

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-selectin (Homo sapiens (Human)) | BDBM50061136 ((2S,3R,4E)-3-(Benzoyloxy)-2-(hexadecanoylamino)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061130 ((2S,3R,4E)-3-(Benzoyloxy)-2-(hexadecanoylamino)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061124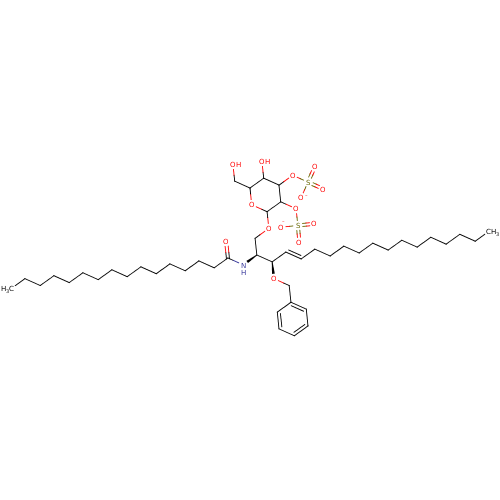 ((2S,3R,4E)-3-(Benzoyloxy)-2-(hexadecanoylamino)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50369315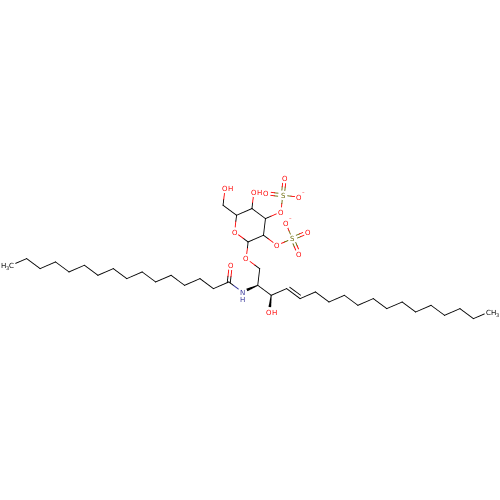 (CHEMBL1627019) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061125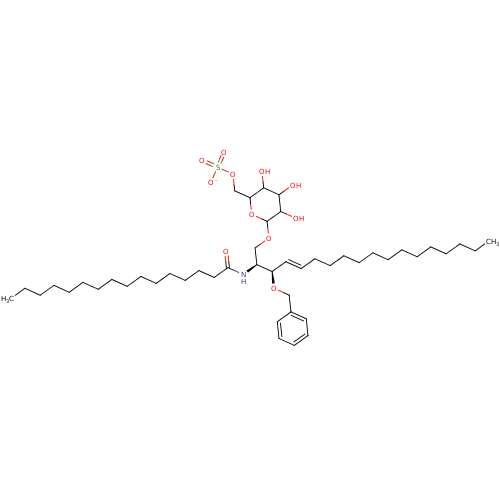 ((2S,3R,4E)-2-(hexadecanoylamino)-3-(benzoyloxy)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061133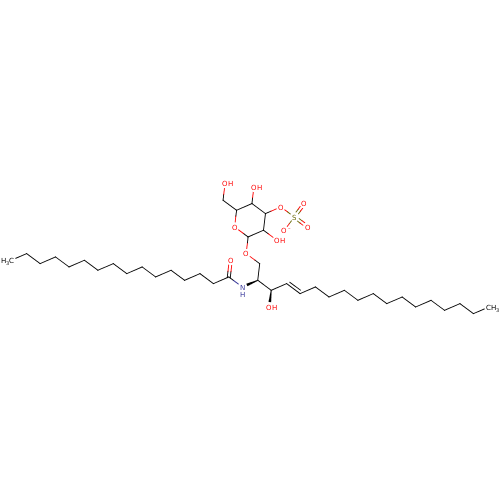 ((2S,3R,4E)-2-(Hexadecanoylamino)-3-hydroxy-1-[[3-O...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061128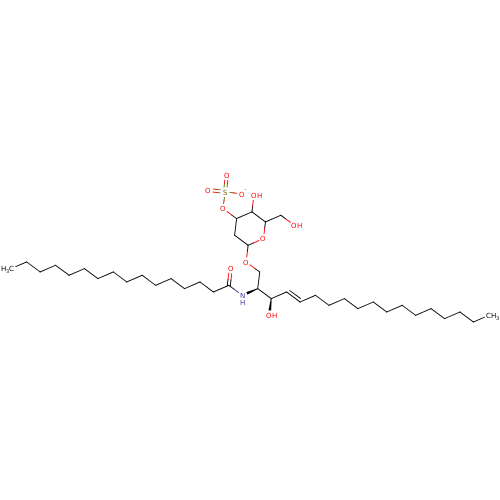 (1N-[2-hydroxy-1-(5-hydroxy-6-hydroxymethyltetrahyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061122 ((2S,3R,4E)-2-(Hexadecanoylamino)-3-(benzoyloxy)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061132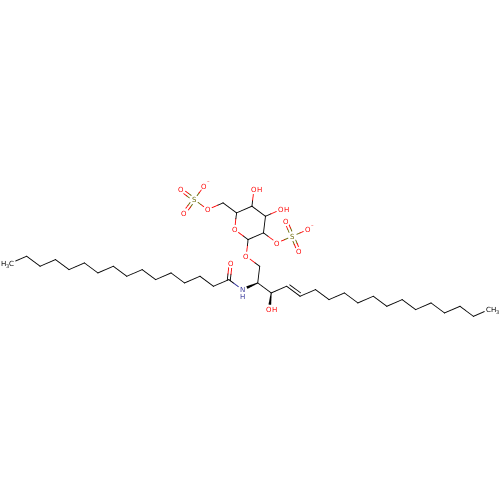 ((2S,3R,4E)-2-(Hexadecanoylamino)-3-hydroxy-1-[[2,6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061135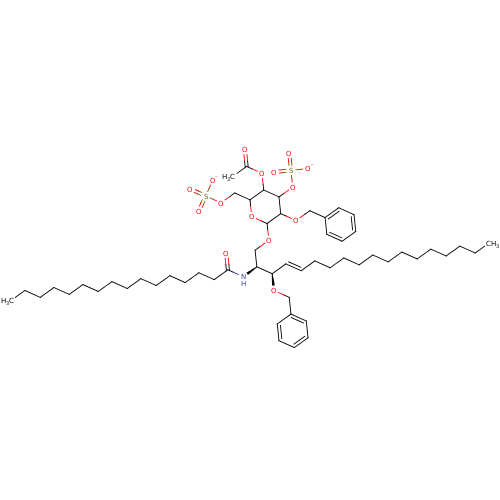 ((2S,3R,4E)-3-(Benzoyloxy)-2-(hexadecanoylamino)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061137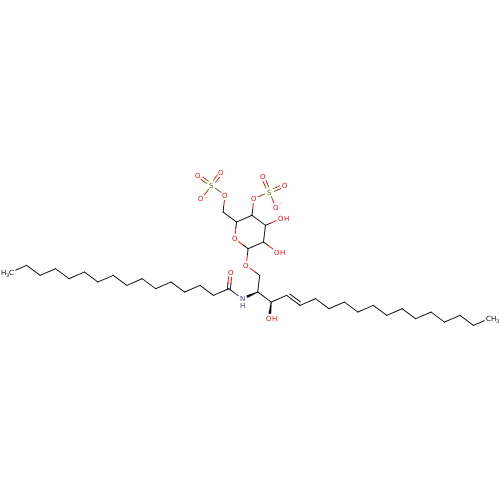 ((2S,3R,4E)-3-Hydroxy-2-(hexadecanoylamino)-1-[[4,6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061131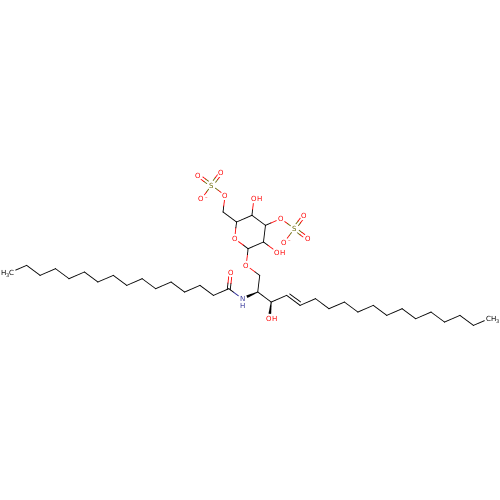 ((2S,3R,4E)-3-Hydroxy-2-(hexadecanoylamino)-1-[[3,6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061127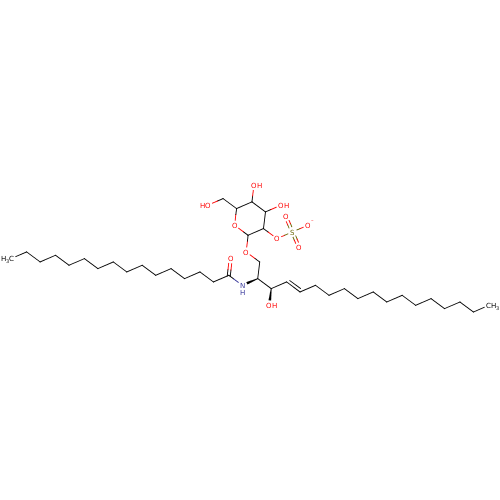 ((2S,3R,4E)-2-(Hexadecanoylamino)-3-hydroxy-1-[[2-O...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061129 ((2S,3R,4E)-2-(hexadecanoylamino)-3-(benzoyloxy)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50496099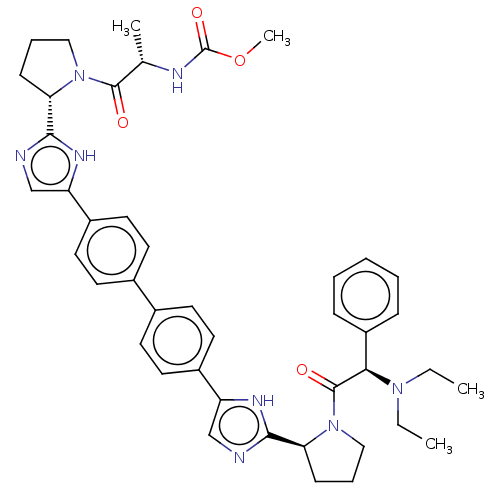 (CHEMBL3121151) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG by whole cell patch clamp assay | J Med Chem 57: 2013-32 (2014) Article DOI: 10.1021/jm401836p BindingDB Entry DOI: 10.7270/Q28918V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061121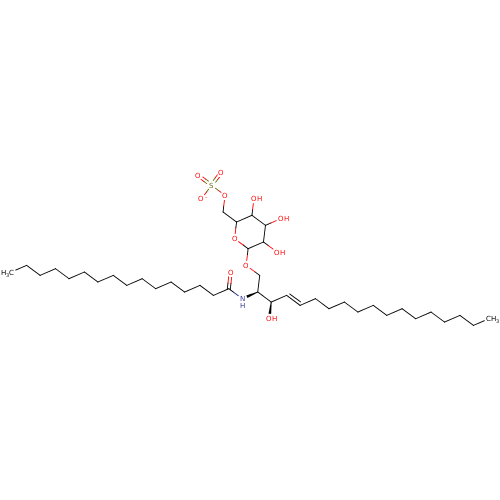 ((2S,3R,4E)-2-(Hexadecanoylamino)-1-[[6-O-(sodium o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061134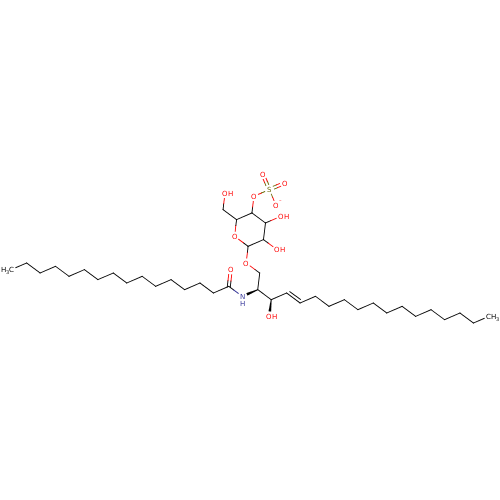 ((2S,3R,4E)-2-(Hexadecanoylamino)-3-hydroxy-1-[[4-O...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50387084 (BMS-790052 | DACLATASVIR) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG by whole cell patch clamp assay | J Med Chem 57: 2013-32 (2014) Article DOI: 10.1021/jm401836p BindingDB Entry DOI: 10.7270/Q28918V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061123 (((Z)-2-Hydroxy-1-hydroxymethyl-heptadec-3-enyl)-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50164201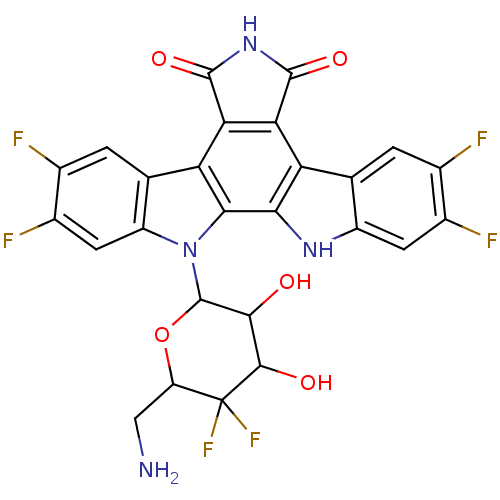 (12-(6-aminomethyl-5,5-difluoro-3,4-dihydroxytetrah...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Topoisomerase I activity for single-strand breaks in the DNA substrate | J Med Chem 48: 2258-61 (2005) Article DOI: 10.1021/jm049090z BindingDB Entry DOI: 10.7270/Q20G3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50164206 (3,9-difluoro-13-(6-fluoromethyl-3,4,5-trihydroxyte...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 67.2 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Topoisomerase I activity for single-strand breaks in the DNA substrate | J Med Chem 48: 2258-61 (2005) Article DOI: 10.1021/jm049090z BindingDB Entry DOI: 10.7270/Q20G3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50164203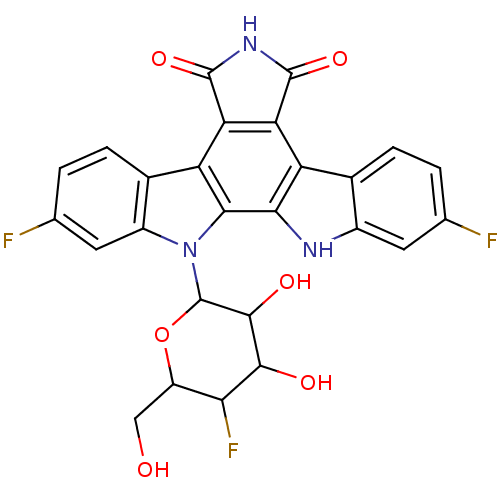 (2,10-difluoro-12-(5-fluoro-3,4-dihydroxy-6-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Topoisomerase I activity for single-strand breaks in the DNA substrate | J Med Chem 48: 2258-61 (2005) Article DOI: 10.1021/jm049090z BindingDB Entry DOI: 10.7270/Q20G3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50164205 (2,3,9,10-tetrafluoro-12-(5-fluoro-3,4-dihydroxy-6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Topoisomerase I activity for single-strand breaks in the DNA substrate | J Med Chem 48: 2258-61 (2005) Article DOI: 10.1021/jm049090z BindingDB Entry DOI: 10.7270/Q20G3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50164196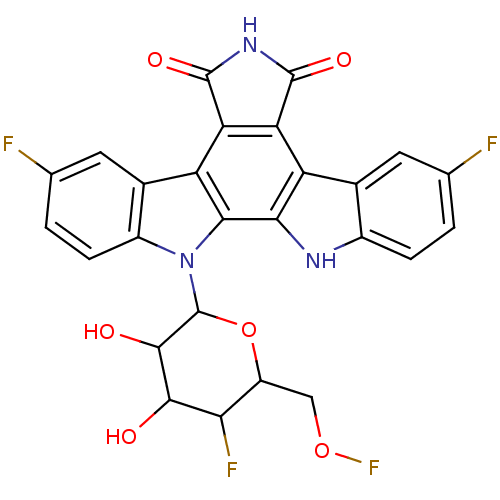 (3,9-difluoro-12-(5-fluoro-3,4-dihydroxy-6-hydroxym...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 65.6 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Topoisomerase I activity for single-strand breaks in the DNA substrate | J Med Chem 48: 2258-61 (2005) Article DOI: 10.1021/jm049090z BindingDB Entry DOI: 10.7270/Q20G3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50164197 (3,9-difluoro-12-(5-fluoro-3,4-dihydroxy-6-hydroxym...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 17.6 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Topoisomerase I activity for single-strand breaks in the DNA substrate | J Med Chem 48: 2258-61 (2005) Article DOI: 10.1021/jm049090z BindingDB Entry DOI: 10.7270/Q20G3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50002738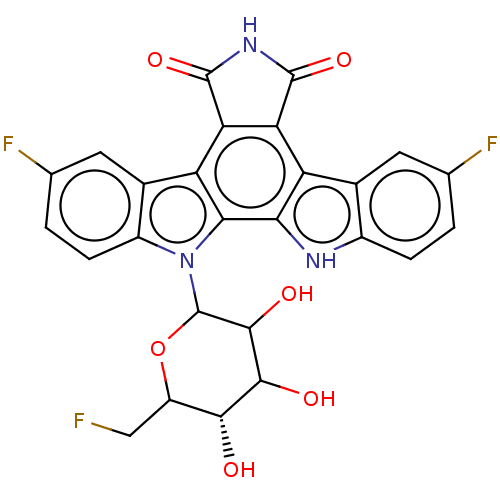 | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 704 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Topoisomerase I activity for single-strand breaks in the DNA substrate | J Med Chem 48: 2258-61 (2005) Article DOI: 10.1021/jm049090z BindingDB Entry DOI: 10.7270/Q20G3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50164202 (2,3,9,10-tetrafluoro-13-(6-fluoromethyl-3,4,5-trih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Topoisomerase I activity for single-strand breaks in the DNA substrate | J Med Chem 48: 2258-61 (2005) Article DOI: 10.1021/jm049090z BindingDB Entry DOI: 10.7270/Q20G3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50164199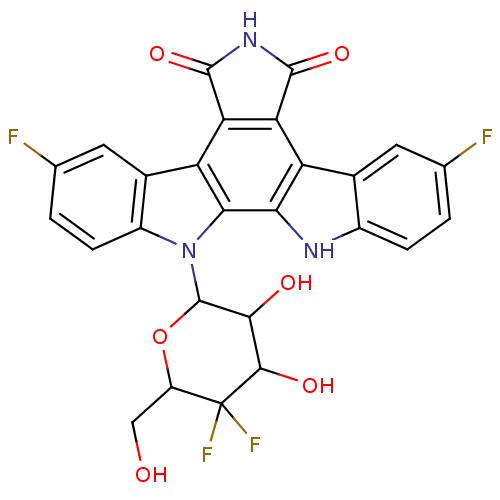 (3,9-difluoro-12-(5-fluoro-3,4,5-trihydroxy-6-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12.8 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Topoisomerase I activity for single-strand breaks in the DNA substrate | J Med Chem 48: 2258-61 (2005) Article DOI: 10.1021/jm049090z BindingDB Entry DOI: 10.7270/Q20G3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50164198 (3,9-difluoro-12-(6-fluoromethyl-3,4,5-trihydroxyte...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 76.8 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Topoisomerase I activity for single-strand breaks in the DNA substrate | J Med Chem 48: 2258-61 (2005) Article DOI: 10.1021/jm049090z BindingDB Entry DOI: 10.7270/Q20G3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50164200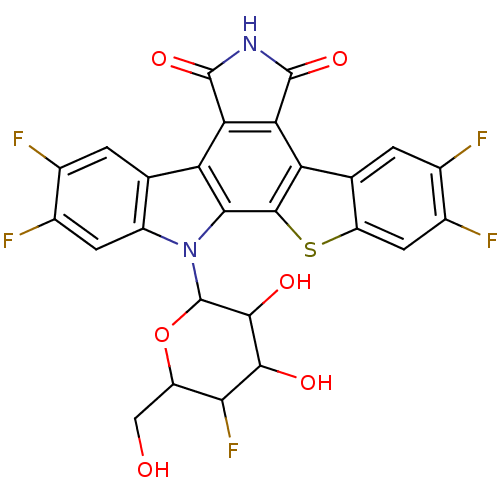 (2,3,9,10-tetrafluoro-13-(5-fluoro-3,4-dihydroxy-6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Topoisomerase I activity for single-strand breaks in the DNA substrate | J Med Chem 48: 2258-61 (2005) Article DOI: 10.1021/jm049090z BindingDB Entry DOI: 10.7270/Q20G3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50164204 (13-(5,5-difluoro-3,4-dihydroxy-6-hydroxymethyltetr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Topoisomerase I activity for single-strand breaks in the DNA substrate | J Med Chem 48: 2258-61 (2005) Article DOI: 10.1021/jm049090z BindingDB Entry DOI: 10.7270/Q20G3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50164207 (2,3,9,10-tetrafluoro-12-(6-fluoromethyl-3,4,5-trih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Topoisomerase I activity for single-strand breaks in the DNA substrate | J Med Chem 48: 2258-61 (2005) Article DOI: 10.1021/jm049090z BindingDB Entry DOI: 10.7270/Q20G3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50142927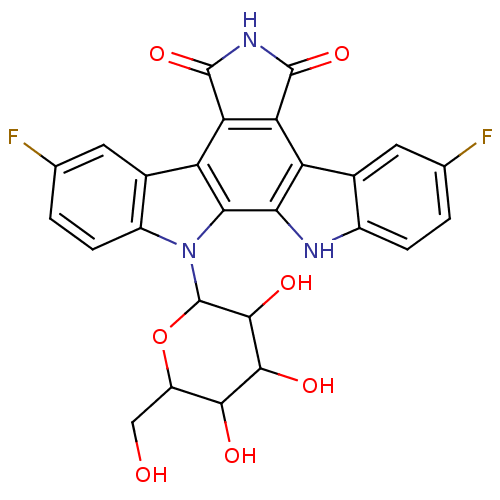 (12-(3,4-dihydroxy-6-hydroxymethyl-5-methoxytetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 38.4 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Topoisomerase I activity for single-strand breaks in the DNA substrate | J Med Chem 48: 2258-61 (2005) Article DOI: 10.1021/jm049090z BindingDB Entry DOI: 10.7270/Q20G3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50164195 (12-(6-aminomethyl-5-fluoro-3,4-dihydroxytetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11.2 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Topoisomerase I activity for single-strand breaks in the DNA substrate | J Med Chem 48: 2258-61 (2005) Article DOI: 10.1021/jm049090z BindingDB Entry DOI: 10.7270/Q20G3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||