

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291665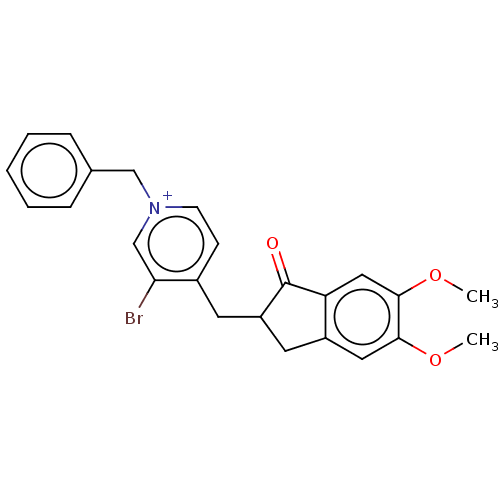 (CHEMBL4165327) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291432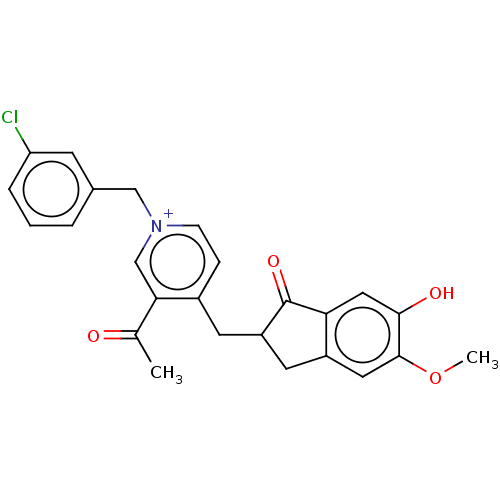 (CHEMBL4159705) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238227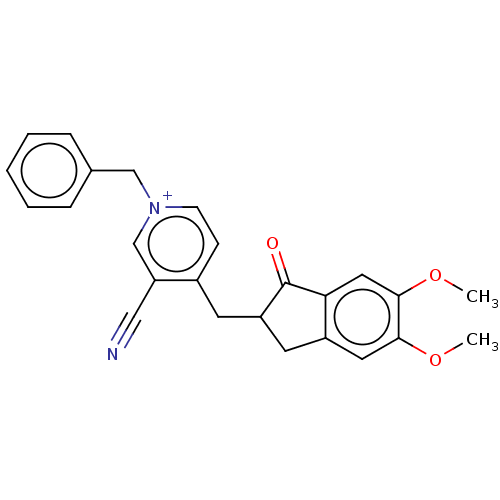 (CHEMBL4071377) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238223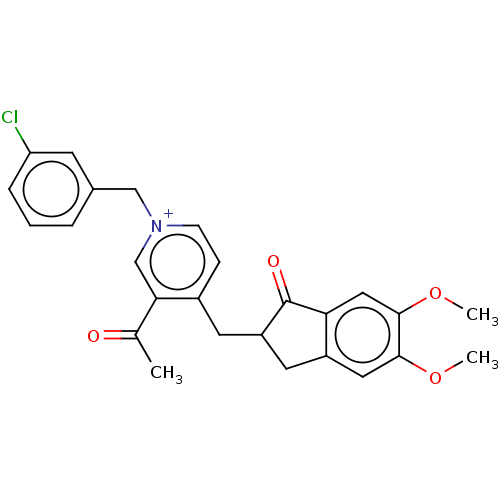 (CHEMBL4102333) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238216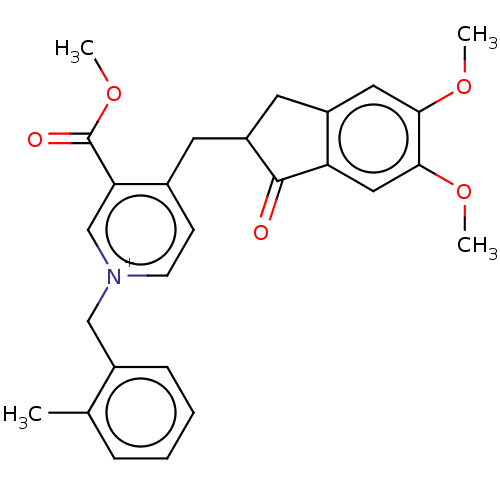 (CHEMBL4095535) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238235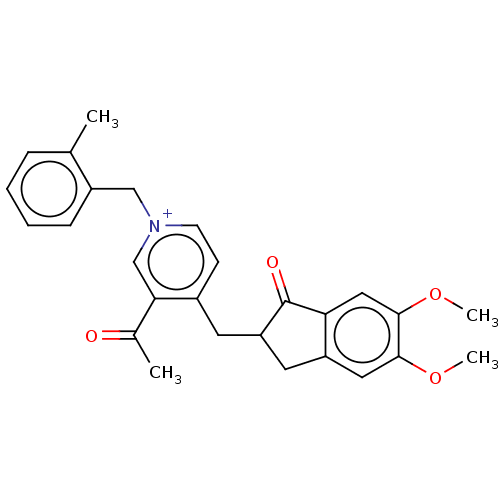 (CHEMBL4081915) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291438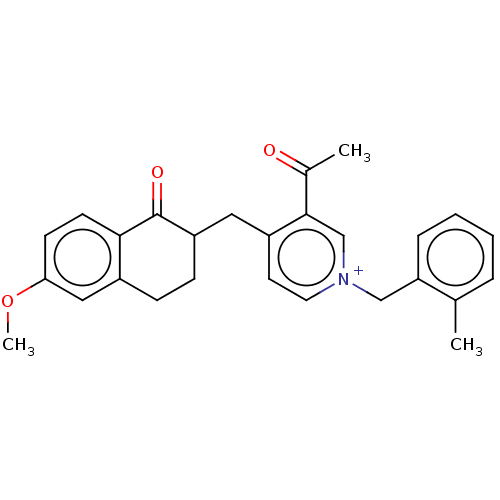 (CHEMBL4161065) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291612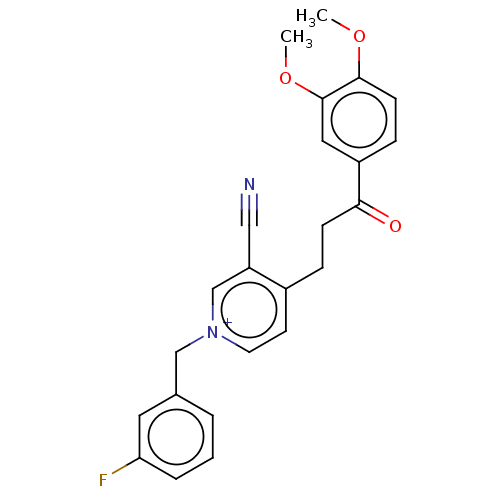 (CHEMBL4163678) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238224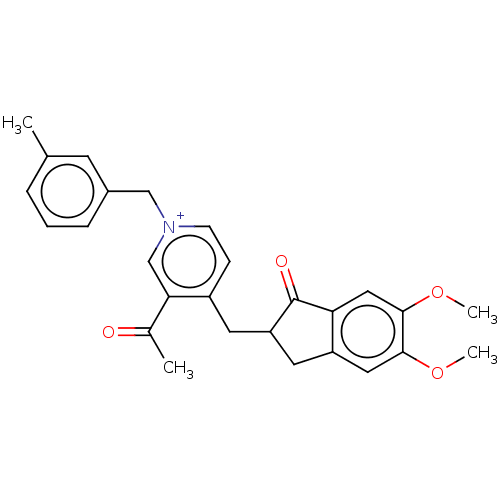 (CHEMBL4094081) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291664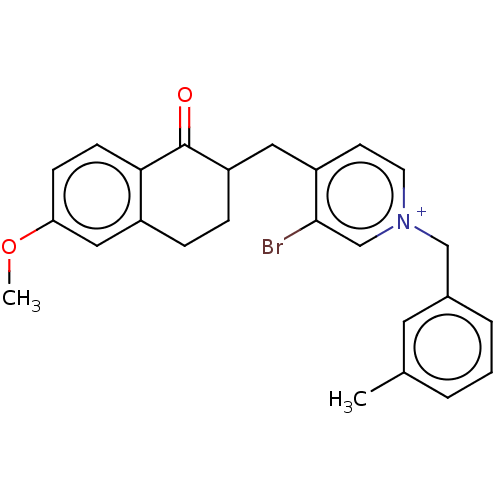 (CHEMBL4173239) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238213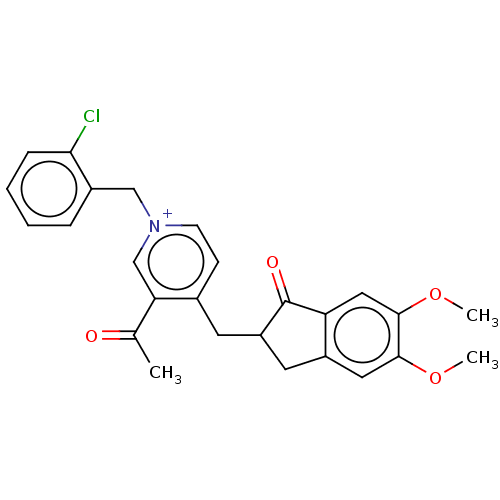 (CHEMBL4066548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238225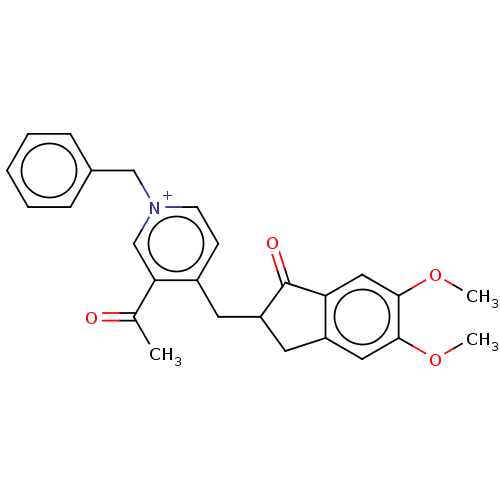 (CHEMBL4060011) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291633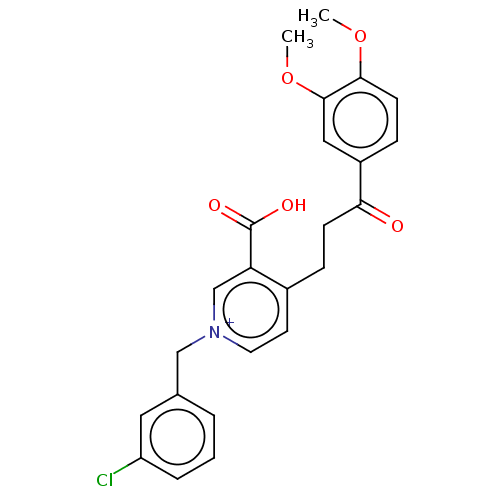 (CHEMBL4165973) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238212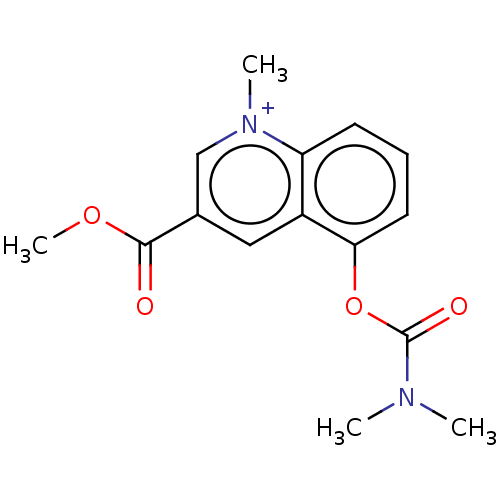 (CHEMBL4077952) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291636 (CHEMBL4169938) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291610 (CHEMBL4159880) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291635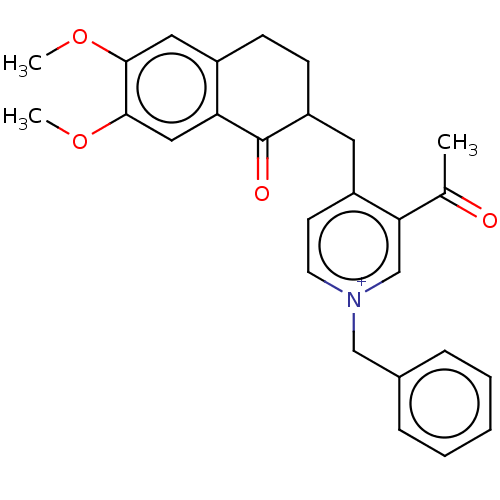 (CHEMBL4162015) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238236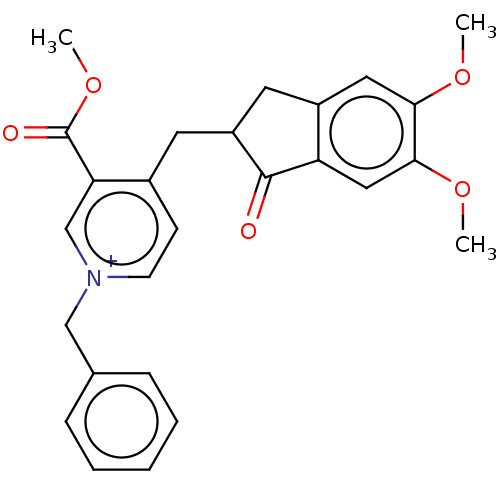 (CHEMBL4068149) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238220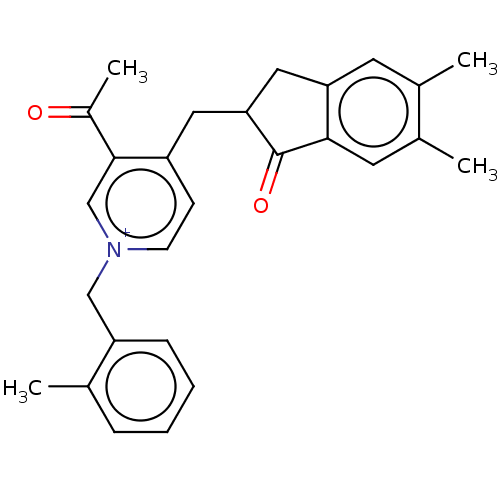 (CHEMBL4074062) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291435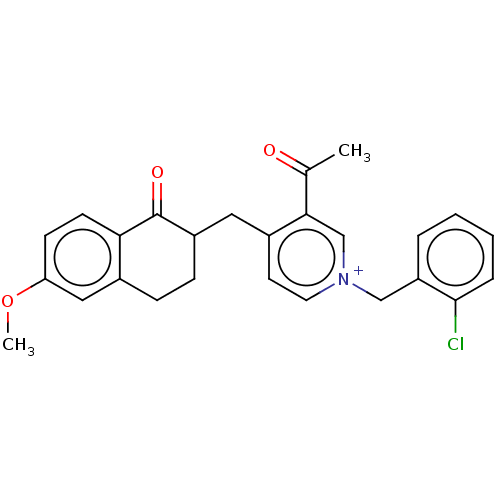 (CHEMBL4177133) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238222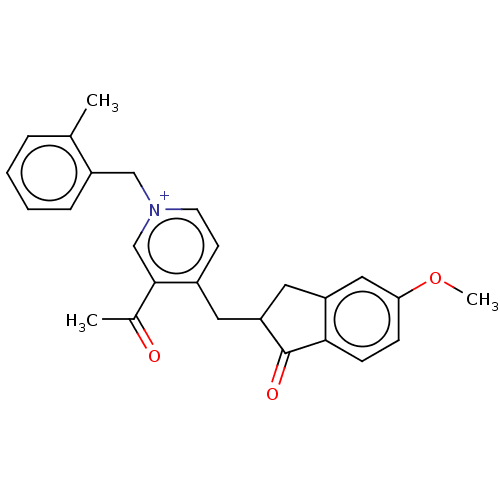 (CHEMBL4094591) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117592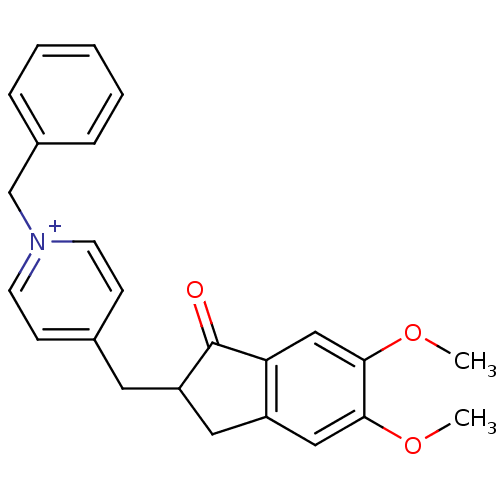 (1-Benzyl-4-(5,6-dimethoxy-1-oxo-indan-2-ylmethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291637 (CHEMBL4173784) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238228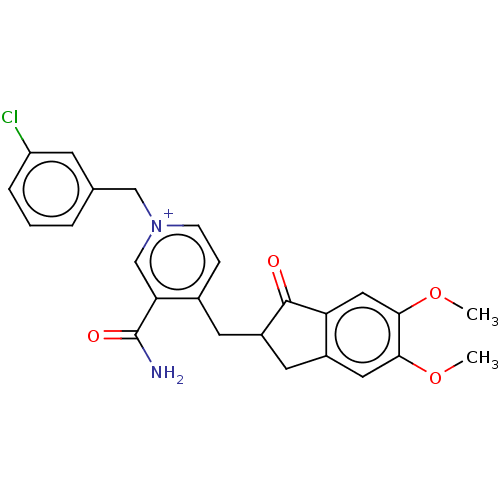 (CHEMBL4092048) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291436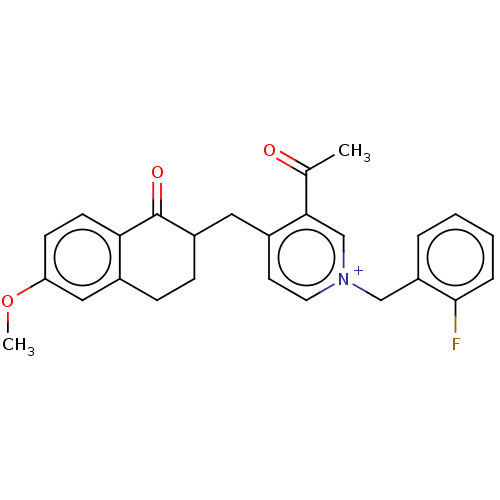 (CHEMBL4168037) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291437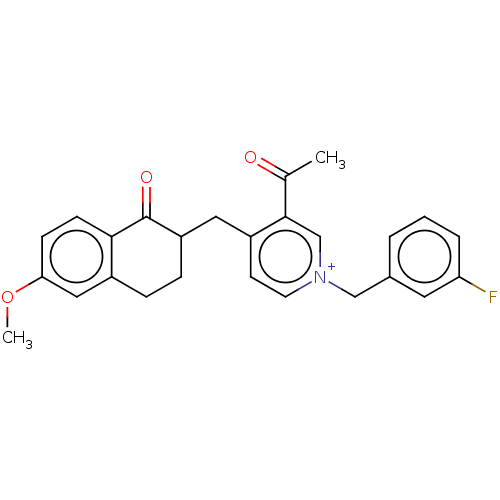 (CHEMBL4160115) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238240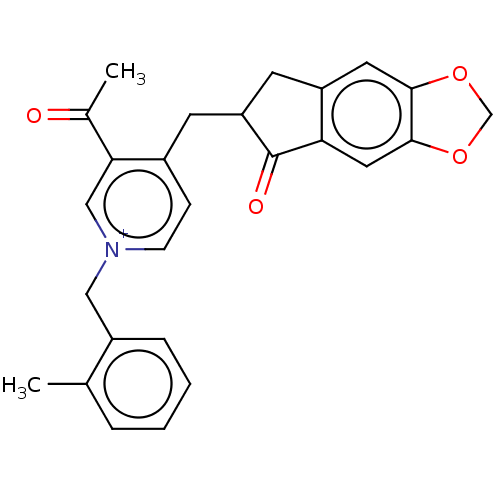 (CHEMBL4084371) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291577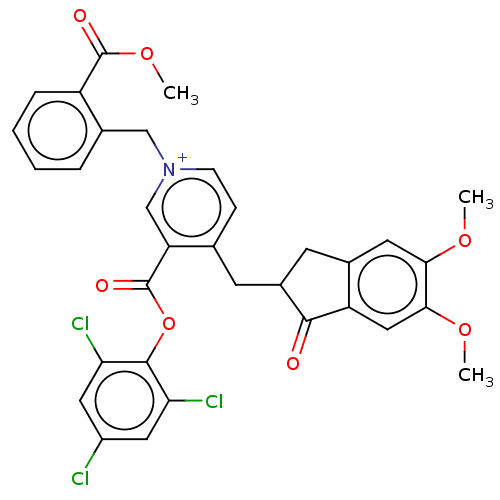 (CHEMBL4159243) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291434 (CHEMBL4166513) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238219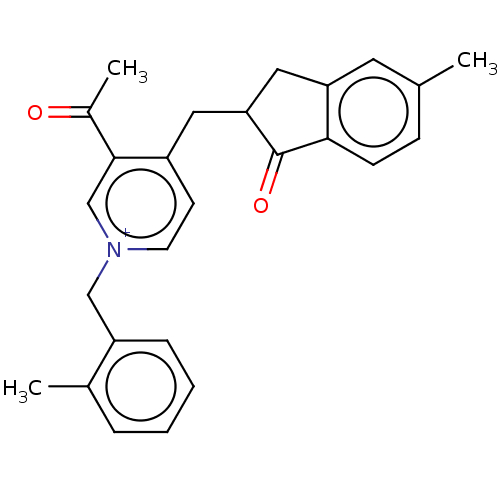 (CHEMBL4072999) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291439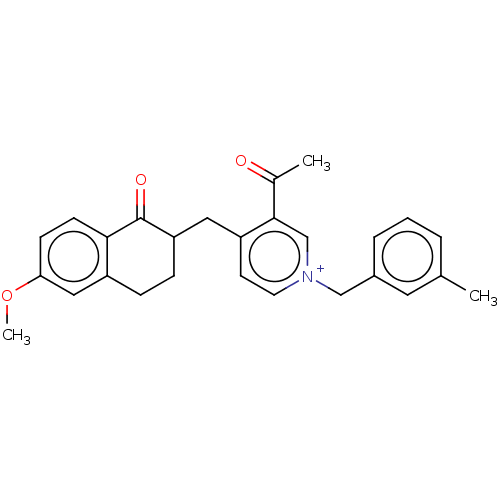 (CHEMBL4168987) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291433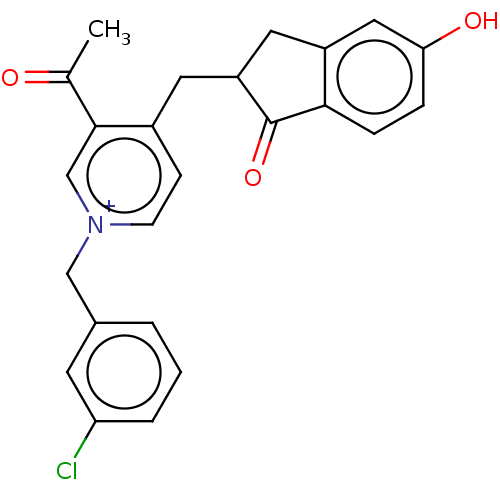 (CHEMBL4170331) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291575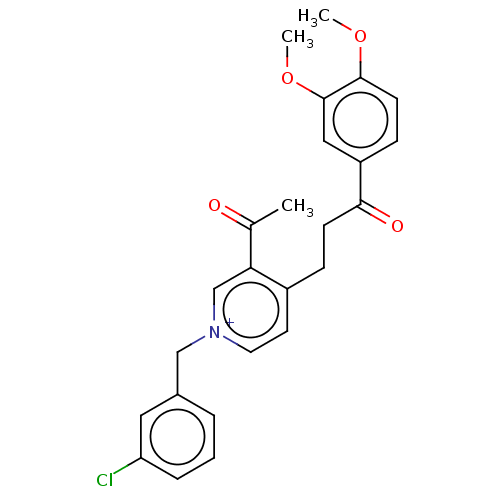 (CHEMBL4165562) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291616 (CHEMBL4171568) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291584 (CHEMBL4160696) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291568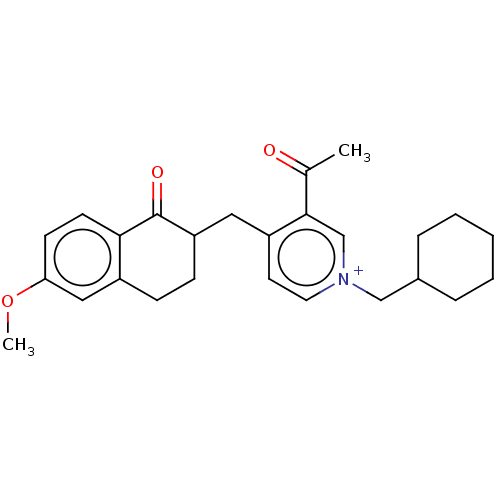 (CHEMBL4176179) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291606 (CHEMBL4164981) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50291435 (CHEMBL4177133) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 256 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238239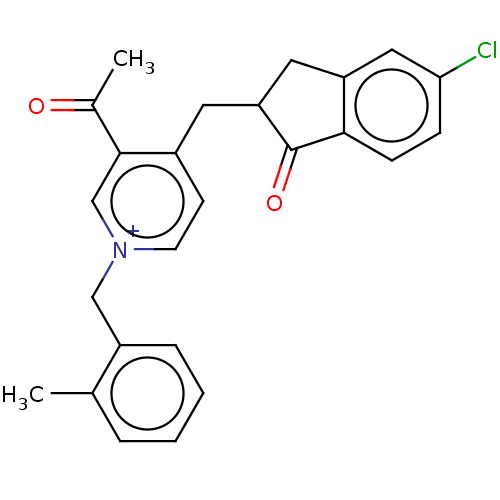 (CHEMBL4099873) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50238220 (CHEMBL4074062) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291630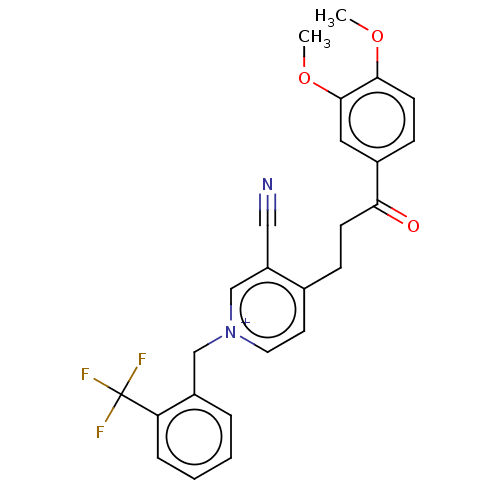 (CHEMBL4175003) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291584 (CHEMBL4160696) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 286 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291440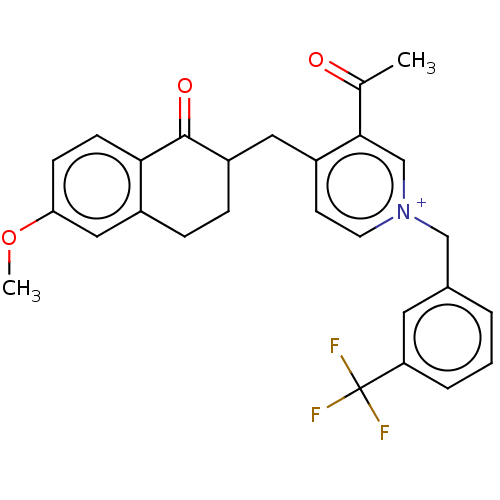 (CHEMBL4173405) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 308 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50291568 (CHEMBL4176179) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 341 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50291577 (CHEMBL4159243) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 373 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291588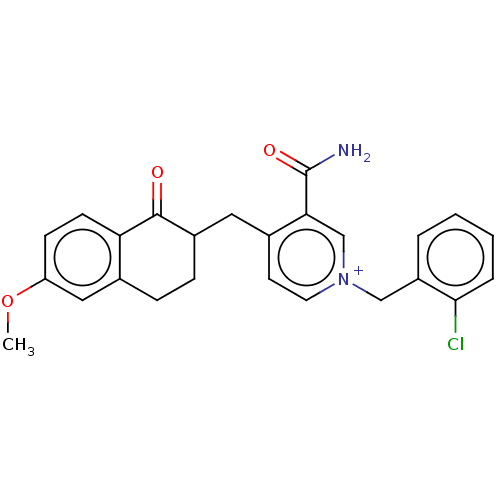 (CHEMBL4166464) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 379 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50291437 (CHEMBL4160115) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 412 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50291584 (CHEMBL4160696) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 415 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... | Eur J Med Chem 145: 165-190 (2018) Article DOI: 10.1016/j.ejmech.2017.12.084 BindingDB Entry DOI: 10.7270/Q2FN18RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 158 total ) | Next | Last >> |