

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439646 (CHEMBL2419600 | US8993586, 110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013412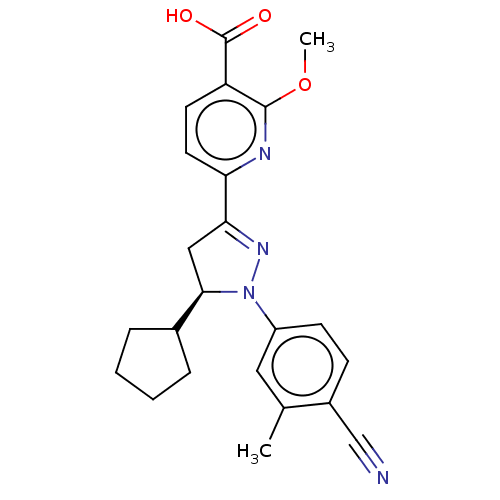 (CHEMBL3263752) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to mineralocorticoid receptor (unknown origin) | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439646 (CHEMBL2419600 | US8993586, 110) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013430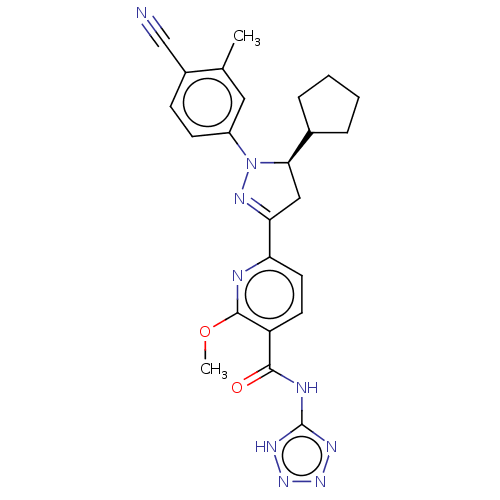 (CHEMBL3263768) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439642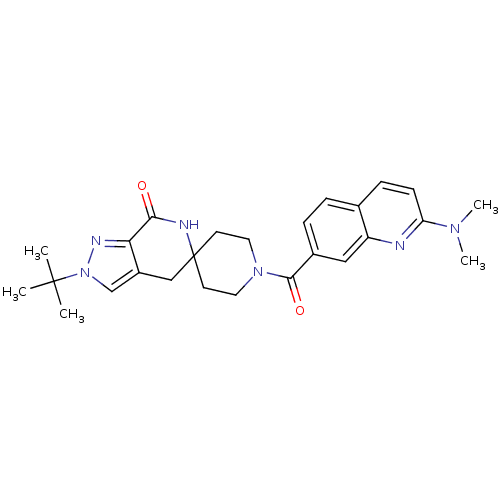 (CHEMBL2419589 | US8993586, 105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013422 (CHEMBL3263760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013428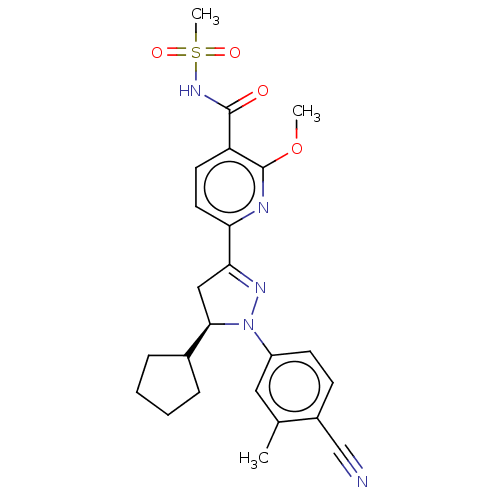 (CHEMBL3263766) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439644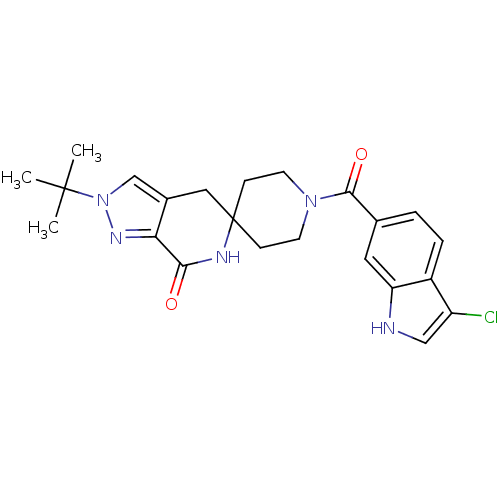 (CHEMBL2419593 | US8993586, 86) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013426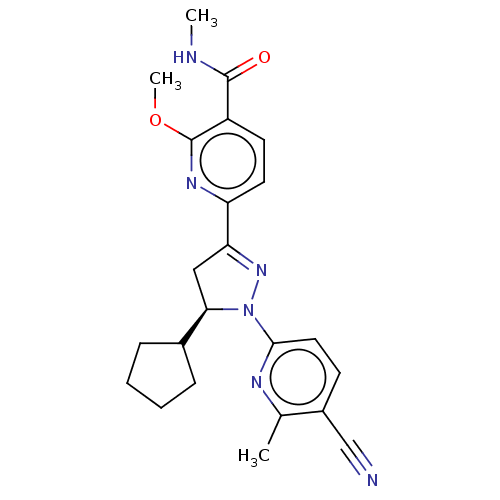 (CHEMBL3263764) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013424 (CHEMBL3263762) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439634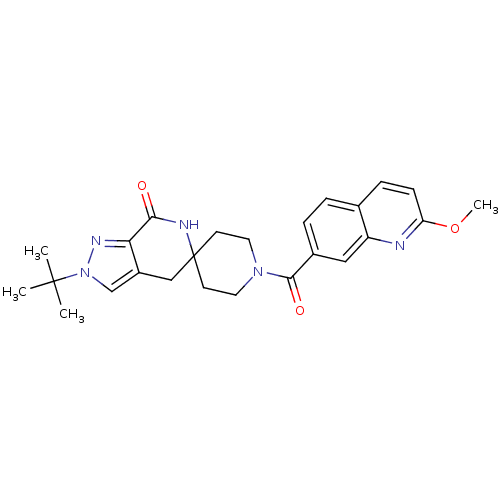 (CHEMBL2419596 | US8993586, 71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013429 (CHEMBL3263767) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439641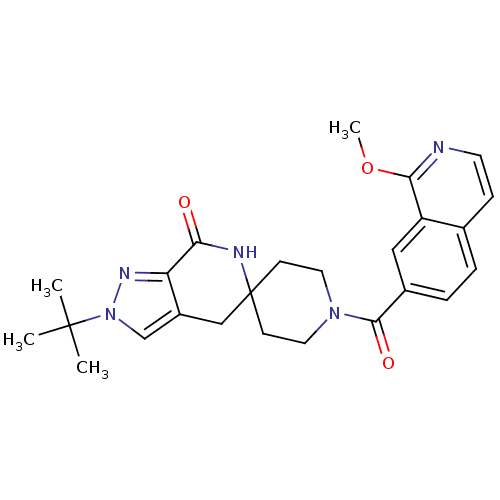 (CHEMBL2419597 | US8993586, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439643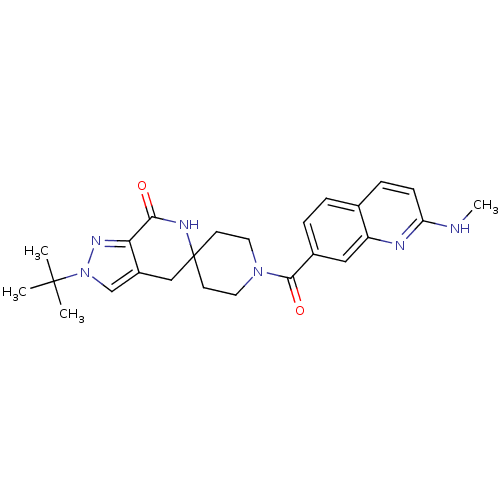 (CHEMBL2419598 | US8993586, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013425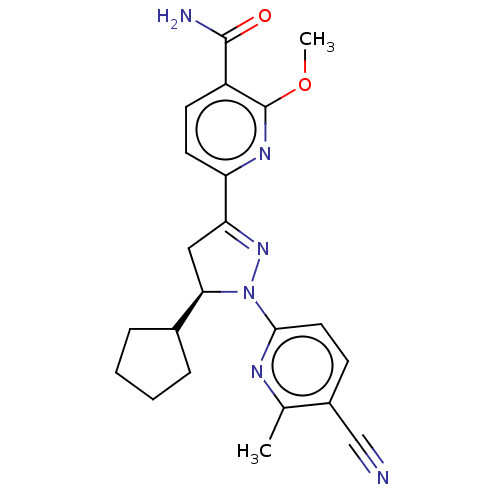 (CHEMBL3263763) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013419 (CHEMBL3263757) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013412 (CHEMBL3263752) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439645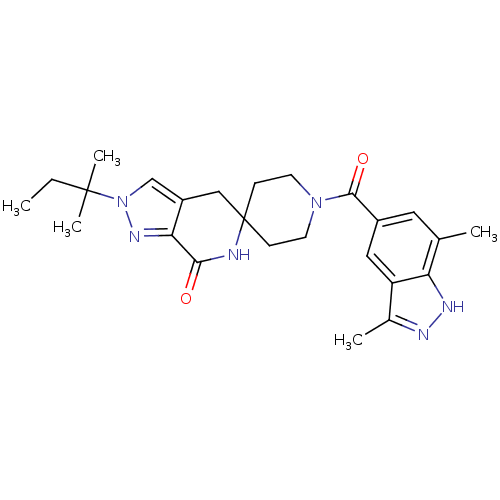 (CHEMBL2419607) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439642 (CHEMBL2419589 | US8993586, 105) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439635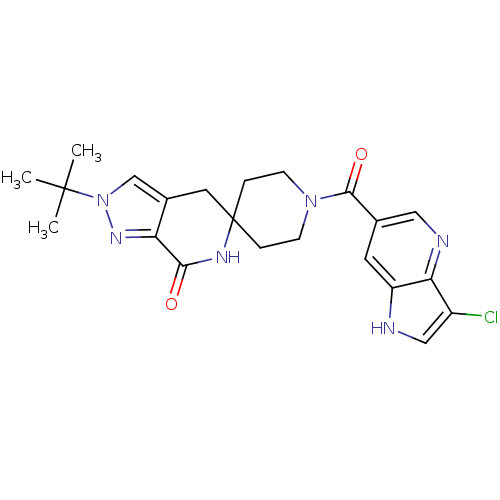 (CHEMBL2419594 | US8993586, 88) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439643 (CHEMBL2419598 | US8993586, 76) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439633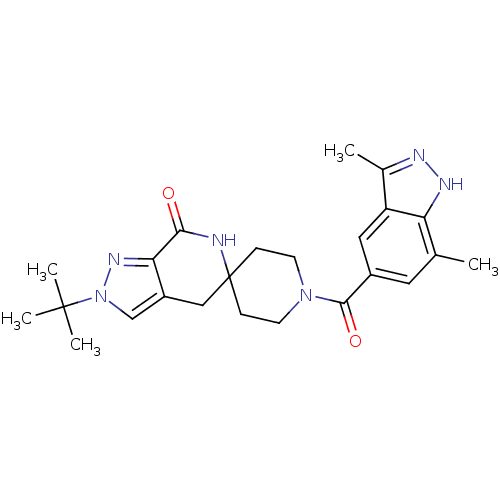 (CHEMBL2419604) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439638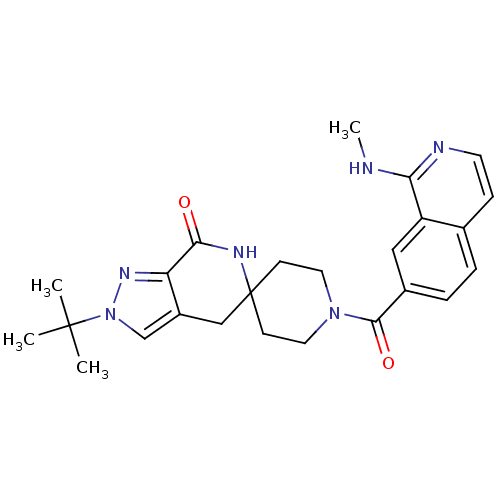 (CHEMBL2419599 | US8993586, 82) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439639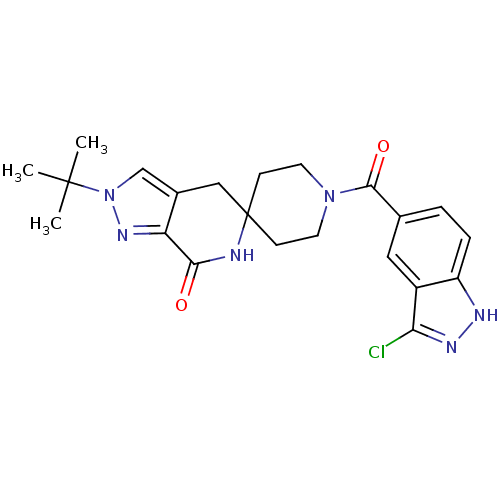 (CHEMBL2419591 | US8993586, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439636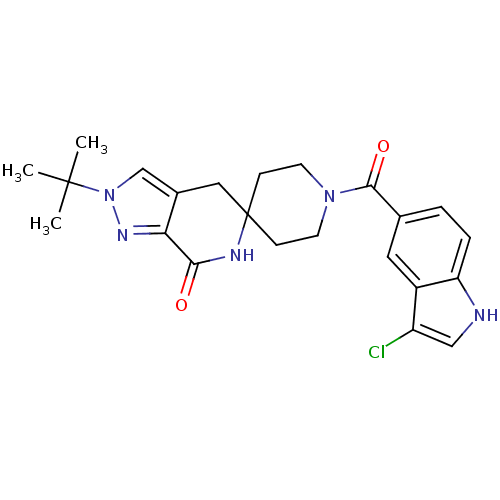 (CHEMBL2419592 | US8993586, 85) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439644 (CHEMBL2419593 | US8993586, 86) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013416 (CHEMBL3263754) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50324210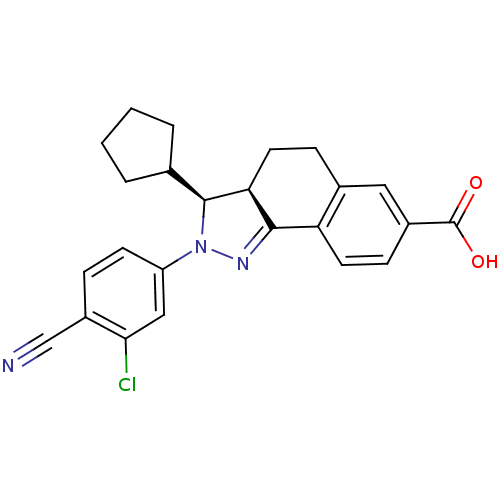 ((+/-)-(3SR,3aRS)-2-(3-Chloro-4-cyanophenyl)-3-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to mineralocorticoid receptor (unknown origin) | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013413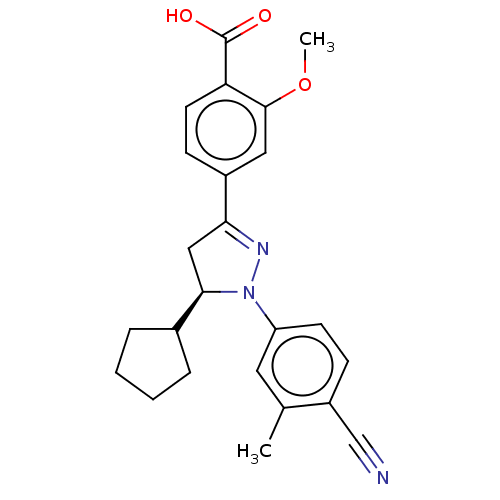 (CHEMBL3263750) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50324210 ((+/-)-(3SR,3aRS)-2-(3-Chloro-4-cyanophenyl)-3-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013427 (CHEMBL3263765) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439637 (CHEMBL2419601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439626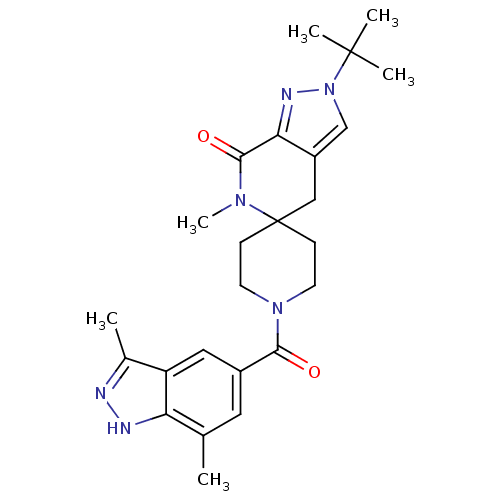 (CHEMBL2419610) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439634 (CHEMBL2419596 | US8993586, 71) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013414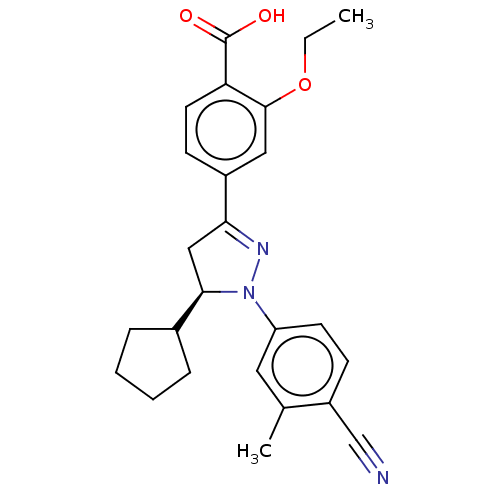 (CHEMBL3263751) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439641 (CHEMBL2419597 | US8993586, 55) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439635 (CHEMBL2419594 | US8993586, 88) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439636 (CHEMBL2419592 | US8993586, 85) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50324199 (4-[(5R)-1-(3-Chloro-4-cyanophenyl)-5-cyclopentyl-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50127711 (CHEMBL3629719) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Curated by ChEMBL | Assay Description Inhibition of rat liver ACC1 preincubated for 10 mins followed by acetyl-CoA/KHCO3/[14C]-NaHCO3/ATP addition measured after 20 mins by liquid scintil... | Bioorg Med Chem Lett 25: 5352-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.035 BindingDB Entry DOI: 10.7270/Q2W37Z4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439640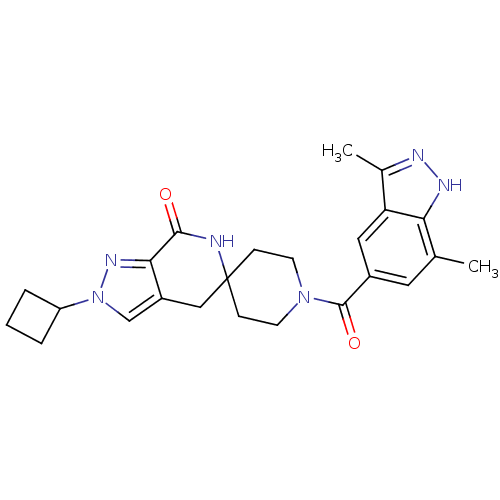 (CHEMBL2419606 | US8993586, 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439639 (CHEMBL2419591 | US8993586, 64) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013423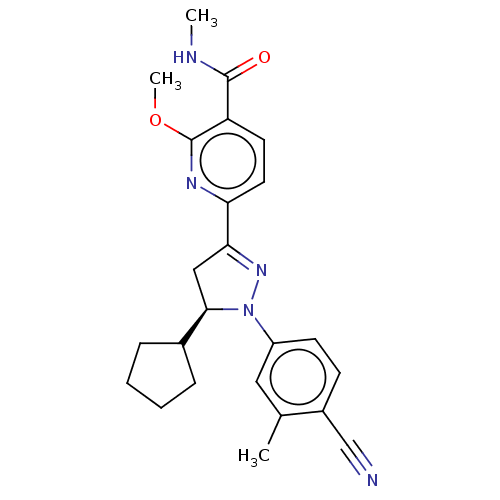 (CHEMBL3263761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013417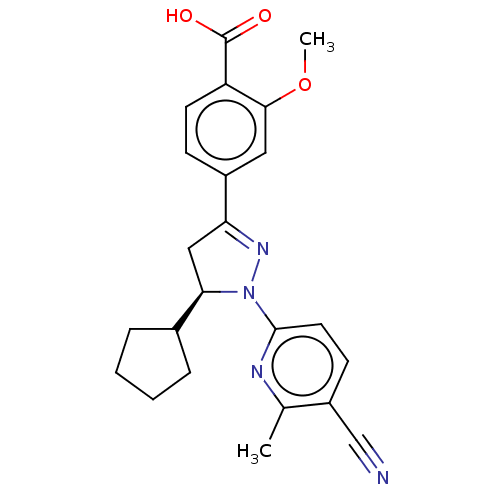 (CHEMBL3263755) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439638 (CHEMBL2419599 | US8993586, 82) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439630 (CHEMBL2419590 | US8993586, 80) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439637 (CHEMBL2419601) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013418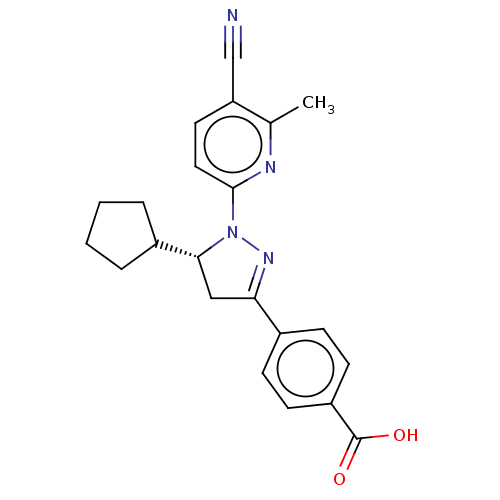 (CHEMBL3263756) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439645 (CHEMBL2419607) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50324201 (4-[(5R)-1-(3-Chloro-4-cyanophenyl)-5-cyclopentyl-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 165 total ) | Next | Last >> |