

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pteridine reductase 1 (Leishmania major) | BDBM50551183 (CHEMBL4747846) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551184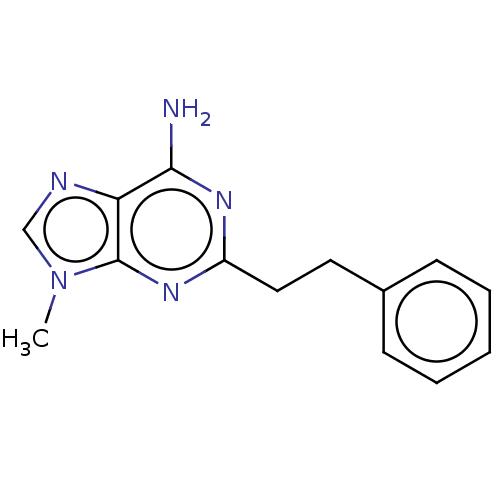 (CHEMBL4797185) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551180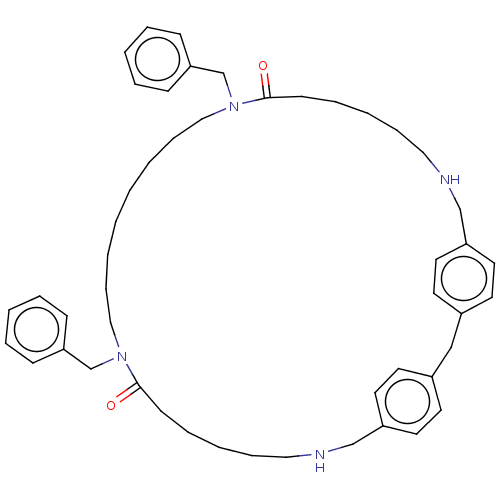 (CHEMBL4760251) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551177 (CHEMBL4762279) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551178 (CHEMBL4785591) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551181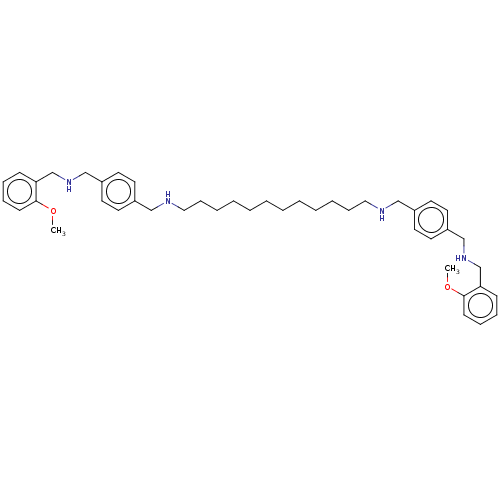 (CHEMBL54725) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551182 (CHEMBL4754292) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551179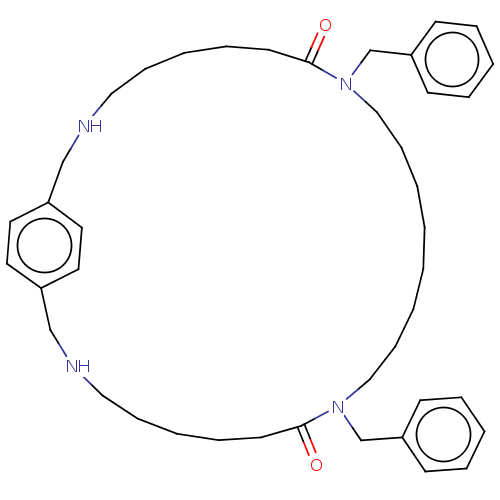 (CHEMBL4778279) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551176 (CHEMBL4748094) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551175 (CHEMBL1993081) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551174 (CHEMBL158919) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551173 (CHEMBL4753331) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50062821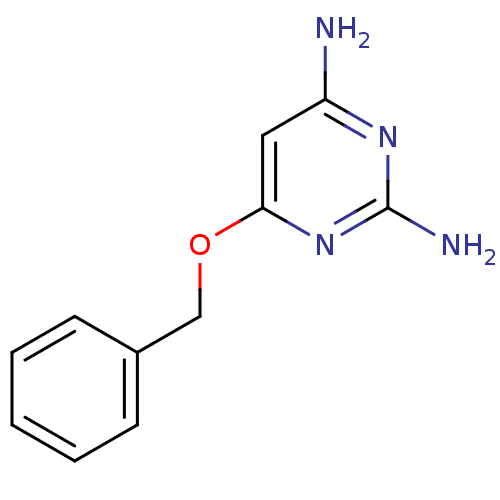 (6-Benzyloxy-pyrimidine-2,4-diamine | CHEMBL121445) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50386758 (CHEMBL2046893) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid ceramidase (Homo sapiens (Human)) | BDBM29080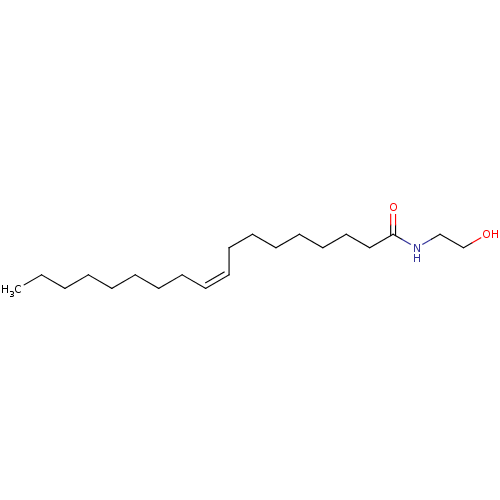 (CHEMBL280065 | N-oleoylethanolamine | Oleamide MEA...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of acid ceramidase (unknown origin) | J Med Chem 56: 3518-30 (2013) Article DOI: 10.1021/jm301879g BindingDB Entry DOI: 10.7270/Q27D2WH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM359728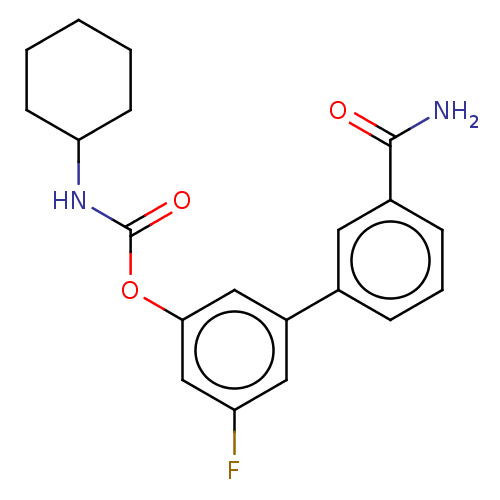 (US10435355, Example 12 | US9822068, 12 | [3-(3-car...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California; Fondazione Istituto Italiano di Technologia US Patent | Assay Description Rat FAAH was prepared from male Sprague Dawley rat brains, homogenized in a potter in 20 mM of Tris HCl pH 7.4, 0.32 M sucrose.The radiometric assay ... | US Patent US9822068 (2017) BindingDB Entry DOI: 10.7270/Q2Q242HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM359728 (US10435355, Example 12 | US9822068, 12 | [3-(3-car...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description Rat FAAH was prepared from male Sprague Dawley rat brains, homogenized in a potter in 20 mM of Tris HCl pH 7.4, 0.32 M sucrose.The radiometric assay ... | US Patent US10435355 (2019) BindingDB Entry DOI: 10.7270/Q29C70TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50437233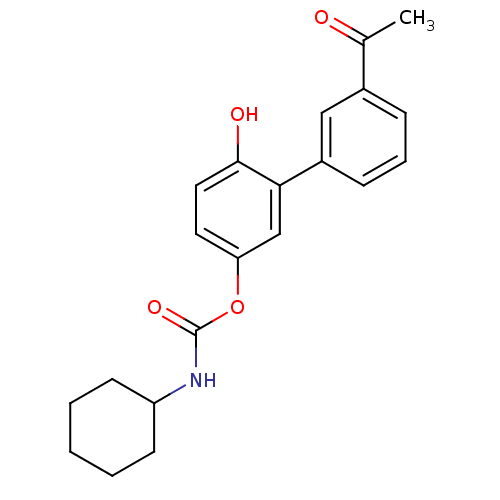 (CHEMBL2402911) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of Wistar rat brain FAAH using [3H]-ethanolamine as substrate preincubated for 20 mins followed by substrate addition by liquid scintillat... | J Med Chem 56: 5917-30 (2014) Article DOI: 10.1021/jm4007017 BindingDB Entry DOI: 10.7270/Q2KP83KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50437232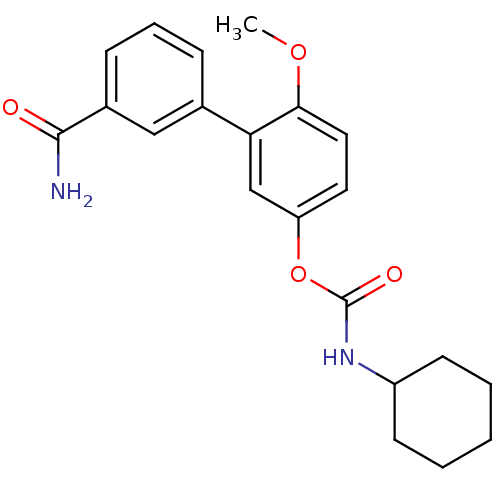 (3‐(3‐carbamoylphenyl)‐4‐me...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of Wistar rat brain FAAH using [3H]-ethanolamine as substrate preincubated for 20 mins followed by substrate addition by liquid scintillat... | J Med Chem 56: 5917-30 (2014) Article DOI: 10.1021/jm4007017 BindingDB Entry DOI: 10.7270/Q2KP83KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50437231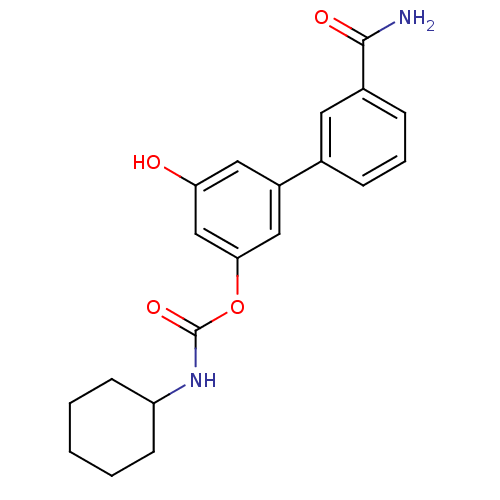 (CHEMBL2402925) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of Wistar rat brain FAAH using [3H]-ethanolamine as substrate preincubated for 20 mins followed by substrate addition by liquid scintillat... | J Med Chem 56: 5917-30 (2014) Article DOI: 10.1021/jm4007017 BindingDB Entry DOI: 10.7270/Q2KP83KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236331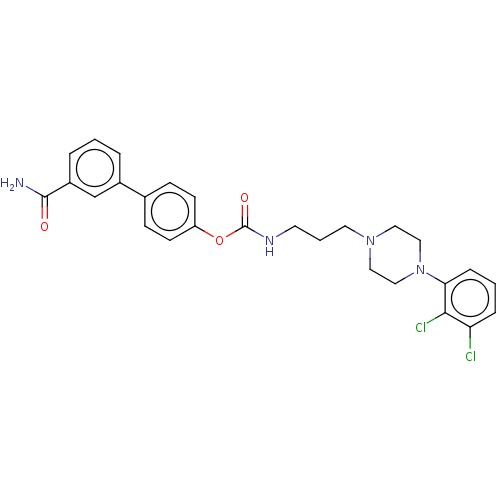 (CHEMBL4091498) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236330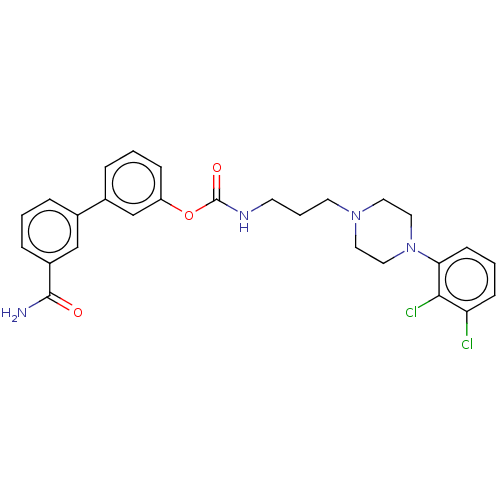 (CHEMBL4070196) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236343 (CHEMBL4086944) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236333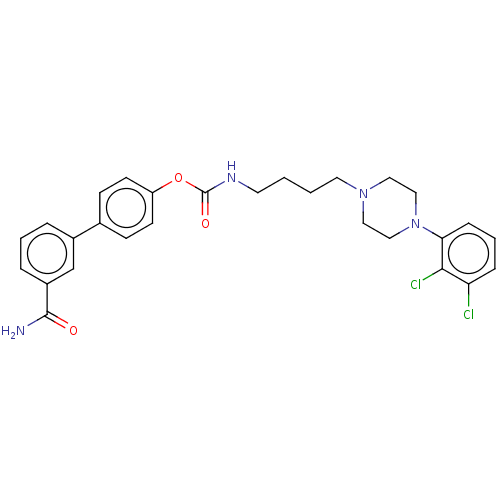 (CHEMBL4092052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM359718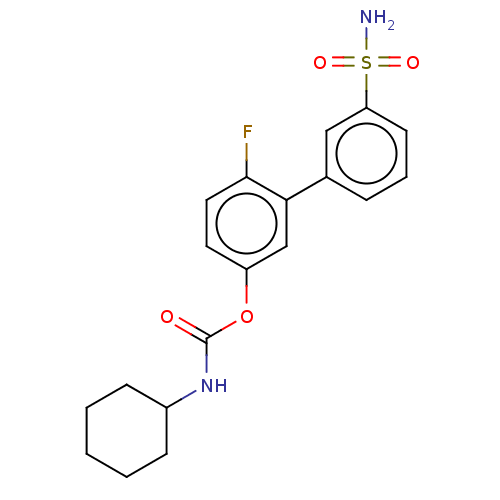 (US10435355, Example 2 | US9822068, 2 | [4-fluoro-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California; Fondazione Istituto Italiano di Technologia US Patent | Assay Description Rat FAAH was prepared from male Sprague Dawley rat brains, homogenized in a potter in 20 mM of Tris HCl pH 7.4, 0.32 M sucrose.The radiometric assay ... | US Patent US9822068 (2017) BindingDB Entry DOI: 10.7270/Q2Q242HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM359718 (US10435355, Example 2 | US9822068, 2 | [4-fluoro-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description Rat FAAH was prepared from male Sprague Dawley rat brains, homogenized in a potter in 20 mM of Tris HCl pH 7.4, 0.32 M sucrose.The radiometric assay ... | US Patent US10435355 (2019) BindingDB Entry DOI: 10.7270/Q29C70TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM359721 (US10435355, Example 5 | US9822068, 5 | [3-(3-carba...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California; Fondazione Istituto Italiano di Technologia US Patent | Assay Description Human recombinant FAAH was obtained from a HEK-293 cell line stably overexpressing human FAAH-1 enzyme. Cells were grown in Dulbecco's Modified E... | US Patent US9822068 (2017) BindingDB Entry DOI: 10.7270/Q2Q242HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM359721 (US10435355, Example 5 | US9822068, 5 | [3-(3-carba...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description Human recombinant FAAH was obtained from a HEK-293 cell line stably overexpressing human FAAH-1 enzyme. Cells were grown in Dulbecco's Modified E... | US Patent US10435355 (2019) BindingDB Entry DOI: 10.7270/Q29C70TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM359728 (US10435355, Example 12 | US9822068, 12 | [3-(3-car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description Human recombinant FAAH was obtained from a HEK-293 cell line stably overexpressing human FAAH-1 enzyme. Cells were grown in Dulbecco's Modified E... | US Patent US10435355 (2019) BindingDB Entry DOI: 10.7270/Q29C70TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM359728 (US10435355, Example 12 | US9822068, 12 | [3-(3-car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California; Fondazione Istituto Italiano di Technologia US Patent | Assay Description Human recombinant FAAH was obtained from a HEK-293 cell line stably overexpressing human FAAH-1 enzyme. Cells were grown in Dulbecco's Modified E... | US Patent US9822068 (2017) BindingDB Entry DOI: 10.7270/Q2Q242HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM359729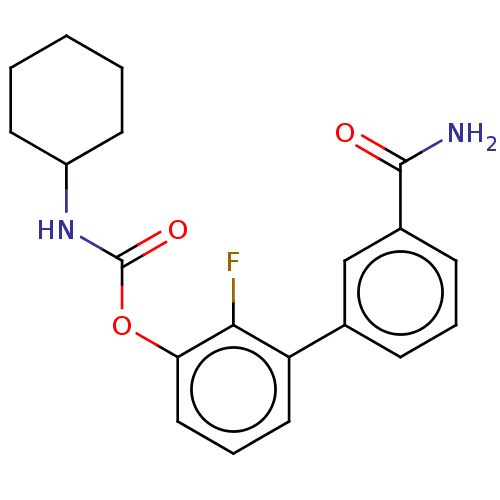 (US10435355, Example 13 | US9822068, 13 | [3-(3-car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description Human recombinant FAAH was obtained from a HEK-293 cell line stably overexpressing human FAAH-1 enzyme. Cells were grown in Dulbecco's Modified E... | US Patent US10435355 (2019) BindingDB Entry DOI: 10.7270/Q29C70TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM359729 (US10435355, Example 13 | US9822068, 13 | [3-(3-car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California; Fondazione Istituto Italiano di Technologia US Patent | Assay Description Human recombinant FAAH was obtained from a HEK-293 cell line stably overexpressing human FAAH-1 enzyme. Cells were grown in Dulbecco's Modified E... | US Patent US9822068 (2017) BindingDB Entry DOI: 10.7270/Q2Q242HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM359719 (US10435355, Example 3 | US9822068, 3 | [3-(3-carba...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California; Fondazione Istituto Italiano di Technologia US Patent | Assay Description Rat FAAH was prepared from male Sprague Dawley rat brains, homogenized in a potter in 20 mM of Tris HCl pH 7.4, 0.32 M sucrose.The radiometric assay ... | US Patent US9822068 (2017) BindingDB Entry DOI: 10.7270/Q2Q242HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM359719 (US10435355, Example 3 | US9822068, 3 | [3-(3-carba...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description Rat FAAH was prepared from male Sprague Dawley rat brains, homogenized in a potter in 20 mM of Tris HCl pH 7.4, 0.32 M sucrose.The radiometric assay ... | US Patent US10435355 (2019) BindingDB Entry DOI: 10.7270/Q29C70TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236345 (CHEMBL4079093) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236340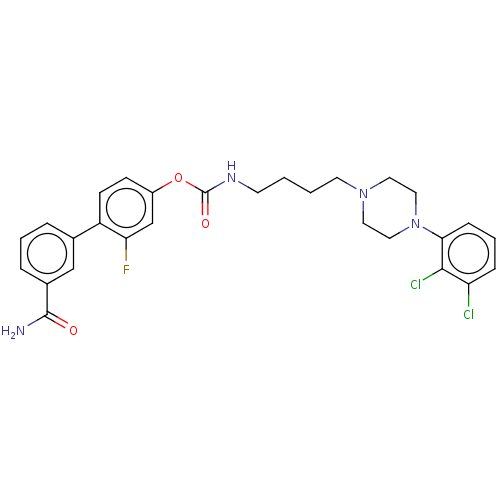 (CHEMBL4069565) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236326 (CHEMBL4073966) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236342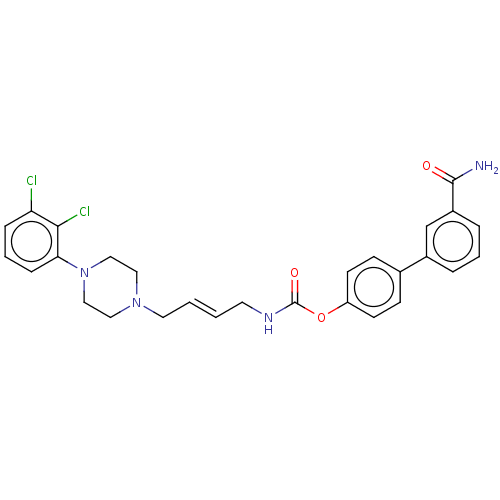 (CHEMBL4065510) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50437230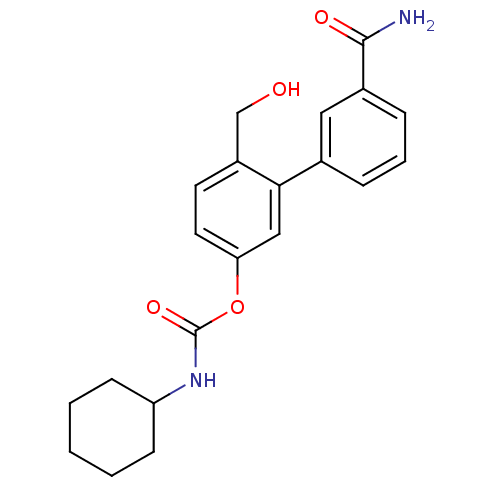 (CHEMBL2402922) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of Wistar rat brain FAAH using [3H]-ethanolamine as substrate preincubated for 20 mins followed by substrate addition by liquid scintillat... | J Med Chem 56: 5917-30 (2014) Article DOI: 10.1021/jm4007017 BindingDB Entry DOI: 10.7270/Q2KP83KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236327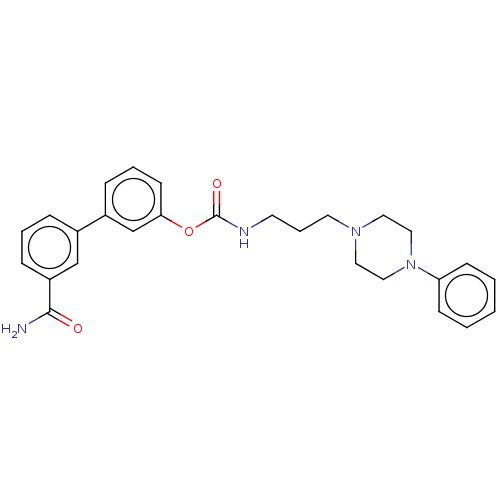 (CHEMBL4100735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Binding affinity against human Alpha-1a adrenergic receptor | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM359724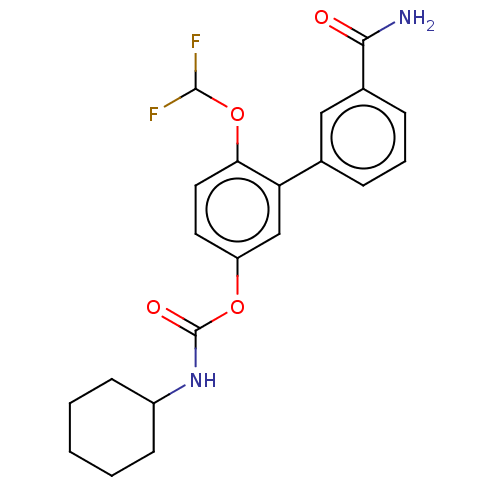 (US10435355, Example 8 | US9822068, 8 | [3-(3-carba...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description Rat FAAH was prepared from male Sprague Dawley rat brains, homogenized in a potter in 20 mM of Tris HCl pH 7.4, 0.32 M sucrose.The radiometric assay ... | US Patent US10435355 (2019) BindingDB Entry DOI: 10.7270/Q29C70TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM359717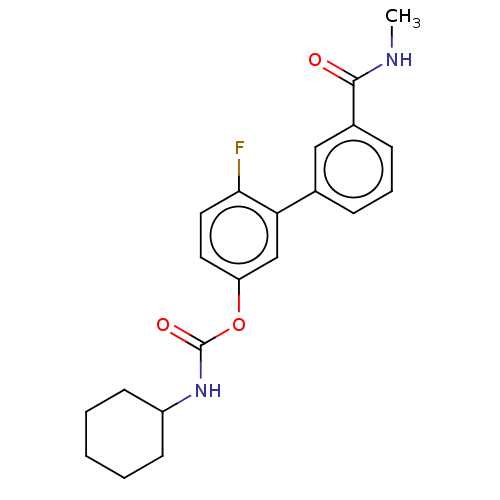 (US10435355, Example 1 | US9822068, 1 | [4-fluoro-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California; Fondazione Istituto Italiano di Technologia US Patent | Assay Description Rat FAAH was prepared from male Sprague Dawley rat brains, homogenized in a potter in 20 mM of Tris HCl pH 7.4, 0.32 M sucrose.The radiometric assay ... | US Patent US9822068 (2017) BindingDB Entry DOI: 10.7270/Q2Q242HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM359724 (US10435355, Example 8 | US9822068, 8 | [3-(3-carba...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California; Fondazione Istituto Italiano di Technologia US Patent | Assay Description Rat FAAH was prepared from male Sprague Dawley rat brains, homogenized in a potter in 20 mM of Tris HCl pH 7.4, 0.32 M sucrose.The radiometric assay ... | US Patent US9822068 (2017) BindingDB Entry DOI: 10.7270/Q2Q242HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM359717 (US10435355, Example 1 | US9822068, 1 | [4-fluoro-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description Rat FAAH was prepared from male Sprague Dawley rat brains, homogenized in a potter in 20 mM of Tris HCl pH 7.4, 0.32 M sucrose.The radiometric assay ... | US Patent US10435355 (2019) BindingDB Entry DOI: 10.7270/Q29C70TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50437229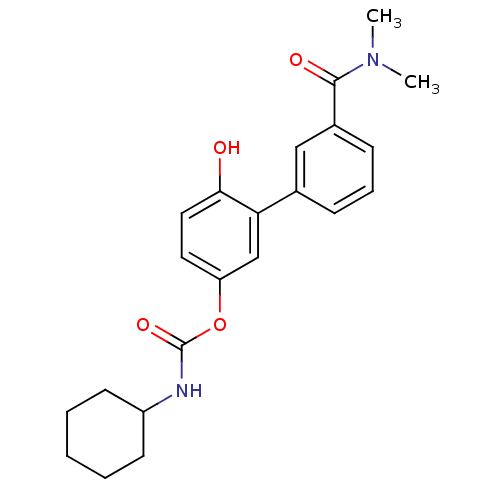 (CHEMBL2402915) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of Wistar rat brain FAAH using [3H]-ethanolamine as substrate preincubated for 20 mins followed by substrate addition by liquid scintillat... | J Med Chem 56: 5917-30 (2014) Article DOI: 10.1021/jm4007017 BindingDB Entry DOI: 10.7270/Q2KP83KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50437228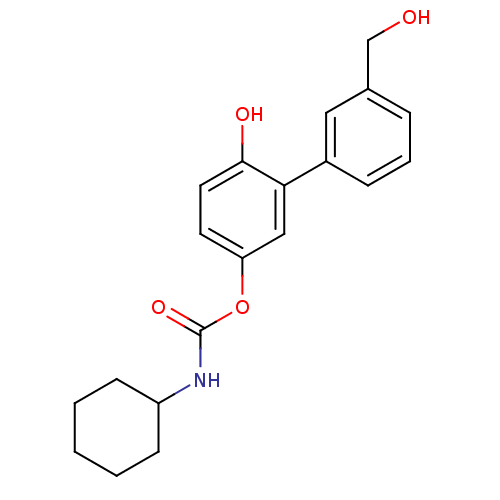 (CHEMBL2402920) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of Wistar rat brain FAAH using [3H]-ethanolamine as substrate preincubated for 20 mins followed by substrate addition by liquid scintillat... | J Med Chem 56: 5917-30 (2014) Article DOI: 10.1021/jm4007017 BindingDB Entry DOI: 10.7270/Q2KP83KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236334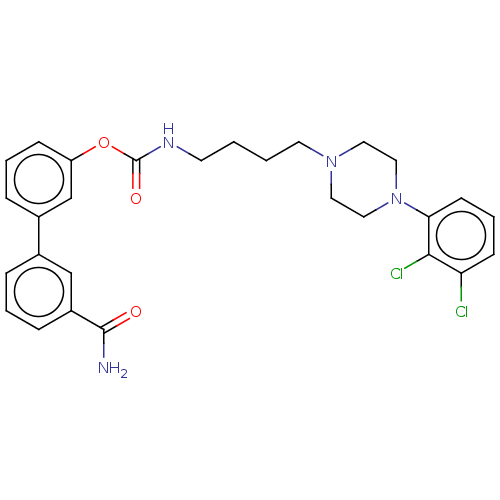 (CHEMBL4071240) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM359719 (US10435355, Example 3 | US9822068, 3 | [3-(3-carba...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California; Fondazione Istituto Italiano di Technologia US Patent | Assay Description Human recombinant FAAH was obtained from a HEK-293 cell line stably overexpressing human FAAH-1 enzyme. Cells were grown in Dulbecco's Modified E... | US Patent US9822068 (2017) BindingDB Entry DOI: 10.7270/Q2Q242HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236329 (CHEMBL4081780) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM359719 (US10435355, Example 3 | US9822068, 3 | [3-(3-carba...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description Human recombinant FAAH was obtained from a HEK-293 cell line stably overexpressing human FAAH-1 enzyme. Cells were grown in Dulbecco's Modified E... | US Patent US10435355 (2019) BindingDB Entry DOI: 10.7270/Q29C70TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 780 total ) | Next | Last >> |