

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Enterobacter cloacae) | BDBM50339869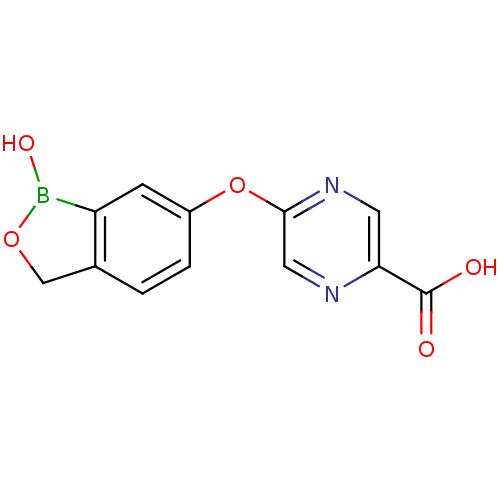 (5-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339868 (4-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339870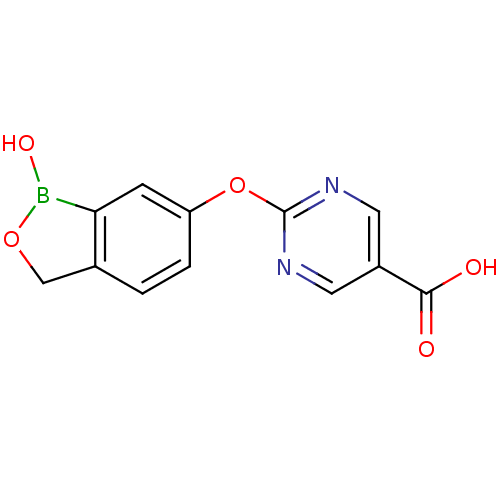 (2-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM25028 (4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 580 | -35.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Piramed Pharma | Assay Description Mammalian target of rapamycin (mTOR) was assayed by monitoring phosphorylation of GFP-4EBP using a homogeneous time-resolved fluorescence resonance e... | J Med Chem 51: 5522-32 (2008) Article DOI: 10.1021/jm800295d BindingDB Entry DOI: 10.7270/Q2222S23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339846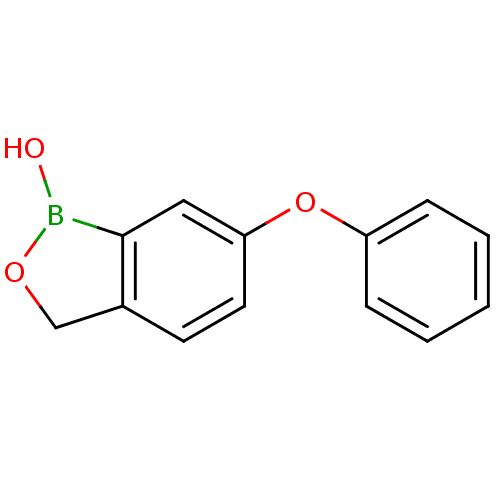 (6-phenoxybenzo[c][1,2]oxaborol-1(3H)-ol | CHEMBL17...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339856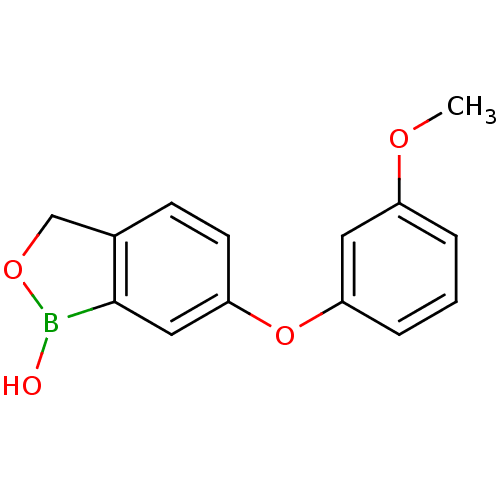 (6-(3-methoxyphenoxy)benzo[c][1,2]oxaborol-1(3H)-ol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339862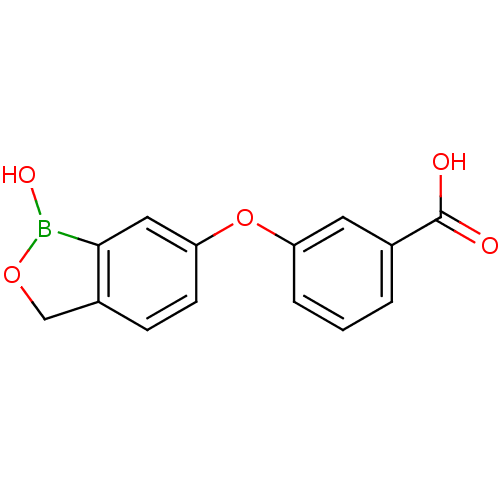 (3-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Saccharomyces cerevisiae S288c) | BDBM50370987 (TAVABOROLE) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase after 20 mins | Science 316: 1759-1761 (2007) Article DOI: 10.1126/science.1142189 BindingDB Entry DOI: 10.7270/Q2P84CQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339860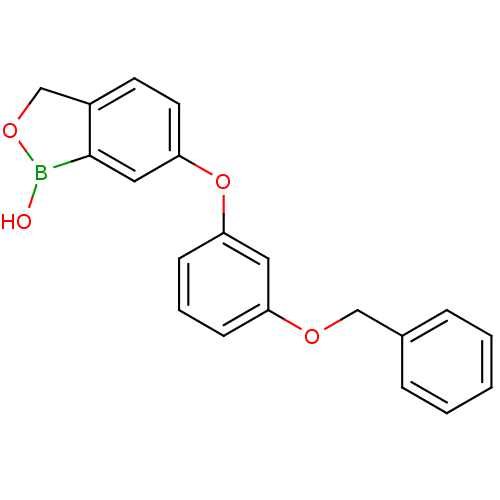 (6-(3-(benzyloxy)phenoxy)benzo[c][1,2]oxaborol-1(3H...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339857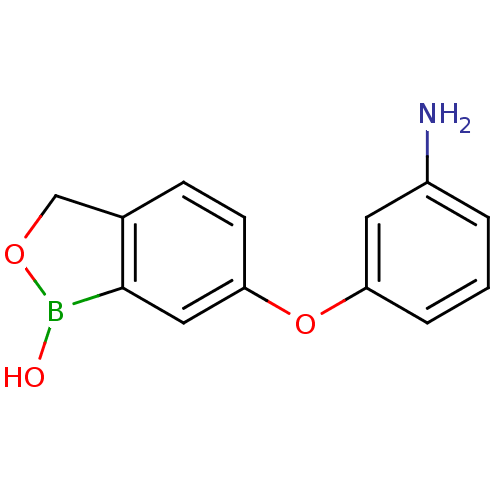 (6-(3-aminophenoxy)benzo[c][1,2]oxaborol-1(3H)-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339859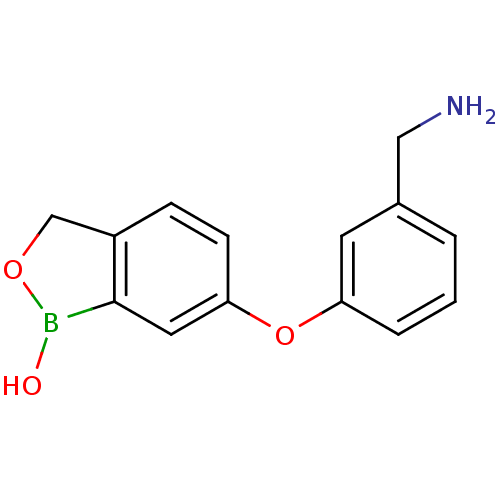 (6-(3-(aminomethyl)phenoxy)benzo[c][1,2]oxaborol-1(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339855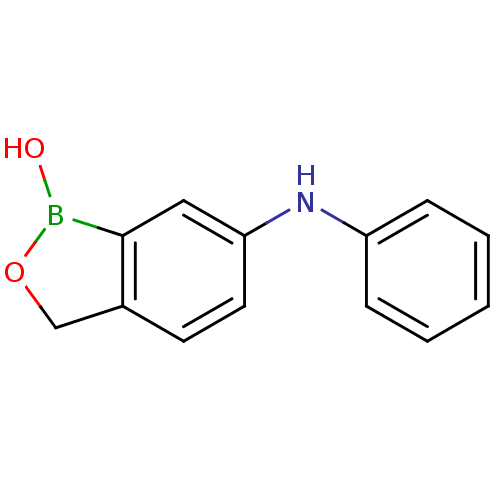 (6-(phenylamino)benzo[c][1,2]oxaborol-1(3H)-ol | CH...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339867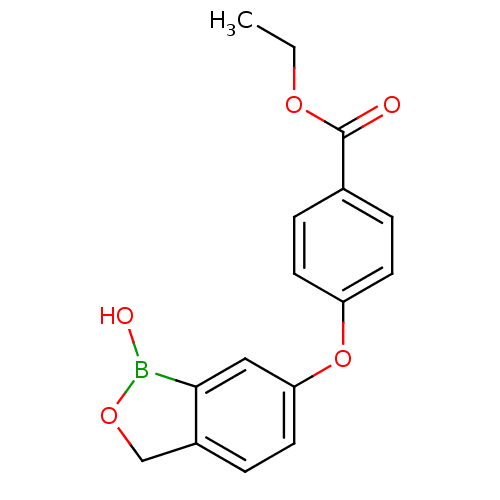 (CHEMBL1761273 | ethyl 4-(1-hydroxy-1,3-dihydrobenz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339863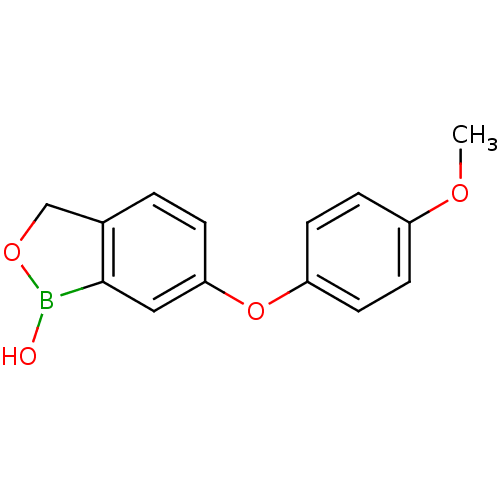 (6-(4-methoxyphenoxy)benzo[c][1,2]oxaborol-1(3H)-ol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339850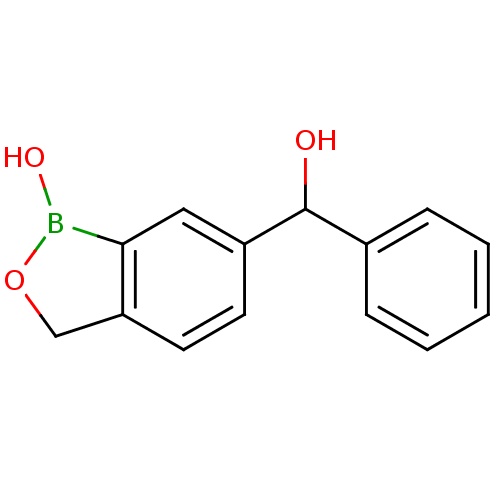 (6-(hydroxy(phenyl)methyl)benzo[c][1,2]oxaborol-1(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339861 (6-(3-((dimethylamino)methyl)phenoxy)benzo[c][1,2]o...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339858 (6-(3-(hydroxymethyl)phenoxy)benzo[c][1,2]oxaborol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339864 (6-(4-aminophenoxy)benzo[c][1,2]oxaborol-1(3H)-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339847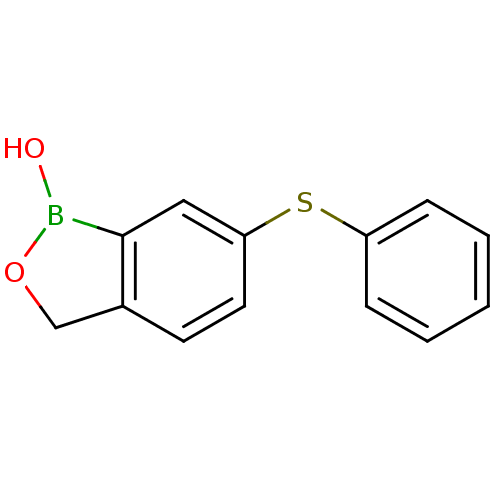 (6-(phenylthio)benzo[c][1,2]oxaborol-1(3H)-ol | CHE...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339865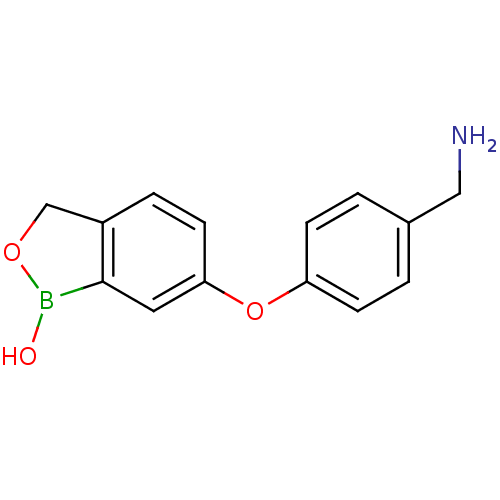 (6-(4-(aminomethyl)phenoxy)benzo[c][1,2]oxaborol-1(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339854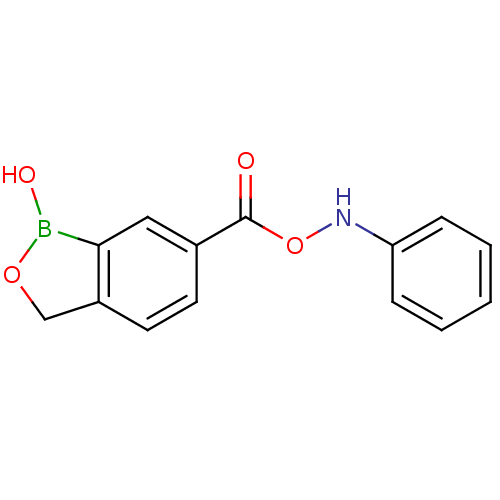 (6-((phenylaminooxy)carbonyl)benzo[c][1,2]oxaborol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50089572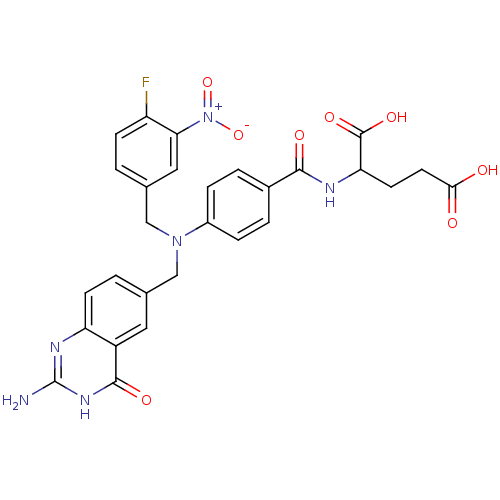 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Aminoimidazole carboxamide ribonucleotide transformylase | Bioorg Med Chem Lett 10: 1471-5 (2000) BindingDB Entry DOI: 10.7270/Q2GM86HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339848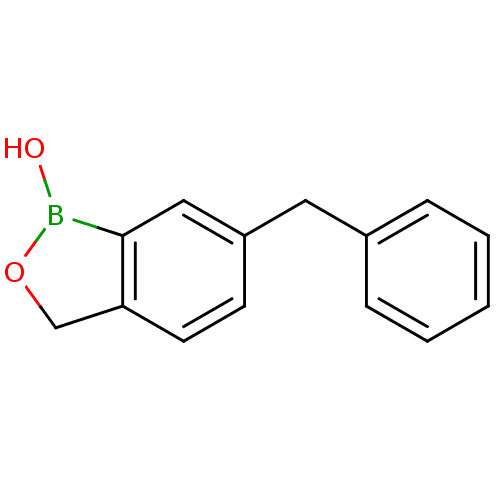 (6-benzylbenzo[c][1,2]oxaborol-1(3H)-ol | CHEMBL176...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339849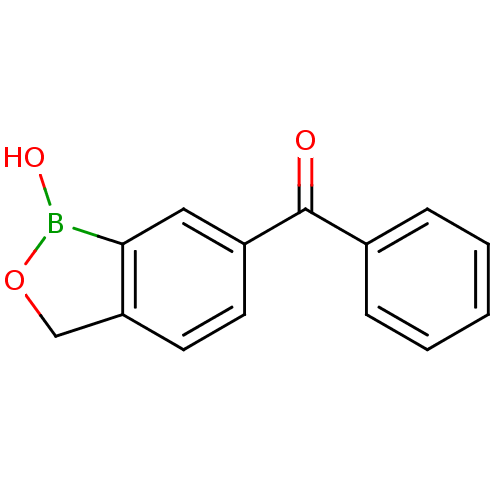 ((1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Saccharomyces cerevisiae S288c) | BDBM50370987 (TAVABOROLE) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 3.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase after 2 mins | Science 316: 1759-1761 (2007) Article DOI: 10.1126/science.1142189 BindingDB Entry DOI: 10.7270/Q2P84CQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50089585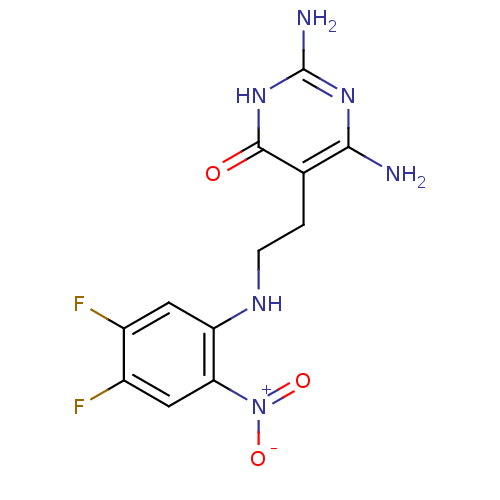 (2,6-Diamino-5-[2-(4,5-difluoro-2-nitro-phenylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Glycinamide ribonucleotide transformylase (GAR Tfase) after 3 min at 250 uM | Bioorg Med Chem Lett 10: 1471-5 (2000) BindingDB Entry DOI: 10.7270/Q2GM86HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339866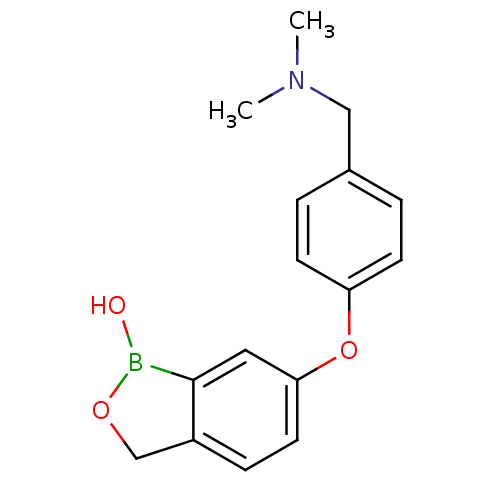 (6-(4-((dimethylamino)methyl)phenoxy)benzo[c][1,2]o...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50089594 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Glycinamide ribonucleotide transformylase (GAR Tfase) | Bioorg Med Chem Lett 10: 1471-5 (2000) BindingDB Entry DOI: 10.7270/Q2GM86HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339853 (CHEMBL1761258 | N-(1-hydroxy-1,3-dihydrobenzo[c][1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 4.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50089594 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Aminoimidazole carboxamide ribonucleotide transformylase | Bioorg Med Chem Lett 10: 1471-5 (2000) BindingDB Entry DOI: 10.7270/Q2GM86HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339851 (6-(phenylsulfinyl)benzo[c][1,2]oxaborol-1(3H)-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339852 (6-(phenylsulfonyl)benzo[c][1,2]oxaborol-1(3H)-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50089572 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Glycinamide ribonucleotide transformylase (GAR Tfase) | Bioorg Med Chem Lett 10: 1471-5 (2000) BindingDB Entry DOI: 10.7270/Q2GM86HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101068 (4-Chloro-10,10-dioxo-9,10-dihydro-10lambda*6*-thio...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101051 (4,5-Dichloro-9H-xanthene-9-carboxylic acid [(2R,3R...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101050 (10,10-Dioxo-9,10-dihydro-10lambda*6*-thioxanthene-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101069 (10,10-Bis-trifluoromethyl-9,10-dihydro-anthracene-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101045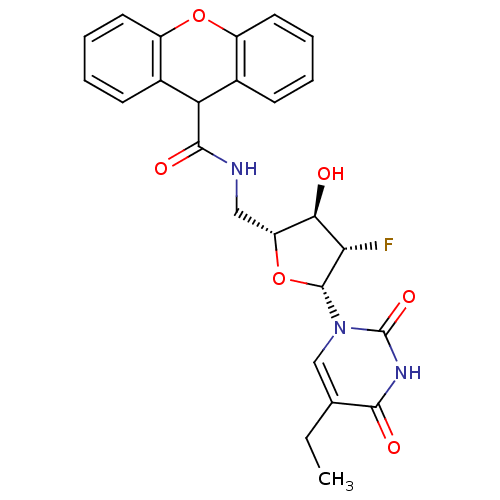 (9H-Xanthene-9-carboxylic acid [(2R,3R,4S,5R)-5-(5-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101071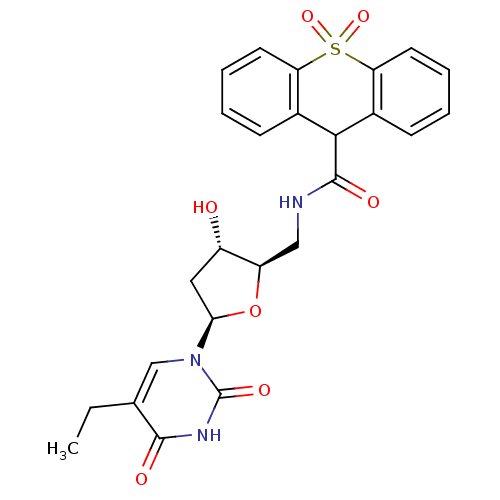 (10,10-Dioxo-9,10-dihydro-10lambda*6*-thioxanthene-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101053 (9H-Xanthene-9-carboxylic acid [(2R,3S,5R)-5-(5-eth...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101058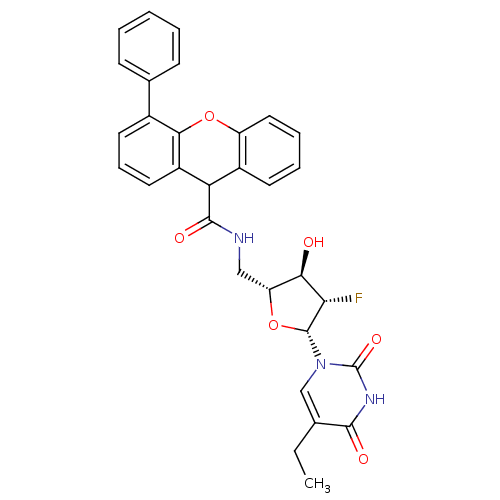 (4-Phenyl-9H-xanthene-9-carboxylic acid [(2R,3R,4S,...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101060 (4-Trifluoromethyl-9H-xanthene-9-carboxylic acid [(...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101048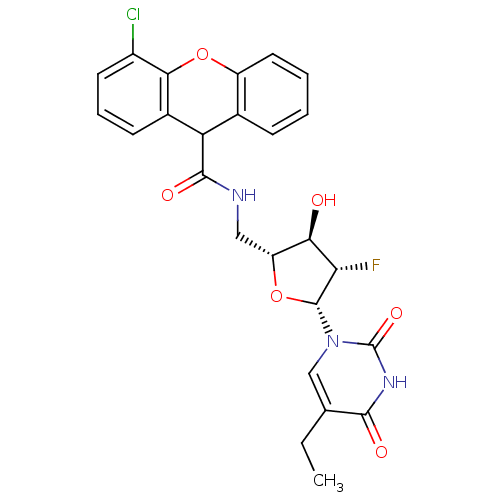 (4-Chloro-9H-xanthene-9-carboxylic acid [(2R,3R,4S,...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101069 (10,10-Bis-trifluoromethyl-9,10-dihydro-anthracene-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101047 (4-Methyl-9H-xanthene-9-carboxylic acid [(2R,3R,4S,...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101067 ((S)-2-(2,4-Dichloro-5-methoxy-phenoxy)-N-[(2R,3R,4...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101074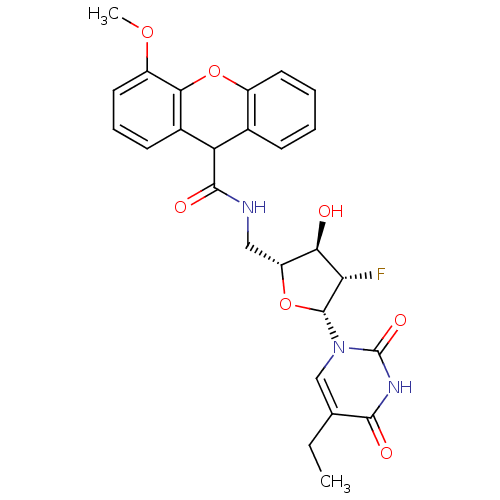 (4-Methoxy-9H-xanthene-9-carboxylic acid [(2R,3R,4S...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101062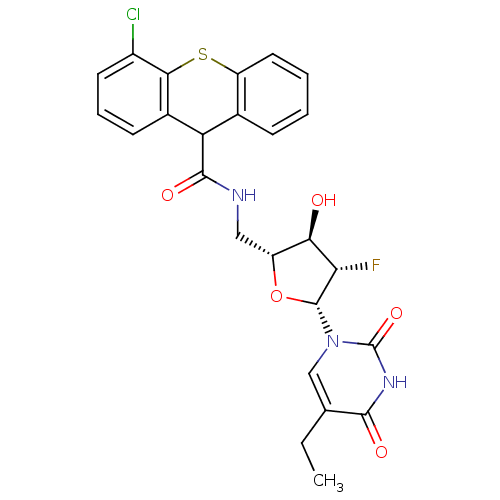 (4-Chloro-9H-thioxanthene-9-carboxylic acid [(2R,3R...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101068 (4-Chloro-10,10-dioxo-9,10-dihydro-10lambda*6*-thio...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101049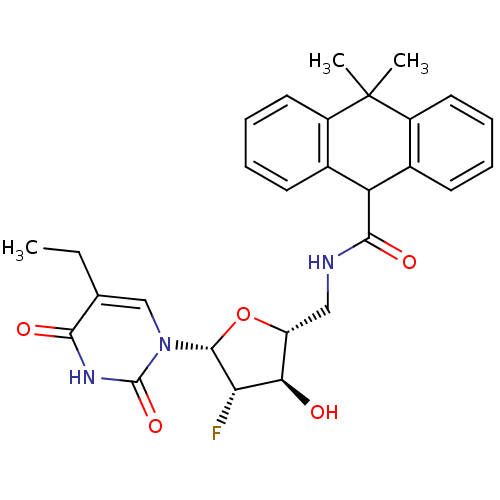 (10,10-Dimethyl-9,10-dihydro-anthracene-9-carboxyli...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 238 total ) | Next | Last >> |