

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50216040 (3-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Antagonist activity at human Dopamine receptor D2 isoform long expressed in HEK293 cells assessed as change in quinpirole-induced intracellular calci... | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50216045 (2-(4-(4-(2-methoxyphenyl)-1-piperazinyl)butyl)-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Antagonist activity at human Dopamine receptor D2 isoform long expressed in HEK293 cells assessed as change in quinpirole-induced intracellular calci... | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50080151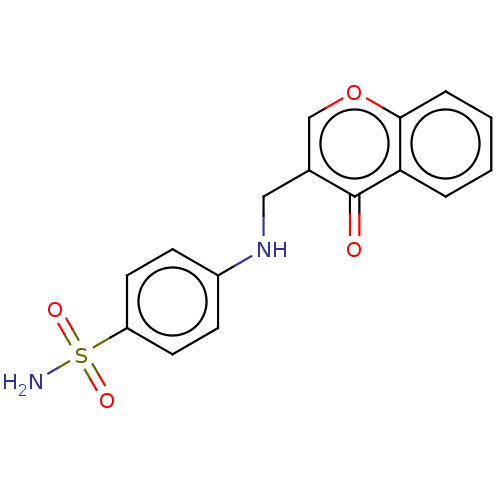 (CHEMBL3040927) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50080145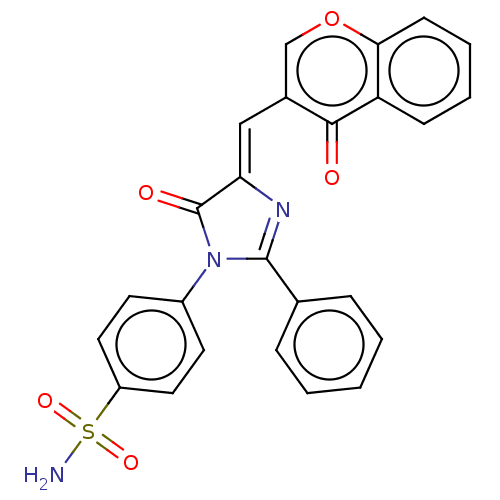 (CHEMBL3416241) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50080147 (CHEMBL3416247) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50216046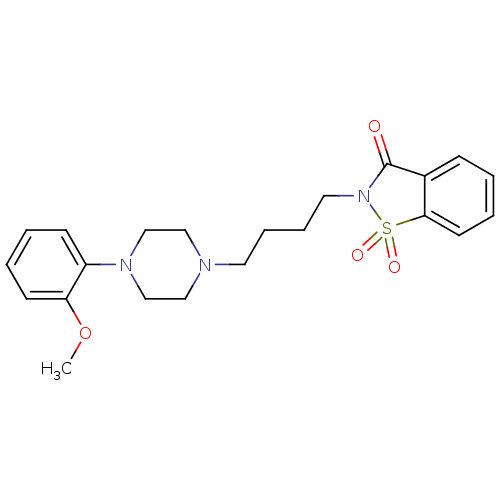 (2-(4-(4-phenyl-1-piperazinyl)butyl)-1,2-benzisothi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Antagonist activity at human Dopamine receptor D2 isoform long expressed in HEK293 cells assessed as change in quinpirole-induced intracellular calci... | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50080146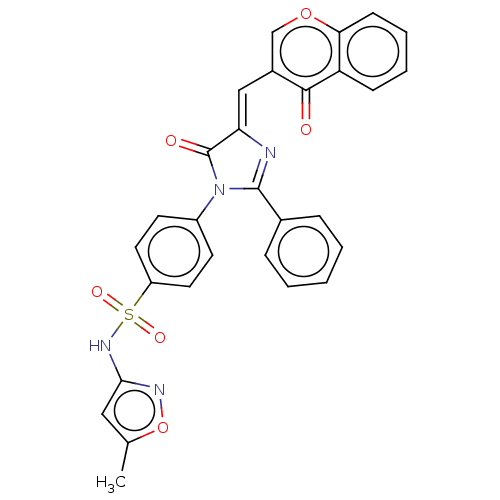 (CHEMBL3416242) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50080154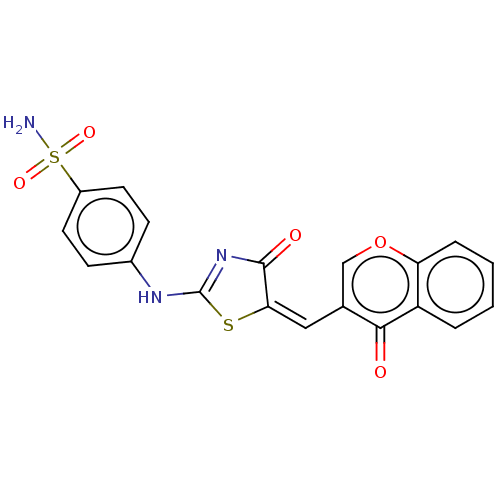 (CHEMBL3416235) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50080152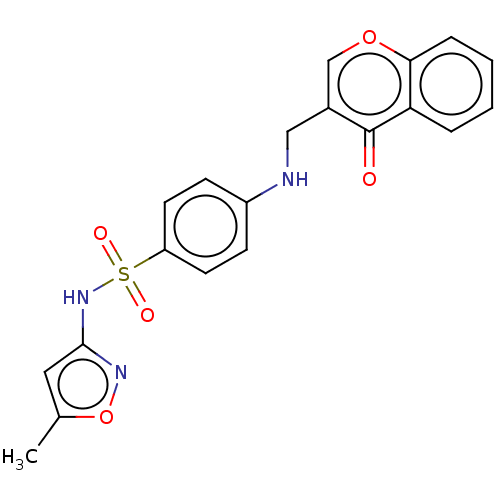 (CHEMBL3416230) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human cytosolic form of carbonic anhydrase-2 assessed as CO2 hydration activity incubated for 15 mins prior to testing by stopped flow ... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50080154 (CHEMBL3416235) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human cytosolic form of carbonic anhydrase-2 assessed as CO2 hydration activity incubated for 15 mins prior to testing by stopped flow ... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216044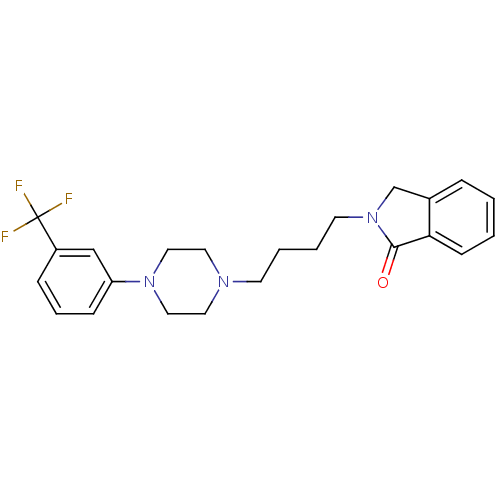 (2-(4-(4-(3-a,a,a-trifluorotolyl)-1-piperazinyl)but...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human Dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50080154 (CHEMBL3416235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-9 assessed as CO2 hydration activity incubated for 15 mins prior to testing by sto... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50080147 (CHEMBL3416247) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-9 assessed as CO2 hydration activity incubated for 15 mins prior to testing by sto... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50080150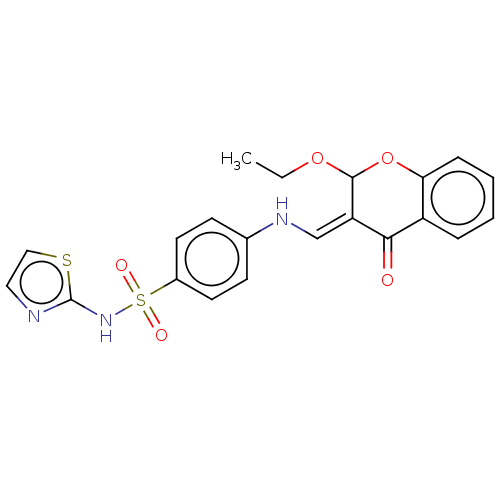 (CHEMBL3416229) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-9 assessed as CO2 hydration activity incubated for 15 mins prior to testing by sto... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50437929 (CHEMBL2408560) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-9 assessed as CO2 hydration activity incubated for 15 mins prior to testing by sto... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50080145 (CHEMBL3416241) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-9 assessed as CO2 hydration activity incubated for 15 mins prior to testing by sto... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50080150 (CHEMBL3416229) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50080148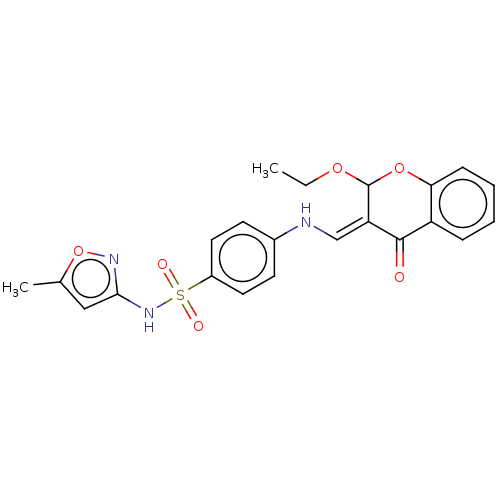 (CHEMBL3416226) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-9 assessed as CO2 hydration activity incubated for 15 mins prior to testing by sto... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-9 assessed as CO2 hydration activity incubated for 15 mins prior to testing by sto... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50080146 (CHEMBL3416242) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-9 assessed as CO2 hydration activity incubated for 15 mins prior to testing by sto... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50080155 (CHEMBL3416237) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-9 assessed as CO2 hydration activity incubated for 15 mins prior to testing by sto... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50216044 (2-(4-(4-(3-a,a,a-trifluorotolyl)-1-piperazinyl)but...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Antagonist activity at human Dopamine receptor D2 isoform long expressed in HEK293 cells assessed as change in quinpirole-induced intracellular calci... | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50080144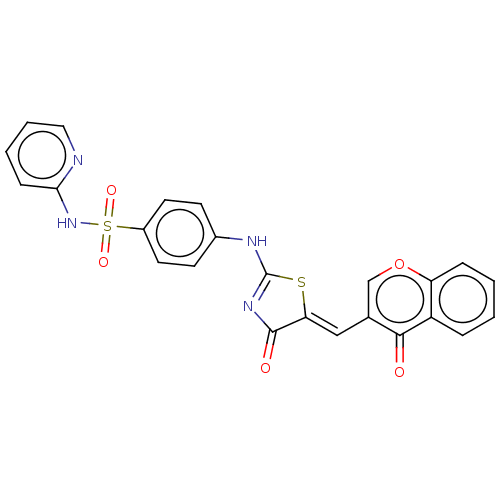 (CHEMBL3416240) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-9 assessed as CO2 hydration activity incubated for 15 mins prior to testing by sto... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50080151 (CHEMBL3040927) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-9 assessed as CO2 hydration activity incubated for 15 mins prior to testing by sto... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216047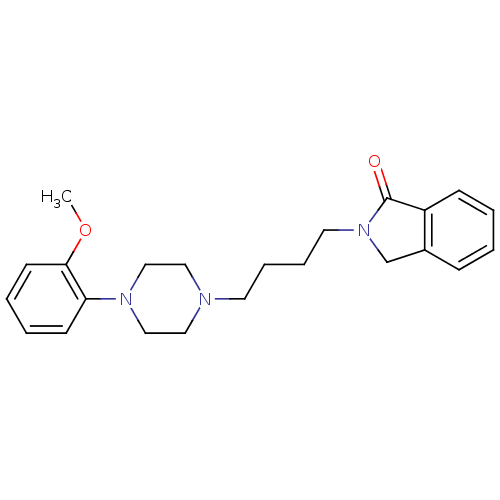 (2-(4-(4-phenyl-1-piperazinyl)butyl)-1-isoindolinon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human Dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50080152 (CHEMBL3416230) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-9 assessed as CO2 hydration activity incubated for 15 mins prior to testing by sto... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50216052 (2-(4-(4-(4-chlorophenyl)-1-piperazinyl)butyl)-1-is...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Displacement of [3H]SCH 23390 from human Dopamine D5 receptor expressed in HEK293 | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50216047 (2-(4-(4-phenyl-1-piperazinyl)butyl)-1-isoindolinon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Antagonist activity at human Dopamine receptor D2 isoform long expressed in HEK293 cells assessed as change in quinpirole-induced intracellular calci... | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216048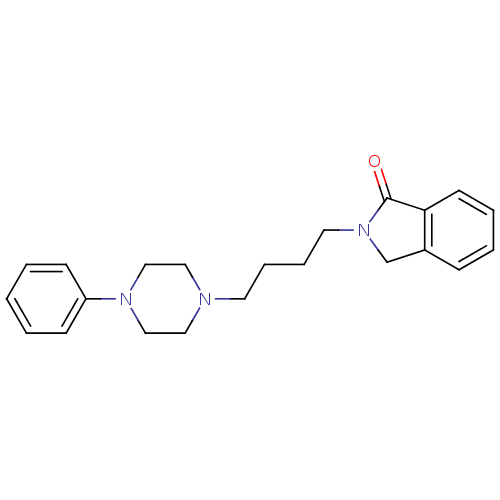 (2-(4-(4-(2-methoxyphenyl)-1-piperazinyl)butyl)-1-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human Dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50080149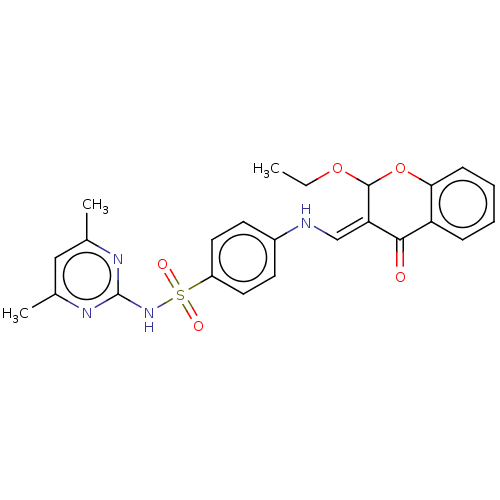 (CHEMBL3416228) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-9 assessed as CO2 hydration activity incubated for 15 mins prior to testing by sto... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50216045 (2-(4-(4-(2-methoxyphenyl)-1-piperazinyl)butyl)-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human Dopamine receptor D2 isoform long expressed in HEK293 cells | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50216041 (3-(4-(4-(2-methoxyphenyl)-1-piperazinyl)butyl)quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human Dopamine receptor D2 isoform long expressed in HEK293 cells | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50216048 (2-(4-(4-(2-methoxyphenyl)-1-piperazinyl)butyl)-1-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Antagonist activity at human Dopamine receptor D2 isoform long expressed in HEK293 cells assessed as change in quinpirole-induced intracellular calci... | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50437929 (CHEMBL2408560) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216045 (2-(4-(4-(2-methoxyphenyl)-1-piperazinyl)butyl)-1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human Dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50216053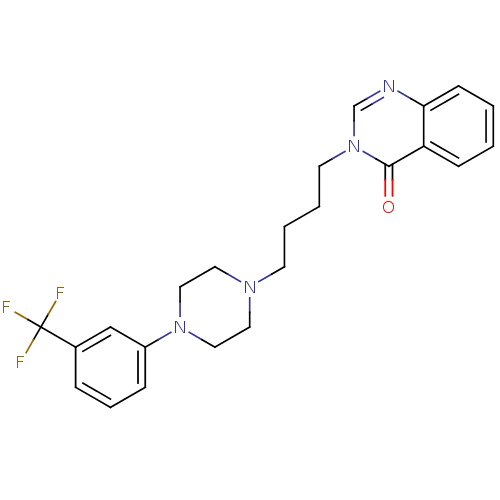 (3-(4-(4-(3-a,a,a-trifluorotolyl)-1-piperazinyl)but...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human Dopamine receptor D2 isoform long expressed in HEK293 cells | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50080145 (CHEMBL3416241) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human cytosolic form of carbonic anhydrase-2 assessed as CO2 hydration activity incubated for 15 mins prior to testing by stopped flow ... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50216047 (2-(4-(4-phenyl-1-piperazinyl)butyl)-1-isoindolinon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Displacement of [3H]SCH 23390 from human Dopamine D5 receptor expressed in HEK293 | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50080149 (CHEMBL3416228) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50216048 (2-(4-(4-(2-methoxyphenyl)-1-piperazinyl)butyl)-1-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Displacement of [3H]SCH 23390 from human Dopamine D5 receptor expressed in HEK293 | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216039 (1-(4-(4-(2-methoxyphenyl)-1-piperazinyl)butyl)-ind...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human Dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50216044 (2-(4-(4-(3-a,a,a-trifluorotolyl)-1-piperazinyl)but...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Displacement of [3H]SCH 23390 from human Dopamine D5 receptor expressed in HEK293 | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50080155 (CHEMBL3416237) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st... | Eur J Med Chem 96: 425-35 (2015) Article DOI: 10.1016/j.ejmech.2015.04.033 BindingDB Entry DOI: 10.7270/Q2T155BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216053 (3-(4-(4-(3-a,a,a-trifluorotolyl)-1-piperazinyl)but...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human Dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216051 (2-(4-(4-(3-a,a,a-trifluorotolyl)-1-piperazinyl)but...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human Dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50216051 (2-(4-(4-(3-a,a,a-trifluorotolyl)-1-piperazinyl)but...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human Dopamine receptor D2 isoform long expressed in HEK293 cells | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50216047 (2-(4-(4-phenyl-1-piperazinyl)butyl)-1-isoindolinon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Antagonist activity at human Dopamine D5 receptor in HEK293 cells assessed as change in SKF 38393-induced intracellular calcium response | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50216050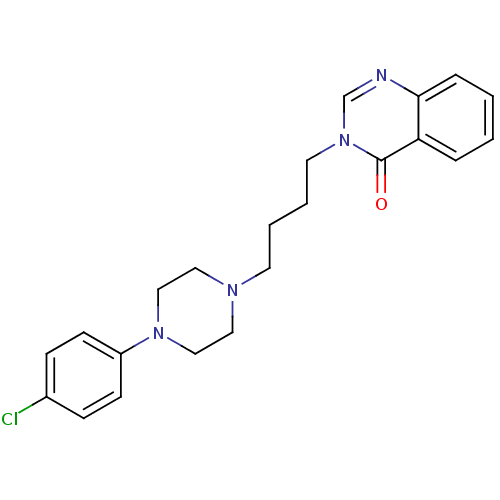 (3-(4-(4-(4-chlorophenyl)-1-piperazinyl)butyl)quina...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human Dopamine receptor D2 isoform long expressed in HEK293 cells | Bioorg Med Chem 15: 5811-8 (2007) Article DOI: 10.1016/j.bmc.2007.06.002 BindingDB Entry DOI: 10.7270/Q27M07MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 243 total ) | Next | Last >> |