 Found 684 hits with Last Name = 'bajorath' and Initial = 'j'
Found 684 hits with Last Name = 'bajorath' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50324477

(Benzylsulfonyl-D-cyclohexylalanyl-proline-(4-amidi...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCCN2C(=O)[C@@H](CC2CCCCC2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C29H39N5O4S/c30-27(31)24-15-13-22(14-16-24)19-32-28(35)26-12-7-17-34(26)29(36)25(18-21-8-3-1-4-9-21)33-39(37,38)20-23-10-5-2-6-11-23/h2,5-6,10-11,13-16,21,25-26,33H,1,3-4,7-9,12,17-20H2,(H3,30,31)(H,32,35)/t25-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 53: 5523-35 (2010)
Article DOI: 10.1021/jm100183e
BindingDB Entry DOI: 10.7270/Q25B02PD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50324478
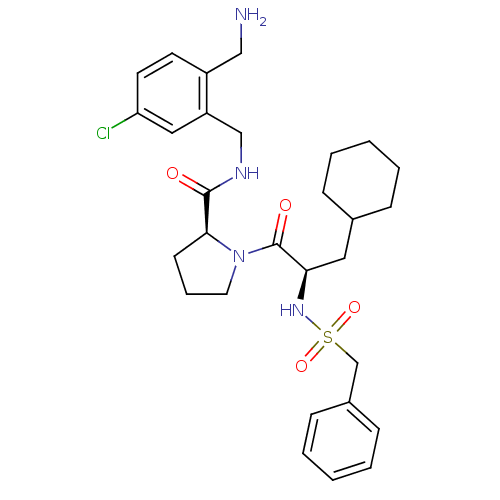
(Benzylsulfonyl-D-cyclohexylalanyl-proline-(2-amino...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NS(=O)(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C29H39ClN4O4S/c30-25-14-13-23(18-31)24(17-25)19-32-28(35)27-12-7-15-34(27)29(36)26(16-21-8-3-1-4-9-21)33-39(37,38)20-22-10-5-2-6-11-22/h2,5-6,10-11,13-14,17,21,26-27,33H,1,3-4,7-9,12,15-16,18-20,31H2,(H,32,35)/t26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 53: 5523-35 (2010)
Article DOI: 10.1021/jm100183e
BindingDB Entry DOI: 10.7270/Q25B02PD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12746
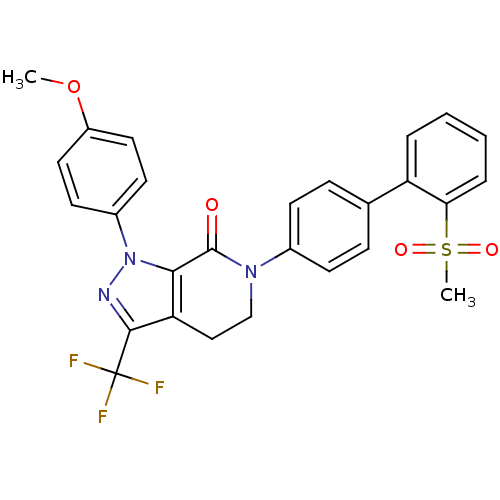
(6-[4-(2-methanesulfonylphenyl)phenyl]-1-(4-methoxy...)Show SMILES COc1ccc(cc1)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)-c1ccccc1S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C27H22F3N3O4S/c1-37-20-13-11-19(12-14-20)33-24-22(25(31-33)27(28,29)30)15-16-32(26(24)34)18-9-7-17(8-10-18)21-5-3-4-6-23(21)38(2,35)36/h3-14H,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 54: 2944-51 (2011)
Article DOI: 10.1021/jm200026b
BindingDB Entry DOI: 10.7270/Q2ZG6TGM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12743
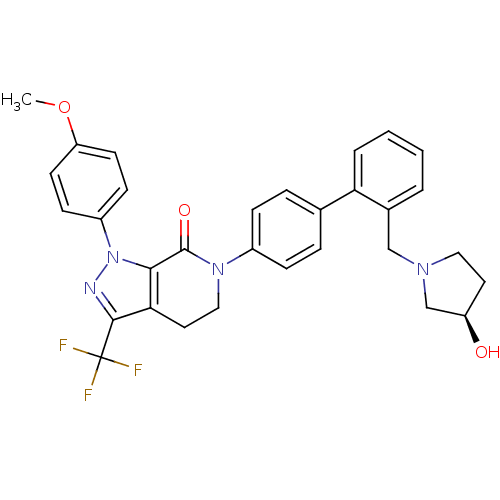
(6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phe...)Show SMILES COc1ccc(cc1)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)-c1ccccc1CN1CC[C@@H](O)C1)C(F)(F)F |r| Show InChI InChI=1S/C31H29F3N4O3/c1-41-25-12-10-23(11-13-25)38-28-27(29(35-38)31(32,33)34)15-17-37(30(28)40)22-8-6-20(7-9-22)26-5-3-2-4-21(26)18-36-16-14-24(39)19-36/h2-13,24,39H,14-19H2,1H3/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 54: 2944-51 (2011)
Article DOI: 10.1021/jm200026b
BindingDB Entry DOI: 10.7270/Q2ZG6TGM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50510762
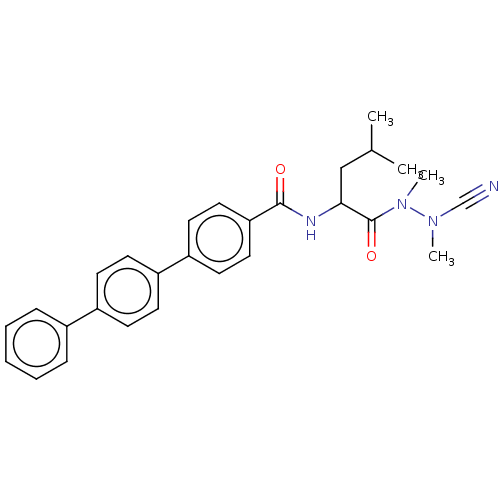
(CHEMBL4442025)Show SMILES CC(C)CC(NC(=O)c1ccc(cc1)-c1ccc(cc1)-c1ccccc1)C(=O)N(C)N(C)C#N Show InChI InChI=1S/C28H30N4O2/c1-20(2)18-26(28(34)32(4)31(3)19-29)30-27(33)25-16-14-24(15-17-25)23-12-10-22(11-13-23)21-8-6-5-7-9-21/h5-17,20,26H,18H2,1-4H3,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B using Z-Phe-Arg-AMC as substrate incubated for 30 mins by fluorescence based assay |
Bioorg Med Chem 27: 1-15 (2019)
Article DOI: 10.1016/j.bmc.2018.10.017
BindingDB Entry DOI: 10.7270/Q21G0QK2 |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Rattus norvegicus (Rat)) | BDBM50378656
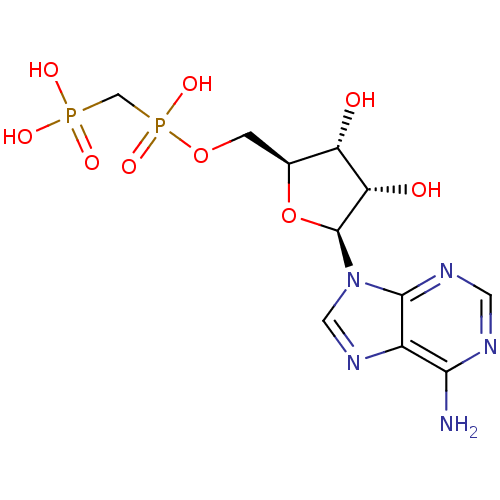
(CHEMBL598619)Show SMILES Nc1ncnc2n(cnc12)[C@H]1O[C@@H](COP(O)(=O)CP(O)(O)=O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C11H17N5O9P2/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(25-11)1-24-27(22,23)4-26(19,20)21/h2-3,5,7-8,11,17-18H,1,4H2,(H,22,23)(H2,12,13,14)(H2,19,20,21)/t5-,7-,8-,11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat ecto-5'-nucleotidase expressed in Sf9 cells by capillary electrophoresis method |
J Med Chem 53: 2076-86 (2010)
Article DOI: 10.1021/jm901851t
BindingDB Entry DOI: 10.7270/Q2DZ097V |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Rattus norvegicus (Rat)) | BDBM50368125

(ADENOSINE DIPHOSPHATE | ADP)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H15N5O10P2/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(24-10)1-23-27(21,22)25-26(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat ecto-5'-nucleotidase expressed in Sf9 cells by capillary electrophoresis method |
J Med Chem 53: 2076-86 (2010)
Article DOI: 10.1021/jm901851t
BindingDB Entry DOI: 10.7270/Q2DZ097V |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50324476

(Benzylsulfonyl-D-arginyl-proline-(2-aminomethyl-5-...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCCNC(N)=N)NS(=O)(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C26H36ClN7O4S/c27-21-11-10-19(15-28)20(14-21)16-32-24(35)23-9-5-13-34(23)25(36)22(8-4-12-31-26(29)30)33-39(37,38)17-18-6-2-1-3-7-18/h1-3,6-7,10-11,14,22-23,33H,4-5,8-9,12-13,15-17,28H2,(H,32,35)(H4,29,30,31)/t22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 53: 5523-35 (2010)
Article DOI: 10.1021/jm100183e
BindingDB Entry DOI: 10.7270/Q25B02PD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50077217
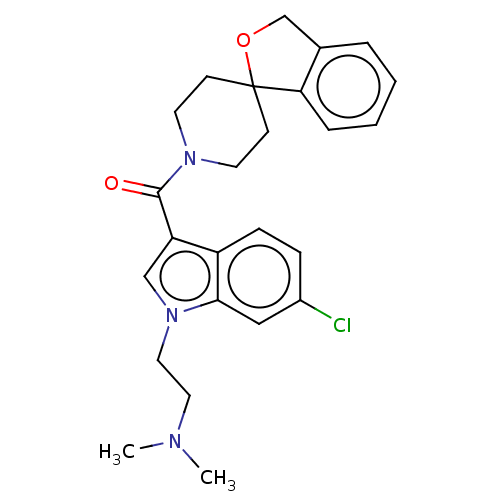
(CHEMBL3416885)Show SMILES CN(C)CCn1cc(C(=O)N2CCC3(CC2)OCc2ccccc32)c2ccc(Cl)cc12 Show InChI InChI=1S/C25H28ClN3O2/c1-27(2)13-14-29-16-21(20-8-7-19(26)15-23(20)29)24(30)28-11-9-25(10-12-28)22-6-4-3-5-18(22)17-31-25/h3-8,15-16H,9-14,17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Antagonist activity at human vasopressin 1a receptor |
J Med Chem 60: 1238-1246 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01437
BindingDB Entry DOI: 10.7270/Q2KK9F10 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50247638

(CHEMBL4101883)Show SMILES CC1NN(C)C(C)=C1c1c(Cl)ccc2c(CCCOc3cc(C)c(Cl)c(C)c3)c3C(=O)N(CCCn3c12)c1cc(cc2n(C)ccc12)C(O)=O |c:6| Show InChI InChI=1S/C39H41Cl2N5O4/c1-21-17-26(18-22(2)35(21)41)50-16-7-9-27-28-10-11-30(40)34(33-23(3)42-44(6)24(33)4)36(28)46-14-8-13-45(38(47)37(27)46)32-20-25(39(48)49)19-31-29(32)12-15-43(31)5/h10-12,15,17-20,23,42H,7-9,13-14,16H2,1-6H3,(H,48,49) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of FITC-Bim binding to human His6-MPB tagged MCL1 expressed in Escherichia coli K-12 by TR-FRET assay |
J Med Chem 61: 1276-1284 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01780
BindingDB Entry DOI: 10.7270/Q2B27XQN |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50324475

(Benzylsulfonyl-D-argininyl-proline-(4-amidinobenzy...)Show SMILES NC(=N)NCCC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C26H36N8O4S/c27-23(28)20-12-10-18(11-13-20)16-32-24(35)22-9-5-15-34(22)25(36)21(8-4-14-31-26(29)30)33-39(37,38)17-19-6-2-1-3-7-19/h1-3,6-7,10-13,21-22,33H,4-5,8-9,14-17H2,(H3,27,28)(H,32,35)(H4,29,30,31)/t21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 53: 5523-35 (2010)
Article DOI: 10.1021/jm100183e
BindingDB Entry DOI: 10.7270/Q25B02PD |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50077213
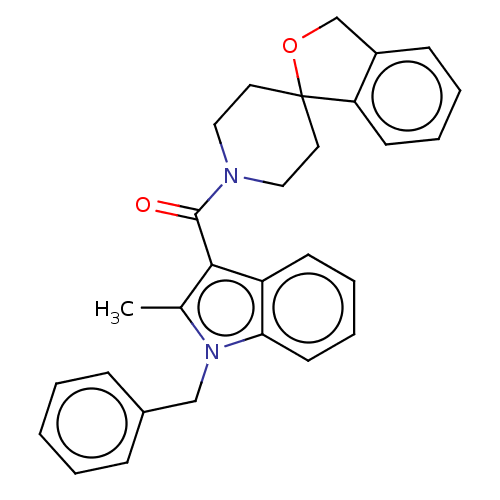
(CHEMBL3416860)Show SMILES Cc1c(C(=O)N2CCC3(CC2)OCc2ccccc32)c2ccccc2n1Cc1ccccc1 Show InChI InChI=1S/C29H28N2O2/c1-21-27(24-12-6-8-14-26(24)31(21)19-22-9-3-2-4-10-22)28(32)30-17-15-29(16-18-30)25-13-7-5-11-23(25)20-33-29/h2-14H,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Antagonist activity at human vasopressin 1a receptor |
J Med Chem 60: 1238-1246 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01437
BindingDB Entry DOI: 10.7270/Q2KK9F10 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50510761
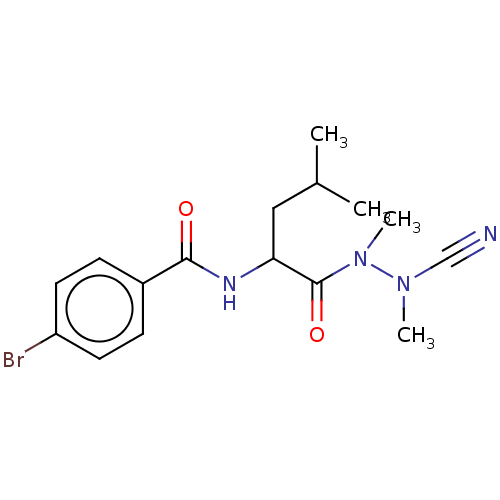
(CHEMBL4440655)Show SMILES CC(C)CC(NC(=O)c1ccc(Br)cc1)C(=O)N(C)N(C)C#N Show InChI InChI=1S/C16H21BrN4O2/c1-11(2)9-14(16(23)21(4)20(3)10-18)19-15(22)12-5-7-13(17)8-6-12/h5-8,11,14H,9H2,1-4H3,(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B using Z-Phe-Arg-AMC as substrate incubated for 30 mins by fluorescence based assay |
Bioorg Med Chem 27: 1-15 (2019)
Article DOI: 10.1016/j.bmc.2018.10.017
BindingDB Entry DOI: 10.7270/Q21G0QK2 |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50505323
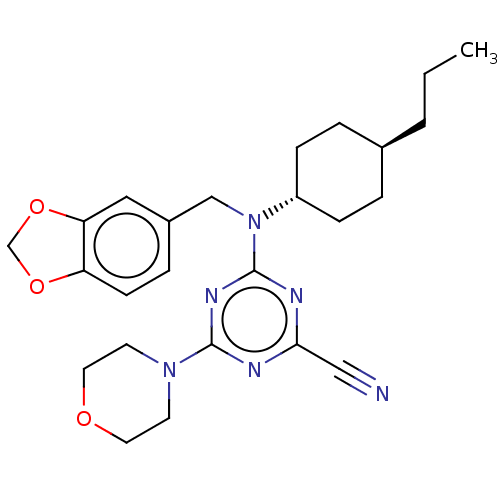
(CHEMBL4556848)Show SMILES CCC[C@H]1CC[C@@H](CC1)N(Cc1ccc2OCOc2c1)c1nc(nc(n1)N1CCOCC1)C#N |r,wU:6.9,wD:3.2,(35.77,-15.92,;34.44,-15.15,;33.11,-15.91,;31.77,-15.14,;31.78,-13.6,;30.44,-12.82,;29.12,-13.59,;29.11,-15.13,;30.44,-15.9,;27.78,-12.82,;26.45,-13.59,;26.45,-15.13,;27.78,-15.89,;27.79,-17.43,;26.45,-18.21,;26.13,-19.72,;24.59,-19.88,;23.96,-18.47,;25.11,-17.43,;25.11,-15.9,;27.78,-11.28,;29.12,-10.51,;29.12,-8.96,;27.78,-8.2,;26.45,-8.97,;26.45,-10.51,;25.12,-8.2,;25.12,-6.65,;23.8,-5.88,;22.46,-6.64,;22.45,-8.18,;23.79,-8.96,;30.44,-8.18,;31.77,-7.41,)| Show InChI InChI=1S/C25H32N6O3/c1-2-3-18-4-7-20(8-5-18)31(16-19-6-9-21-22(14-19)34-17-33-21)25-28-23(15-26)27-24(29-25)30-10-12-32-13-11-30/h6,9,14,18,20H,2-5,7-8,10-13,16-17H2,1H3/t18-,20- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain |
J Med Chem 62: 10497-10525 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00683
BindingDB Entry DOI: 10.7270/Q2K93BT1 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13286
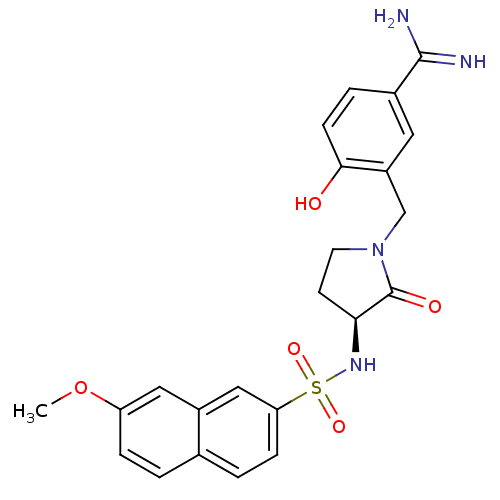
(4-Hydroxy-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@H]1CCN(Cc2cc(ccc2O)C(N)=N)C1=O |r| Show InChI InChI=1S/C23H24N4O5S/c1-32-18-5-2-14-3-6-19(12-16(14)11-18)33(30,31)26-20-8-9-27(23(20)29)13-17-10-15(22(24)25)4-7-21(17)28/h2-7,10-12,20,26,28H,8-9,13H2,1H3,(H3,24,25)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 54: 2944-51 (2011)
Article DOI: 10.1021/jm200026b
BindingDB Entry DOI: 10.7270/Q2ZG6TGM |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50077206

(CHEMBL3416861)Show SMILES Clc1ccc2c(c[nH]c2c1)C(=O)N1CCC2(CC1)OCc1ccccc21 Show InChI InChI=1S/C21H19ClN2O2/c22-15-5-6-16-17(12-23-19(16)11-15)20(25)24-9-7-21(8-10-24)18-4-2-1-3-14(18)13-26-21/h1-6,11-12,23H,7-10,13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Antagonist activity at human vasopressin 1a receptor |
J Med Chem 60: 1238-1246 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01437
BindingDB Entry DOI: 10.7270/Q2KK9F10 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50560498

(CHEMBL4763197)Show SMILES CC(C)C[C@H](NS(=O)(=O)c1cccc(c1)-c1ccccc1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human cathepsin S expressed in insect cells using Z-Phe-Arg-AMC as substrate measured after 60 mins by fluorimetric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127420
BindingDB Entry DOI: 10.7270/Q2S75M1Z |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50510759

(CHEMBL4459206)Show SMILES CC(C)CC(NC(=O)c1cccc(c1)-c1ccncc1)C(=O)N(C)N(C)C#N Show InChI InChI=1S/C21H25N5O2/c1-15(2)12-19(21(28)26(4)25(3)14-22)24-20(27)18-7-5-6-17(13-18)16-8-10-23-11-9-16/h5-11,13,15,19H,12H2,1-4H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B using Z-Phe-Arg-AMC as substrate incubated for 30 mins by fluorescence based assay |
Bioorg Med Chem 27: 1-15 (2019)
Article DOI: 10.1016/j.bmc.2018.10.017
BindingDB Entry DOI: 10.7270/Q21G0QK2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19018
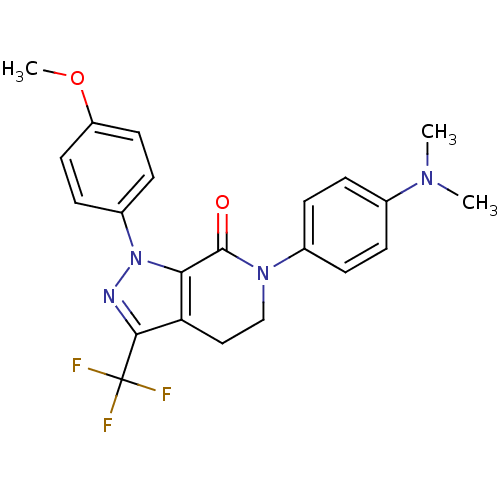
(6-[4-(dimethylamino)phenyl]-1-(4-methoxyphenyl)-3-...)Show SMILES COc1ccc(cc1)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)N(C)C)C(F)(F)F Show InChI InChI=1S/C22H21F3N4O2/c1-27(2)14-4-6-15(7-5-14)28-13-12-18-19(21(28)30)29(26-20(18)22(23,24)25)16-8-10-17(31-3)11-9-16/h4-11H,12-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 54: 2944-51 (2011)
Article DOI: 10.1021/jm200026b
BindingDB Entry DOI: 10.7270/Q2ZG6TGM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081002
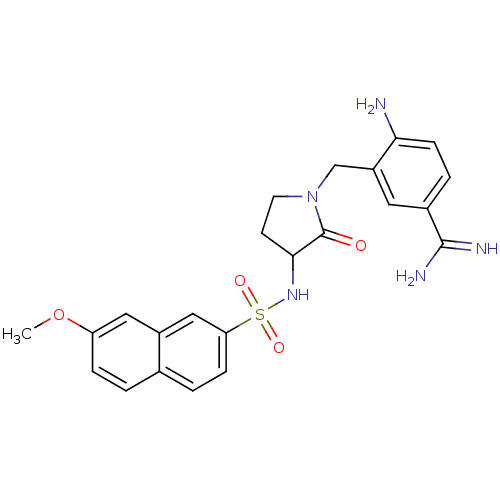
(4-Amino-3-[3-((S)-7-methoxy-naphthalene-2-sulfonyl...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)NC1CCN(Cc2cc(ccc2N)C(N)=N)C1=O Show InChI InChI=1S/C23H25N5O4S/c1-32-18-5-2-14-3-6-19(12-16(14)11-18)33(30,31)27-21-8-9-28(23(21)29)13-17-10-15(22(25)26)4-7-20(17)24/h2-7,10-12,21,27H,8-9,13,24H2,1H3,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 54: 2944-51 (2011)
Article DOI: 10.1021/jm200026b
BindingDB Entry DOI: 10.7270/Q2ZG6TGM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13288

(Sulfonamidopyrrolidinone 27 | methyl 2-(4-carbamim...)Show SMILES COC(=O)COc1ccc(cc1CN1CC[C@H](NS(=O)(=O)c2ccc3ccc(OC)cc3c2)C1=O)C(N)=N |r| Show InChI InChI=1S/C26H28N4O7S/c1-35-20-6-3-16-4-7-21(13-18(16)12-20)38(33,34)29-22-9-10-30(26(22)32)14-19-11-17(25(27)28)5-8-23(19)37-15-24(31)36-2/h3-8,11-13,22,29H,9-10,14-15H2,1-2H3,(H3,27,28)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 54: 2944-51 (2011)
Article DOI: 10.1021/jm200026b
BindingDB Entry DOI: 10.7270/Q2ZG6TGM |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50505321
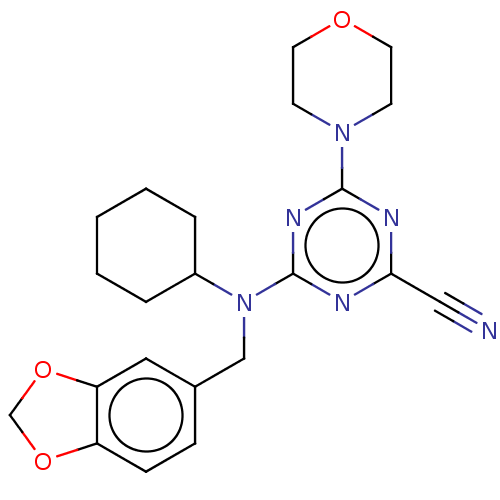
(CHEMBL2402091)Show SMILES N#Cc1nc(nc(n1)N1CCOCC1)N(Cc1ccc2OCOc2c1)C1CCCCC1 Show InChI InChI=1S/C22H26N6O3/c23-13-20-24-21(27-8-10-29-11-9-27)26-22(25-20)28(17-4-2-1-3-5-17)14-16-6-7-18-19(12-16)31-15-30-18/h6-7,12,17H,1-5,8-11,14-15H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain |
J Med Chem 62: 10497-10525 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00683
BindingDB Entry DOI: 10.7270/Q2K93BT1 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13668
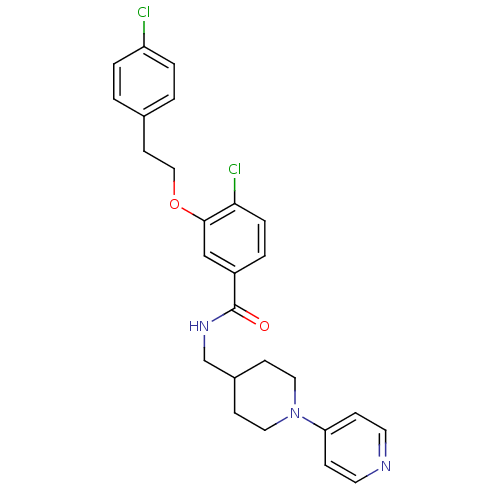
(3-Oxybenzamide 54 | 4-chloro-3-[2-(4-chlorophenyl)...)Show SMILES Clc1ccc(CCOc2cc(ccc2Cl)C(=O)NCC2CCN(CC2)c2ccncc2)cc1 Show InChI InChI=1S/C26H27Cl2N3O2/c27-22-4-1-19(2-5-22)11-16-33-25-17-21(3-6-24(25)28)26(32)30-18-20-9-14-31(15-10-20)23-7-12-29-13-8-23/h1-8,12-13,17,20H,9-11,14-16,18H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 54: 2944-51 (2011)
Article DOI: 10.1021/jm200026b
BindingDB Entry DOI: 10.7270/Q2ZG6TGM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50560496
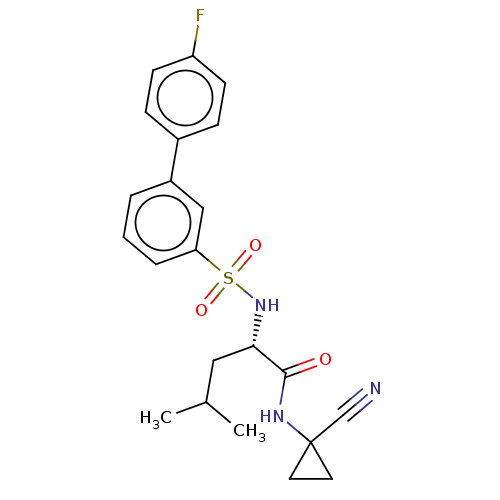
(CHEMBL4790787)Show SMILES CC(C)C[C@H](NS(=O)(=O)c1cccc(c1)-c1ccc(F)cc1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human cathepsin S expressed in insect cells using Z-Phe-Arg-AMC as substrate measured after 60 mins by fluorimetric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127420
BindingDB Entry DOI: 10.7270/Q2S75M1Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50163460

(2-Amino-6-(2-hydroxy-ethylsulfanyl)-4-(3-trifluoro...)Show SMILES Nc1nc(SCCO)c(C#N)c(-c2cccc(c2)C(F)(F)F)c1C#N Show InChI InChI=1S/C16H11F3N4OS/c17-16(18,19)10-3-1-2-9(6-10)13-11(7-20)14(22)23-15(12(13)8-21)25-5-4-24/h1-3,6,24H,4-5H2,(H2,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A1 receptor |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13615
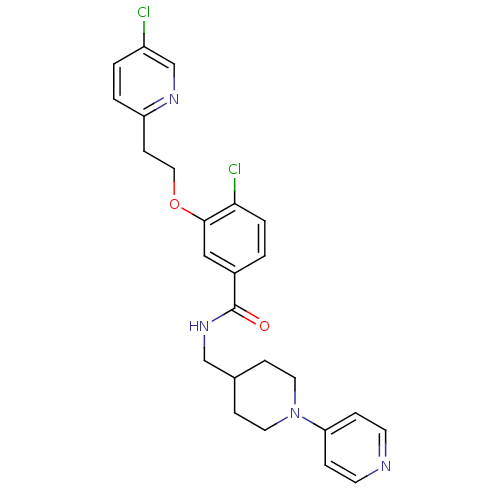
(3-Oxybenzamide 1 | 4-chloro-3-[2-(5-chloropyridin-...)Show SMILES Clc1ccc(CCOc2cc(ccc2Cl)C(=O)NCC2CCN(CC2)c2ccncc2)nc1 Show InChI InChI=1S/C25H26Cl2N4O2/c26-20-2-3-21(29-17-20)9-14-33-24-15-19(1-4-23(24)27)25(32)30-16-18-7-12-31(13-8-18)22-5-10-28-11-6-22/h1-6,10-11,15,17-18H,7-9,12-14,16H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 54: 2944-51 (2011)
Article DOI: 10.1021/jm200026b
BindingDB Entry DOI: 10.7270/Q2ZG6TGM |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50560498

(CHEMBL4763197)Show SMILES CC(C)C[C@H](NS(=O)(=O)c1cccc(c1)-c1ccccc1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human cathepsin K expressed in insect cells using Z-Leu-Arg-AMC as substrate measured after 60 mins by fluorimetric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127420
BindingDB Entry DOI: 10.7270/Q2S75M1Z |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50560495

(CHEMBL4797550)Show SMILES CC(C)C[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccccc1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human cathepsin K expressed in insect cells using Z-Leu-Arg-AMC as substrate measured after 60 mins by fluorimetric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127420
BindingDB Entry DOI: 10.7270/Q2S75M1Z |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50560487
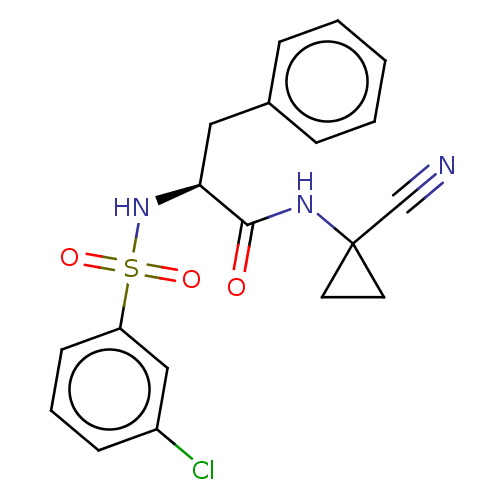
(CHEMBL4760449)Show SMILES Clc1cccc(c1)S(=O)(=O)N[C@@H](Cc1ccccc1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human cathepsin S expressed in insect cells using Z-Phe-Arg-AMC as substrate measured after 60 mins by fluorimetric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127420
BindingDB Entry DOI: 10.7270/Q2S75M1Z |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50560502

(CHEMBL4743577)Show SMILES O=C(NC1(CC1)C#N)[C@H](Cc1ccccc1)NS(=O)(=O)c1cccc(c1)-c1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human cathepsin S expressed in insect cells using Z-Phe-Arg-AMC as substrate measured after 60 mins by fluorimetric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127420
BindingDB Entry DOI: 10.7270/Q2S75M1Z |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50560485

(CHEMBL4779451)Show SMILES Fc1ccc(cc1)-c1cccc(c1)S(=O)(=O)N[C@@H](Cc1ccccc1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human cathepsin S expressed in insect cells using Z-Phe-Arg-AMC as substrate measured after 60 mins by fluorimetric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127420
BindingDB Entry DOI: 10.7270/Q2S75M1Z |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50560489

(CHEMBL4799671)Show SMILES CC(C)C[C@H](NS(=O)(=O)c1cccc(Cl)c1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human cathepsin S expressed in insect cells using Z-Phe-Arg-AMC as substrate measured after 60 mins by fluorimetric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127420
BindingDB Entry DOI: 10.7270/Q2S75M1Z |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50505321

(CHEMBL2402091)Show SMILES N#Cc1nc(nc(n1)N1CCOCC1)N(Cc1ccc2OCOc2c1)C1CCCCC1 Show InChI InChI=1S/C22H26N6O3/c23-13-20-24-21(27-8-10-29-11-9-27)26-22(25-20)28(17-4-2-1-3-5-17)14-16-6-7-18-19(12-16)31-15-30-18/h6-7,12,17H,1-5,8-11,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 62: 10497-10525 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00683
BindingDB Entry DOI: 10.7270/Q2K93BT1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50560492

(CHEMBL4761912)Show SMILES CC(C)C[C@H](NS(=O)(=O)c1cccc(Cl)c1)C(=O)N[C@@H](Cc1ccccc1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human cathepsin K expressed in insect cells using Z-Leu-Arg-AMC as substrate measured after 60 mins by fluorimetric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127420
BindingDB Entry DOI: 10.7270/Q2S75M1Z |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50560501
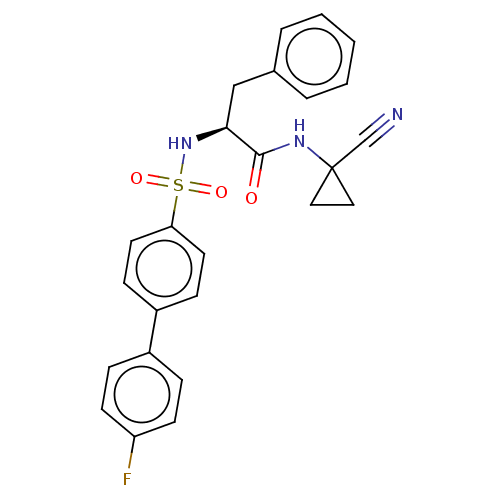
(CHEMBL4788237)Show SMILES Fc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](Cc1ccccc1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human cathepsin S expressed in insect cells using Z-Phe-Arg-AMC as substrate measured after 60 mins by fluorimetric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127420
BindingDB Entry DOI: 10.7270/Q2S75M1Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13667
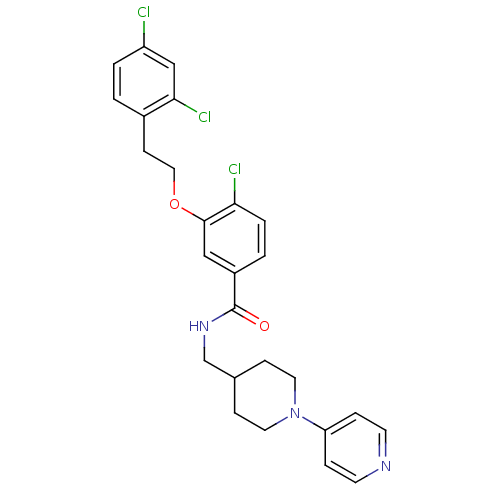
(3-Oxybenzamide 53 | 4-chloro-3-[2-(2,4-dichlorophe...)Show SMILES Clc1ccc(CCOc2cc(ccc2Cl)C(=O)NCC2CCN(CC2)c2ccncc2)c(Cl)c1 Show InChI InChI=1S/C26H26Cl3N3O2/c27-21-3-1-19(24(29)16-21)9-14-34-25-15-20(2-4-23(25)28)26(33)31-17-18-7-12-32(13-8-18)22-5-10-30-11-6-22/h1-6,10-11,15-16,18H,7-9,12-14,17H2,(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 54: 2944-51 (2011)
Article DOI: 10.1021/jm200026b
BindingDB Entry DOI: 10.7270/Q2ZG6TGM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50163452
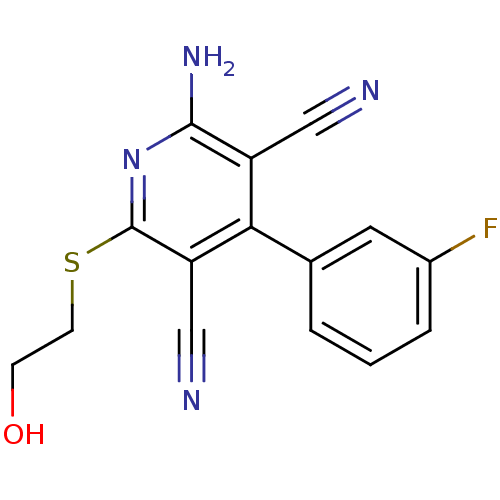
(2-Amino-4-(3-fluoro-phenyl)-6-(2-hydroxy-ethylsulf...)Show InChI InChI=1S/C15H11FN4OS/c16-10-3-1-2-9(6-10)13-11(7-17)14(19)20-15(12(13)8-18)22-5-4-21/h1-3,6,21H,4-5H2,(H2,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A1 receptor |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13618
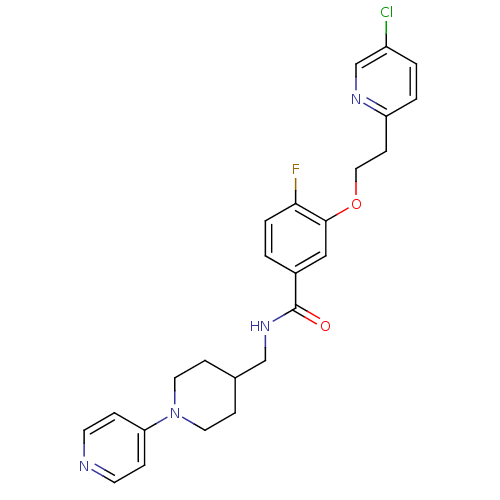
(3-Oxybenzamide 4 | 3-[2-(5-chloropyridin-2-yl)etho...)Show SMILES Fc1ccc(cc1OCCc1ccc(Cl)cn1)C(=O)NCC1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C25H26ClFN4O2/c26-20-2-3-21(29-17-20)9-14-33-24-15-19(1-4-23(24)27)25(32)30-16-18-7-12-31(13-8-18)22-5-10-28-11-6-22/h1-6,10-11,15,17-18H,7-9,12-14,16H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 54: 2944-51 (2011)
Article DOI: 10.1021/jm200026b
BindingDB Entry DOI: 10.7270/Q2ZG6TGM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13619
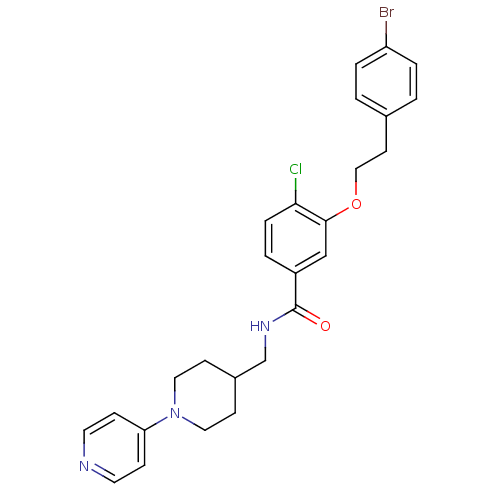
(3-Oxybenzamide 5 | 3-[2-(4-bromophenyl)ethoxy]-4-c...)Show SMILES Clc1ccc(cc1OCCc1ccc(Br)cc1)C(=O)NCC1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C26H27BrClN3O2/c27-22-4-1-19(2-5-22)11-16-33-25-17-21(3-6-24(25)28)26(32)30-18-20-9-14-31(15-10-20)23-7-12-29-13-8-23/h1-8,12-13,17,20H,9-11,14-16,18H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 54: 2944-51 (2011)
Article DOI: 10.1021/jm200026b
BindingDB Entry DOI: 10.7270/Q2ZG6TGM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13617
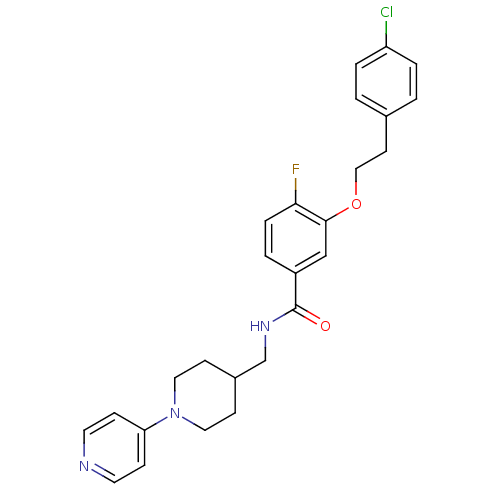
(3-Oxybenzamide 3 | 3-[2-(4-chlorophenyl)ethoxy]-4-...)Show SMILES Fc1ccc(cc1OCCc1ccc(Cl)cc1)C(=O)NCC1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C26H27ClFN3O2/c27-22-4-1-19(2-5-22)11-16-33-25-17-21(3-6-24(25)28)26(32)30-18-20-9-14-31(15-10-20)23-7-12-29-13-8-23/h1-8,12-13,17,20H,9-11,14-16,18H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 54: 2944-51 (2011)
Article DOI: 10.1021/jm200026b
BindingDB Entry DOI: 10.7270/Q2ZG6TGM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50163453
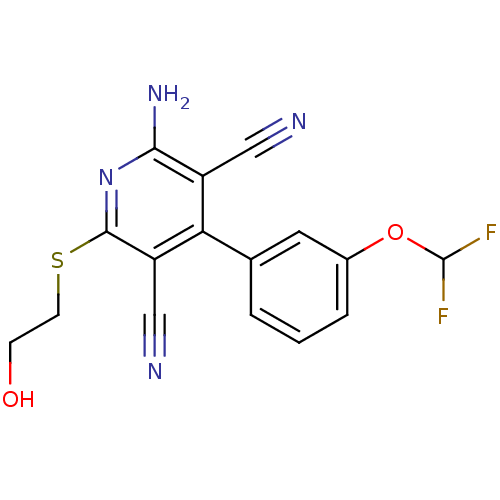
(2-Amino-4-(3-difluoromethoxy-phenyl)-6-(2-hydroxy-...)Show InChI InChI=1S/C16H12F2N4O2S/c17-16(18)24-10-3-1-2-9(6-10)13-11(7-19)14(21)22-15(12(13)8-20)25-5-4-23/h1-3,6,16,23H,4-5H2,(H2,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A1 receptor |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50560494
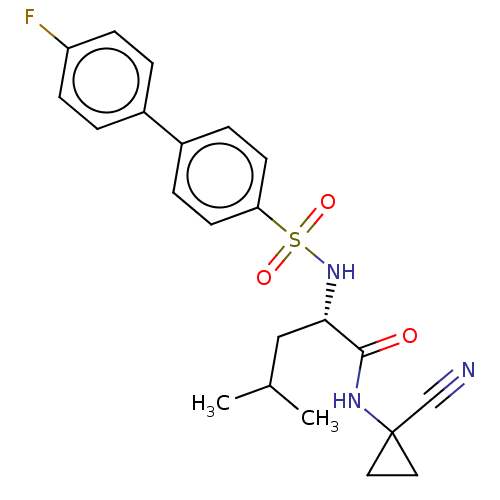
(CHEMBL4748529)Show SMILES CC(C)C[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(F)cc1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human cathepsin K expressed in insect cells using Z-Leu-Arg-AMC as substrate measured after 60 mins by fluorimetric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127420
BindingDB Entry DOI: 10.7270/Q2S75M1Z |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50560492

(CHEMBL4761912)Show SMILES CC(C)C[C@H](NS(=O)(=O)c1cccc(Cl)c1)C(=O)N[C@@H](Cc1ccccc1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human cathepsin S expressed in insect cells using Z-Phe-Arg-AMC as substrate measured after 60 mins by fluorimetric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127420
BindingDB Entry DOI: 10.7270/Q2S75M1Z |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50560491

(CHEMBL4788083)Show SMILES CC(C)C[C@H](NS(=O)(=O)c1cccc(c1)-c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human cathepsin K expressed in insect cells using Z-Leu-Arg-AMC as substrate measured after 60 mins by fluorimetric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127420
BindingDB Entry DOI: 10.7270/Q2S75M1Z |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50560496

(CHEMBL4790787)Show SMILES CC(C)C[C@H](NS(=O)(=O)c1cccc(c1)-c1ccc(F)cc1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human cathepsin K expressed in insect cells using Z-Leu-Arg-AMC as substrate measured after 60 mins by fluorimetric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127420
BindingDB Entry DOI: 10.7270/Q2S75M1Z |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Rattus norvegicus (Rat)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 45.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat ecto-5'-nucleotidase expressed in Sf9 cells by capillary electrophoresis method |
J Med Chem 53: 2076-86 (2010)
Article DOI: 10.1021/jm901851t
BindingDB Entry DOI: 10.7270/Q2DZ097V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081006
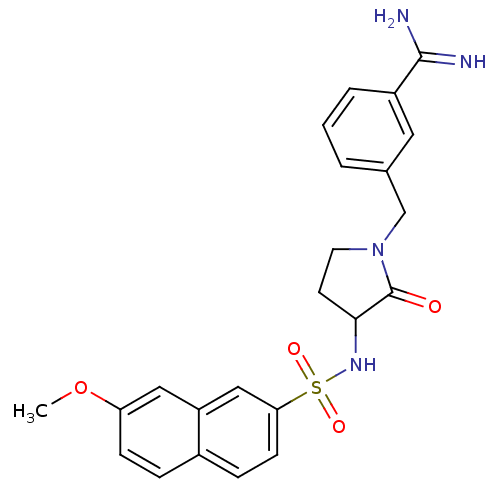
(3-[3-((S)-7-Methoxy-naphthalene-2-sulfonylamino)-2...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)NC1CCN(Cc2cccc(c2)C(N)=N)C1=O Show InChI InChI=1S/C23H24N4O4S/c1-31-19-7-5-16-6-8-20(13-18(16)12-19)32(29,30)26-21-9-10-27(23(21)28)14-15-3-2-4-17(11-15)22(24)25/h2-8,11-13,21,26H,9-10,14H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 54: 2944-51 (2011)
Article DOI: 10.1021/jm200026b
BindingDB Entry DOI: 10.7270/Q2ZG6TGM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13616
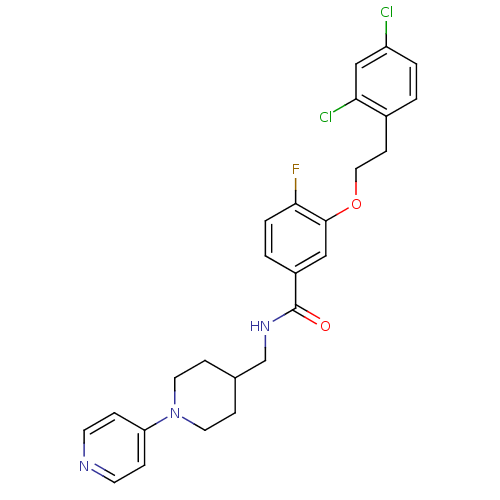
(3-Oxybenzamide 2 | 3-[2-(2,4-dichlorophenyl)ethoxy...)Show SMILES Fc1ccc(cc1OCCc1ccc(Cl)cc1Cl)C(=O)NCC1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C26H26Cl2FN3O2/c27-21-3-1-19(23(28)16-21)9-14-34-25-15-20(2-4-24(25)29)26(33)31-17-18-7-12-32(13-8-18)22-5-10-30-11-6-22/h1-6,10-11,15-16,18H,7-9,12-14,17H2,(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 54: 2944-51 (2011)
Article DOI: 10.1021/jm200026b
BindingDB Entry DOI: 10.7270/Q2ZG6TGM |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50560489

(CHEMBL4799671)Show SMILES CC(C)C[C@H](NS(=O)(=O)c1cccc(Cl)c1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human cathepsin K expressed in insect cells using Z-Leu-Arg-AMC as substrate measured after 60 mins by fluorimetric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127420
BindingDB Entry DOI: 10.7270/Q2S75M1Z |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50324475

(Benzylsulfonyl-D-argininyl-proline-(4-amidinobenzy...)Show SMILES NC(=N)NCCC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C26H36N8O4S/c27-23(28)20-12-10-18(11-13-20)16-32-24(35)22-9-5-15-34(22)25(36)21(8-4-14-31-26(29)30)33-39(37,38)17-19-6-2-1-3-7-19/h1-3,6-7,10-13,21-22,33H,4-5,8-9,14-17H2,(H3,27,28)(H,32,35)(H4,29,30,31)/t21-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matriptase catalytic domain expressed in HEK293 cells after 20 mins |
J Med Chem 53: 5523-35 (2010)
Article DOI: 10.1021/jm100183e
BindingDB Entry DOI: 10.7270/Q25B02PD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data