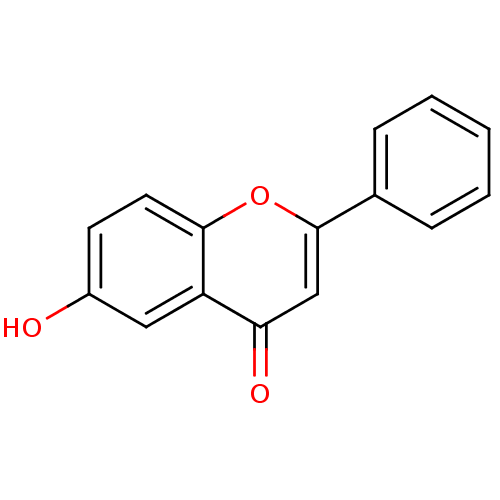 (6-Hydroxy-2-phenyl-chromen-4-one | 6-Hydroxyflavon...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00981 BindingDB Entry DOI: 10.7270/Q2M330SP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyruvate kinase PKM (Homo sapiens (Human)) | BDBM50049385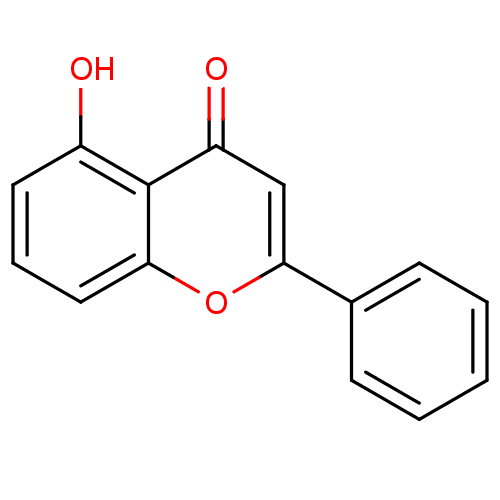 (5-Hydroxy-2-phenyl-chromen-4-one | 5-Hydroxyflavon...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00981 BindingDB Entry DOI: 10.7270/Q2M330SP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50190374 (1-{3-[3-(tert-butyl) phenyl]-5-N,N-dimethylaminome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse mu opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50190374 (1-{3-[3-(tert-butyl) phenyl]-5-N,N-dimethylaminome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from rat kappa opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50190362 (1-{3-[4-(tert-butyl) phenyl]-5-N,N-dimethylaminome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from rat kappa opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50190362 (1-{3-[4-(tert-butyl) phenyl]-5-N,N-dimethylaminome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse mu opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480861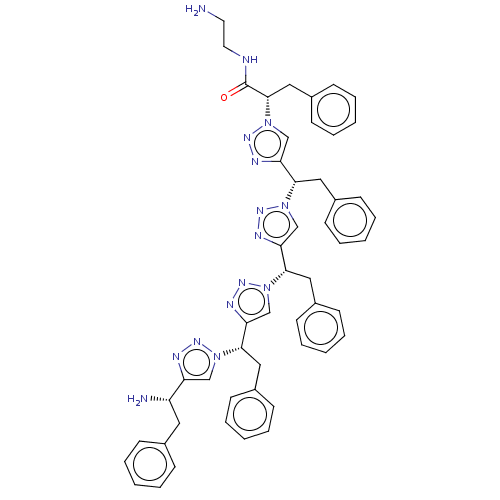 (CHEMBL583925) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET-based assay | Bioorg Med Chem Lett 19: 6023-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.049 BindingDB Entry DOI: 10.7270/Q2BZ68V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480867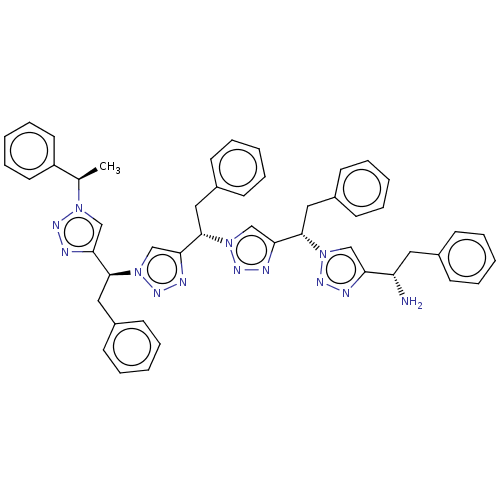 (CHEMBL583710) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET-based assay | Bioorg Med Chem Lett 19: 6023-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.049 BindingDB Entry DOI: 10.7270/Q2BZ68V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyruvate kinase (Leishmania mexicana) | BDBM50179360 (CHEMBL3040216) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00981 BindingDB Entry DOI: 10.7270/Q2M330SP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480864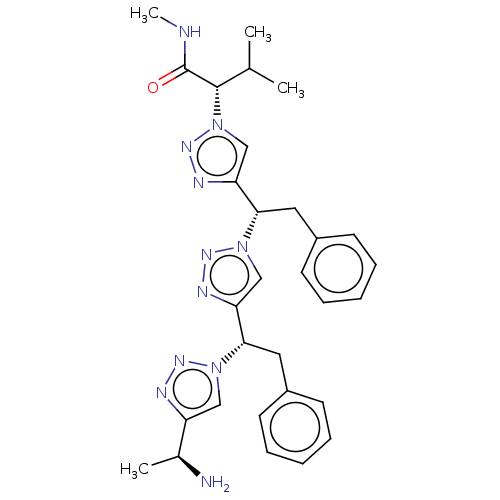 (CHEMBL583926) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET-based assay | Bioorg Med Chem Lett 19: 6023-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.049 BindingDB Entry DOI: 10.7270/Q2BZ68V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480862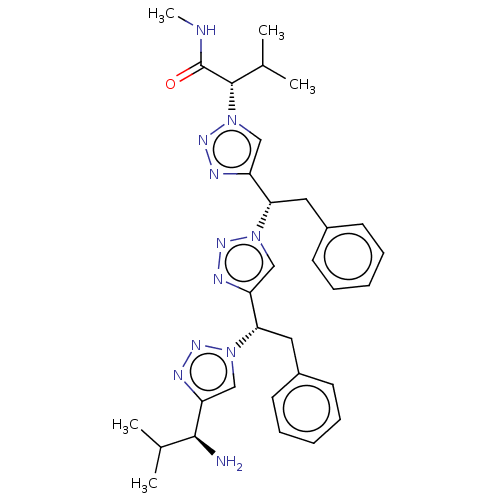 (CHEMBL573582) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by FRET-based assay | Bioorg Med Chem Lett 19: 6023-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.049 BindingDB Entry DOI: 10.7270/Q2BZ68V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541911 (CHEMBL4639983) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541907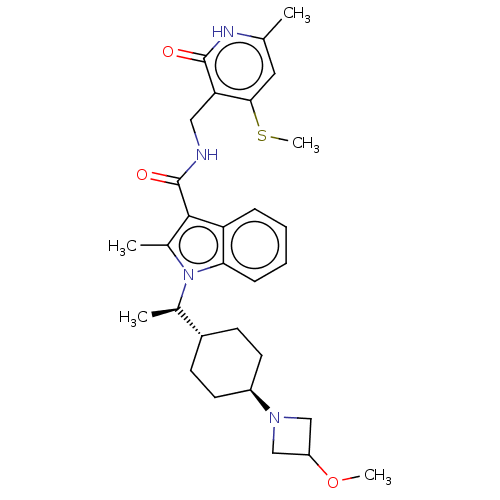 (CHEMBL4640994) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541915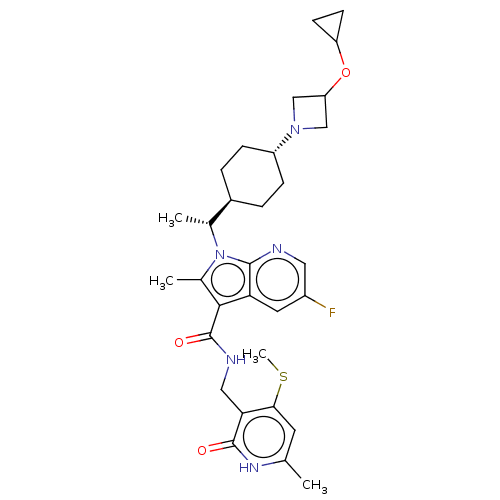 (CHEMBL4638754) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541912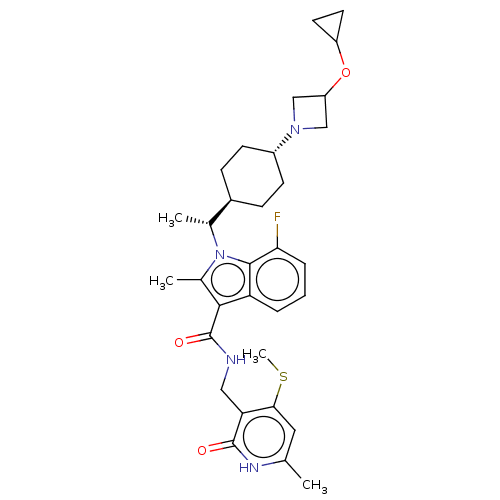 (CHEMBL4637572) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541902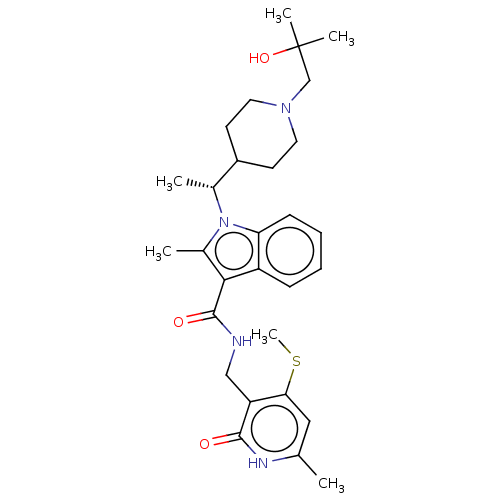 (CHEMBL4635809 | US11459315, Example 11) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541910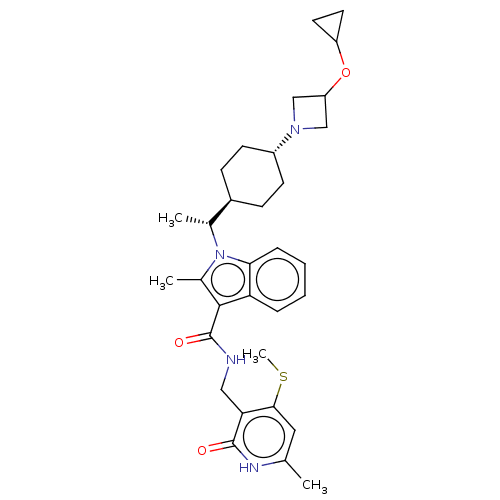 (CHEMBL4635863) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541914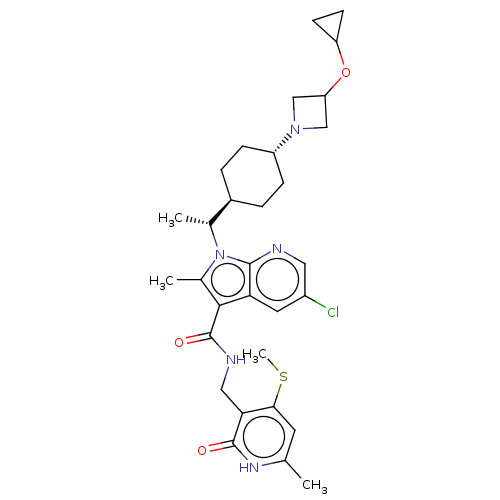 (CHEMBL4635102) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541913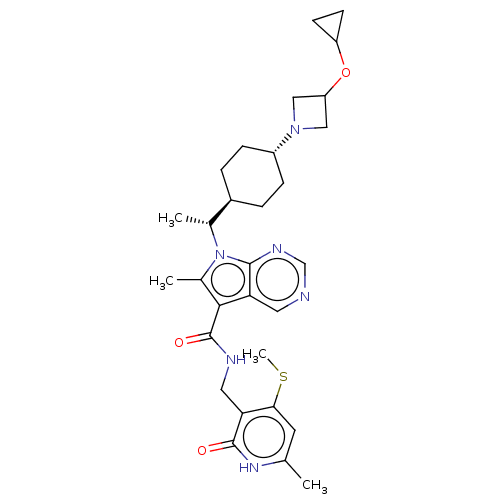 (CHEMBL4633923) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541909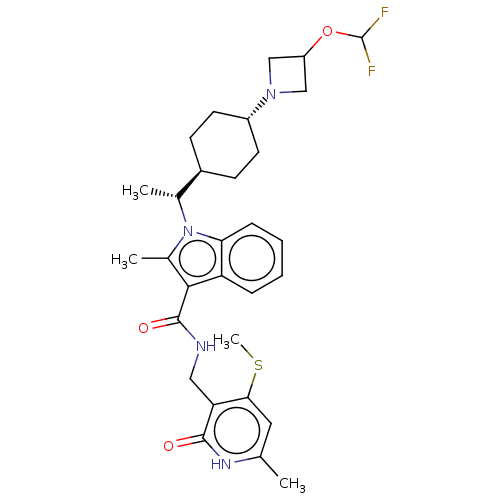 (CHEMBL4639913) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541903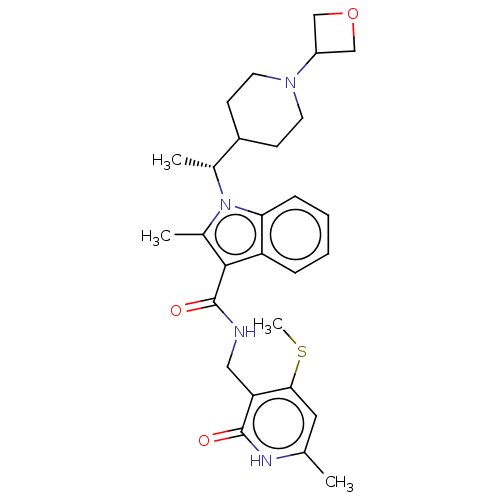 (CHEMBL4633932 | US11459315, Example 133) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541905 (CHEMBL4634677) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541908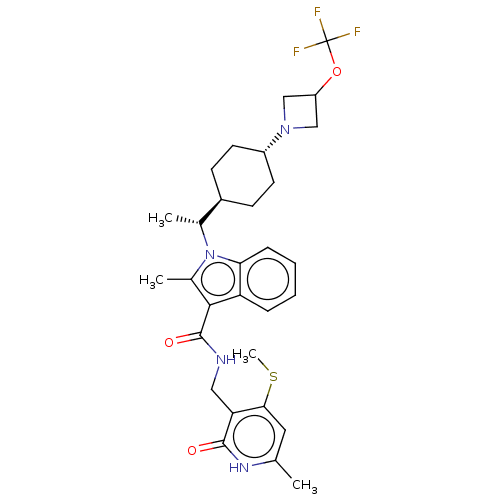 (CHEMBL4637958) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541906 (CHEMBL4649131) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541895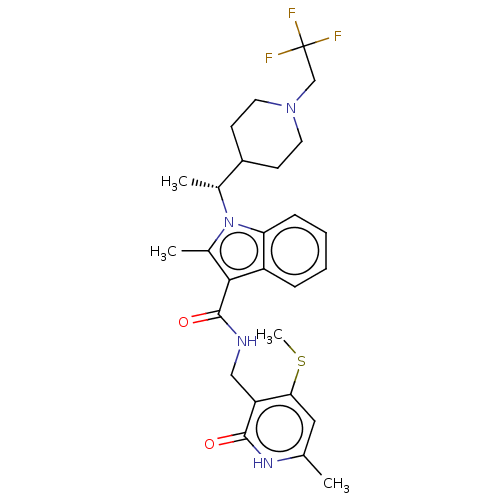 (CHEMBL4639616 | US11459315, Example 12) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541904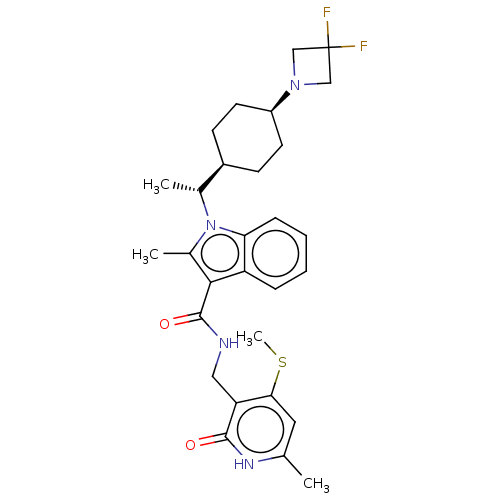 (CHEMBL4639569) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541919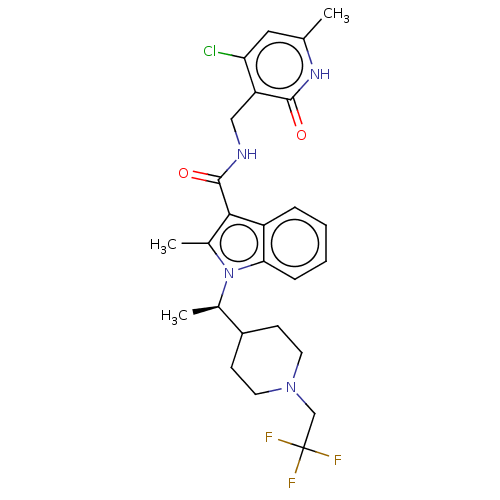 (CHEMBL4632988) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541918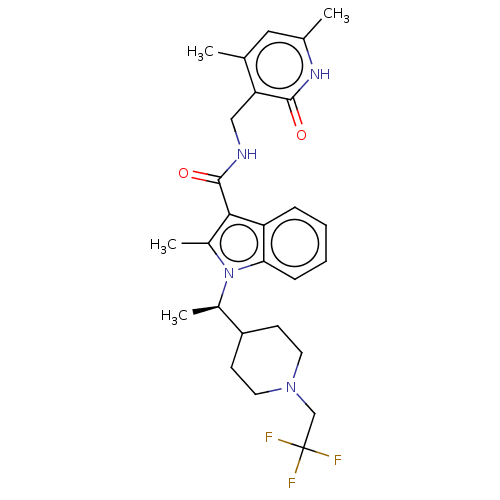 (CHEMBL4641325) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541917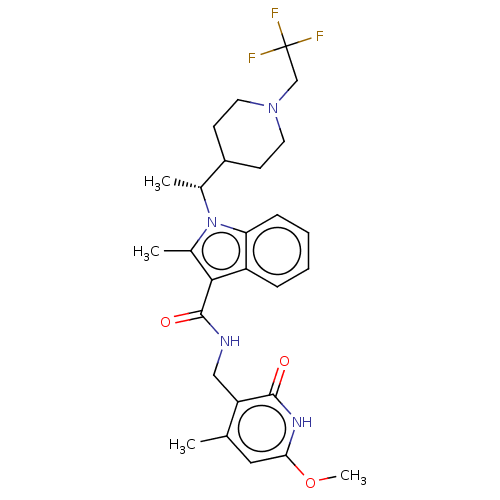 (CHEMBL4644363) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541916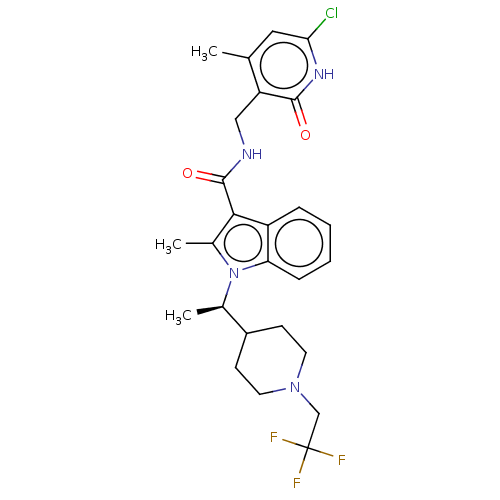 (CHEMBL4633053) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541897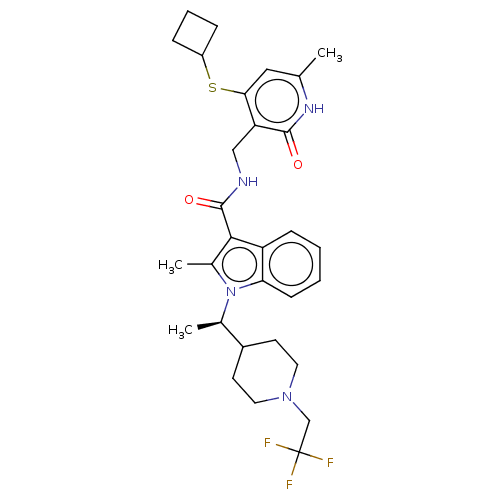 (CHEMBL4634390 | US11459315, Example 109) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541898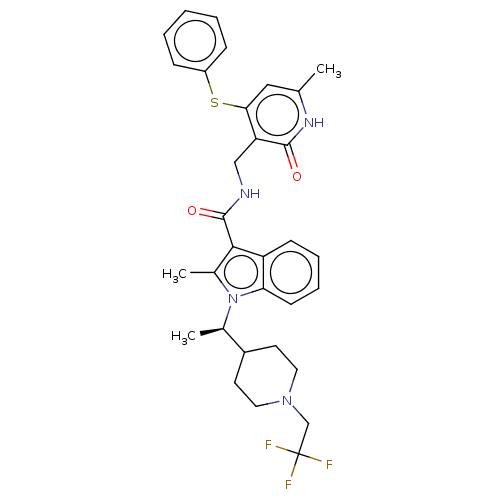 (CHEMBL4638163) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541896 (CHEMBL4639975 | US11459315, Example 86) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50039029 ((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse delta opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50574409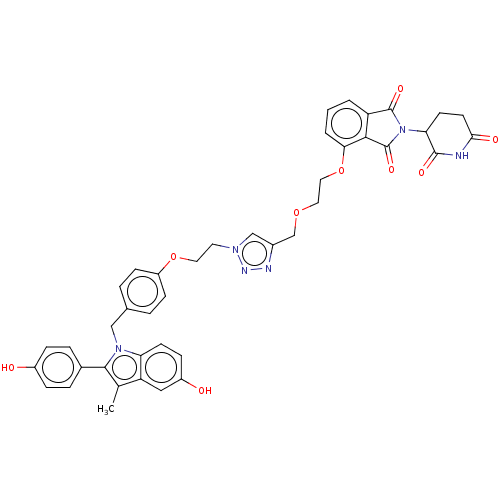 (CHEMBL4873534) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to recombinant His-tagged ERalpha LBD (307 to 554 residue) (unknown origin) expressed in Escherichia coli preincubated for 15 mins f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00127 BindingDB Entry DOI: 10.7270/Q26T0RG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50574409 (CHEMBL4873534) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to ERalpha S463P mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00127 BindingDB Entry DOI: 10.7270/Q26T0RG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27614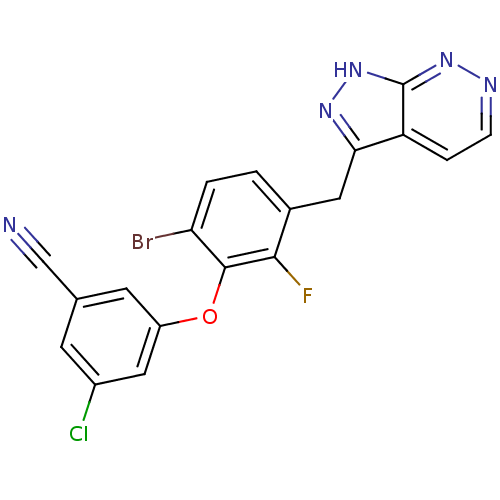 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | 4 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM195608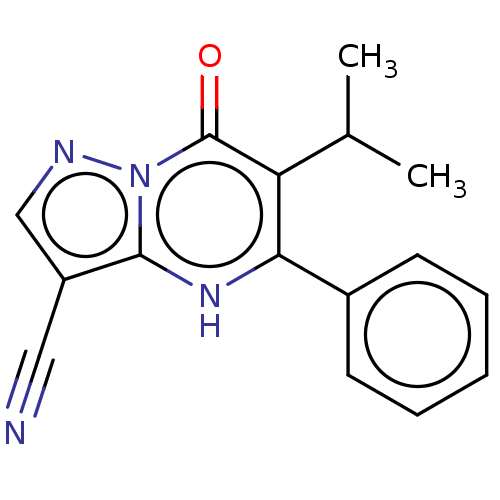 (CPI-455) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using ART(Kme3)QTARKSTGGKAPRKQLA-NovaTagPEG-biotin/2-OG as substrate/co-factor by TR-FRET assay | Bioorg Med Chem Lett 26: 4350-4 (2016) Article DOI: 10.1016/j.bmcl.2016.07.026 BindingDB Entry DOI: 10.7270/Q2MW2MNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM195608 (CPI-455) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using ART(Kme3)QTARKSTGGKAPRKQLA-NovaTagPEG-biotin/2-OG as substrate/co-factor by TR-FRET assay | Bioorg Med Chem Lett 26: 4350-4 (2016) Article DOI: 10.1016/j.bmcl.2016.07.026 BindingDB Entry DOI: 10.7270/Q2MW2MNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM195608 (CPI-455) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Genentech Inc | Assay Description Time-resolved fluorescence resonance energy transfer (TR-FRET) assays were carried out using full-length KDM5 enzymes in 384-well black ProxiPlates (... | Nat Chem Biol 12: 531-8 (2016) Article DOI: 10.1038/nchembio.2085 BindingDB Entry DOI: 10.7270/Q2CF9NXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27615 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-b]pyrazin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | >25 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27614 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | n/a | n/a | 3 | n/a | 1 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50574409 (CHEMBL4873534) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to ERalpha Y537S mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00127 BindingDB Entry DOI: 10.7270/Q26T0RG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541899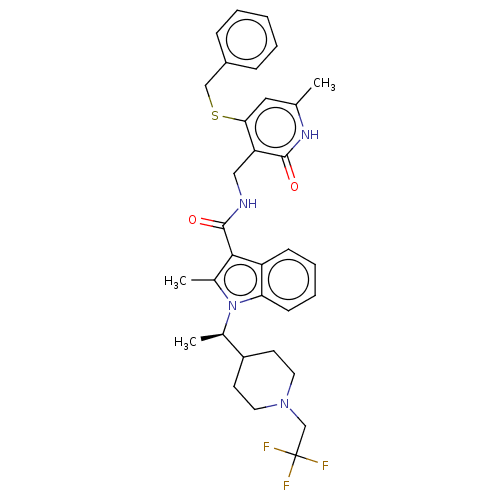 (CHEMBL4638720) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 456 total ) | Next | Last >> |