 Found 376 hits with Last Name = 'banoglu' and Initial = 'e'
Found 376 hits with Last Name = 'banoglu' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50611748

(CHEMBL3957641) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM164972
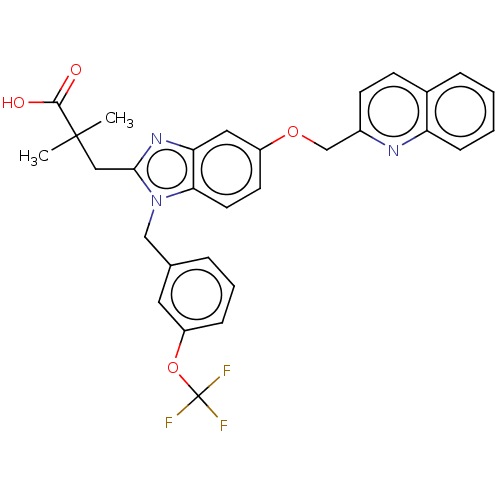
(US9067917, 5 | US9682971, 5)Show SMILES CC(C)(Cc1nc2cc(OCc3ccc4ccccc4n3)ccc2n1Cc1cccc(OC(F)(F)F)c1)C(O)=O Show InChI InChI=1S/C30H26F3N3O4/c1-29(2,28(37)38)16-27-35-25-15-22(39-18-21-11-10-20-7-3-4-9-24(20)34-21)12-13-26(25)36(27)17-19-6-5-8-23(14-19)40-30(31,32)33/h3-15H,16-18H2,1-2H3,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50611744
(CHEMBL5283430) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50611747
(CHEMBL5274418) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50611743
(CHEMBL5269712) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50611745

(CHEMBL5278870) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50029559
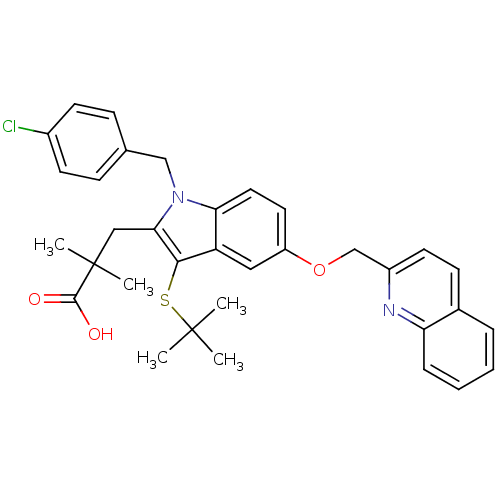
(2-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C(O)=O)n(Cc2ccc(Cl)cc2)c2ccc(OCc3ccc4ccccc4n3)cc12 Show InChI InChI=1S/C34H35ClN2O3S/c1-33(2,3)41-31-27-18-26(40-21-25-15-12-23-8-6-7-9-28(23)36-25)16-17-29(27)37(20-22-10-13-24(35)14-11-22)30(31)19-34(4,5)32(38)39/h6-18H,19-21H2,1-5H3,(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50325646
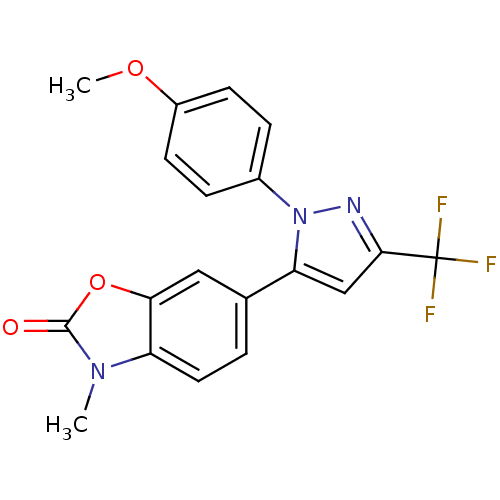
(1-(4-Methoxyphenyl)-3-trifluoromethyl-5-(3-methyl-...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc2n(C)c(=O)oc2c1)C(F)(F)F Show InChI InChI=1S/C19H14F3N3O3/c1-24-14-8-3-11(9-16(14)28-18(24)26)15-10-17(19(20,21)22)23-25(15)12-4-6-13(27-2)7-5-12/h3-10H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX1 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50152151
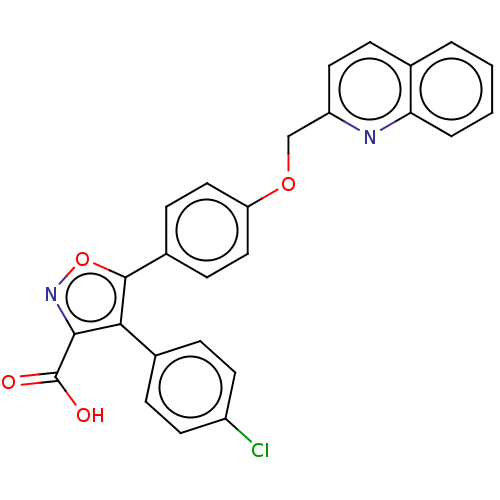
(CHEMBL3781006)Show SMILES OC(=O)c1noc(c1-c1ccc(Cl)cc1)-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C26H17ClN2O4/c27-19-10-5-17(6-11-19)23-24(26(30)31)29-33-25(23)18-8-13-21(14-9-18)32-15-20-12-7-16-3-1-2-4-22(16)28-20/h1-14H,15H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50152151

(CHEMBL3781006)Show SMILES OC(=O)c1noc(c1-c1ccc(Cl)cc1)-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C26H17ClN2O4/c27-19-10-5-17(6-11-19)23-24(26(30)31)29-33-25(23)18-8-13-21(14-9-18)32-15-20-12-7-16-3-1-2-4-22(16)28-20/h1-14H,15H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50325646

(1-(4-Methoxyphenyl)-3-trifluoromethyl-5-(3-methyl-...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc2n(C)c(=O)oc2c1)C(F)(F)F Show InChI InChI=1S/C19H14F3N3O3/c1-24-14-8-3-11(9-16(14)28-18(24)26)15-10-17(19(20,21)22)23-25(15)12-4-6-13(27-2)7-5-12/h3-10H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50325644
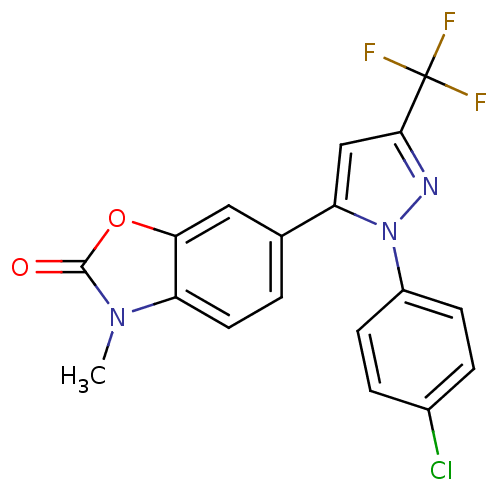
(1-(4-Chlorophenyl)-3-trifluoromethyl-5-(3-methyl-2...)Show SMILES Cn1c2ccc(cc2oc1=O)-c1cc(nn1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C18H11ClF3N3O2/c1-24-13-7-2-10(8-15(13)27-17(24)26)14-9-16(18(20,21)22)23-25(14)12-5-3-11(19)4-6-12/h2-9H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50202473

(CHEMBL3954390)Show SMILES CC(C)Cc1ccc(cc1)C(C)c1nc2cc(ccc2n1Cc1ccccc1Cl)C#N Show InChI InChI=1S/C27H26ClN3/c1-18(2)14-20-8-11-22(12-9-20)19(3)27-30-25-15-21(16-29)10-13-26(25)31(27)17-23-6-4-5-7-24(23)28/h4-13,15,18-19H,14,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human monocytes assessed as suppression of fMLP/LPS-stimulated cys-LT formation preincubated for 15 mins measured after 10 mins... |
Eur J Med Chem 122: 510-519 (2016)
Article DOI: 10.1016/j.ejmech.2016.07.004
BindingDB Entry DOI: 10.7270/Q2H41TCW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50353674
(CHEMBL1830480)Show SMILES CC(C)c1ccc(OC(=O)CCc2cc(-c3ccc(C)cc3)n(n2)-c2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C31H29N3O2/c1-21(2)23-12-16-27(17-13-23)36-31(35)19-15-26-20-29(25-10-8-22(3)9-11-25)34(33-26)30-18-14-24-6-4-5-7-28(24)32-30/h4-14,16-18,20-21H,15,19H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of COX1-mediated 12-HHT production in human platelet after 5 mins by HPLC analysis |
Eur J Med Chem 46: 5021-33 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.009
BindingDB Entry DOI: 10.7270/Q2445MVS |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50006805

(3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...)Show SMILES CC(C)c1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c2c1 Show InChI InChI=1S/C27H34ClNO2S/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18/h8-14,17H,15-16H2,1-7H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins |
Bioorg Med Chem 20: 3728-41 (2012)
Article DOI: 10.1016/j.bmc.2012.04.048
BindingDB Entry DOI: 10.7270/Q26H4JFM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM50085160

(CHEMBL32842 | L-674636 | {4-(4-Chloro-phenyl)-1-[4...)Show SMILES OC(=O)CSC(CCCc1ccc(Cl)cc1)c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C28H26ClNO3S/c29-23-13-8-20(9-14-23)4-3-7-27(34-19-28(31)32)22-11-16-25(17-12-22)33-18-24-15-10-21-5-1-2-6-26(21)30-24/h1-2,5-6,8-17,27H,3-4,7,18-19H2,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50006805

(3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...)Show SMILES CC(C)c1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c2c1 Show InChI InChI=1S/C27H34ClNO2S/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18/h8-14,17H,15-16H2,1-7H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50202473

(CHEMBL3954390)Show SMILES CC(C)Cc1ccc(cc1)C(C)c1nc2cc(ccc2n1Cc1ccccc1Cl)C#N Show InChI InChI=1S/C27H26ClN3/c1-18(2)14-20-8-11-22(12-9-20)19(3)27-30-25-15-21(16-29)10-13-26(25)31(27)17-23-6-4-5-7-24(23)28/h4-13,15,18-19H,14,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human monocytes assessed as suppression of A23187-stimulated 5-LO product formation preincubated for 15 mins followed by A23187... |
Eur J Med Chem 122: 510-519 (2016)
Article DOI: 10.1016/j.ejmech.2016.07.004
BindingDB Entry DOI: 10.7270/Q2H41TCW |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50611748

(CHEMBL3957641) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leukotriene C4 synthase
(Homo sapiens (Human)) | BDBM50462326

(CHEMBL4251024)Show SMILES CC(C)Cc1ccc(cc1)C(C)c1nc2cc(CCC(O)=O)ccc2n1Cc1ccccc1Cl Show InChI InChI=1S/C29H31ClN2O2/c1-19(2)16-21-8-12-23(13-9-21)20(3)29-31-26-17-22(11-15-28(33)34)10-14-27(26)32(29)18-24-6-4-5-7-25(24)30/h4-10,12-14,17,19-20H,11,15-16,18H2,1-3H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of LTC4 synthase in LPS/fMLP-stimulated human monocytes assessed as decrease in cys-LT formation pre-incubated with LPS for 30 mins and la... |
Eur J Med Chem 150: 876-899 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.045
BindingDB Entry DOI: 10.7270/Q25D8VHD |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50603600
(CHEMBL5197602)Show SMILES S=c1[nH]nc(o1)-c1ccc2[nH]c(nc2c1)-c1ccc(Oc2ccccc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114167
BindingDB Entry DOI: 10.7270/Q2TM7G6D |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50603599

(CHEMBL5187474)Show SMILES S=c1[nH]nc(o1)-c1ccc2[nH]c(nc2c1)-c1ccc(OCc2ccccc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114167
BindingDB Entry DOI: 10.7270/Q2TM7G6D |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50202473

(CHEMBL3954390)Show SMILES CC(C)Cc1ccc(cc1)C(C)c1nc2cc(ccc2n1Cc1ccccc1Cl)C#N Show InChI InChI=1S/C27H26ClN3/c1-18(2)14-20-8-11-22(12-9-20)19(3)27-30-25-15-21(16-29)10-13-26(25)31(27)17-23-6-4-5-7-24(23)28/h4-13,15,18-19H,14,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human monocytes assessed as suppression of A23187-stimulated 5-LO product formation using 10 uM arachidonic acid as substrate p... |
Eur J Med Chem 122: 510-519 (2016)
Article DOI: 10.1016/j.ejmech.2016.07.004
BindingDB Entry DOI: 10.7270/Q2H41TCW |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM168655

(US9079866, 25 | US9079866, 32 | US9745328, Compoun...)Show SMILES C[C@H](CO)NS(=O)(=O)c1ccccc1-c1ccc(c(F)c1)-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H19FN4O3S/c1-12(11-25)24-28(26,27)18-5-3-2-4-14(18)13-6-7-15(16(20)8-13)17-9-23-19(21)10-22-17/h2-10,12,24-25H,11H2,1H3,(H2,21,23)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM22334

(BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...)Show InChI InChI=1S/C17H17NO3/c1-14(19)18(20)12-6-8-15-7-5-11-17(13-15)21-16-9-3-2-4-10-16/h2-11,13,20H,12H2,1H3/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 5-Lipoxygenase expressed in Escherichia coli lysate after 10 mins by HPLC analysis |
Eur J Med Chem 46: 5021-33 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.009
BindingDB Entry DOI: 10.7270/Q2445MVS |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50385390
(CHEMBL2036163)Show SMILES CC(C)Cc1ccc(cc1)C(C)c1nc2ccccc2n1Cc1ccccc1Cl Show InChI InChI=1S/C26H27ClN2/c1-18(2)16-20-12-14-21(15-13-20)19(3)26-28-24-10-6-7-11-25(24)29(26)17-22-8-4-5-9-23(22)27/h4-15,18-19H,16-17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50462327
(CHEMBL4246749)Show SMILES CC(C)Cc1ccc(cc1)C(C)c1nc2cc(ccc2n1Cc1ccccc1Cl)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C33H31ClN2O2/c1-21(2)18-23-8-10-24(11-9-23)22(3)32-35-30-19-27(25-12-14-26(15-13-25)33(37)38)16-17-31(30)36(32)20-28-6-4-5-7-29(28)34/h4-17,19,21-22H,18,20H2,1-3H3,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in A23187-stimulated human neutrophils assessed as reduction in 5-LO product formation preincubated for 15 mins followed by A23187... |
Eur J Med Chem 150: 876-899 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.045
BindingDB Entry DOI: 10.7270/Q25D8VHD |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50353666

(CHEMBL1830471)Show SMILES Cc1ccc(cc1)-c1cc(CCC(=O)N2CCN(Cc3ccc(cc3)C(C)(C)C)CC2)nn1-c1ccc2ccccc2n1 Show InChI InChI=1S/C37H41N5O/c1-27-9-13-30(14-10-27)34-25-32(39-42(34)35-19-15-29-7-5-6-8-33(29)38-35)18-20-36(43)41-23-21-40(22-24-41)26-28-11-16-31(17-12-28)37(2,3)4/h5-17,19,25H,18,20-24,26H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of COX1-mediated 12-HHT production in human platelet after 5 mins by HPLC analysis |
Eur J Med Chem 46: 5021-33 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.009
BindingDB Entry DOI: 10.7270/Q2445MVS |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50611742
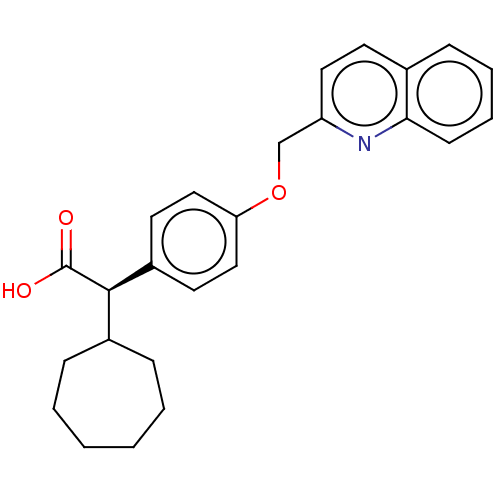
(CHEMBL5275767) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50468648

(CHEMBL4291851)Show SMILES CCOC(=O)c1c(Cc2ccc(Cl)c(Cl)c2)[nH]c2c1cc(O)c1ccccc21 Show InChI InChI=1S/C22H17Cl2NO3/c1-2-28-22(27)20-15-11-19(26)13-5-3-4-6-14(13)21(15)25-18(20)10-12-7-8-16(23)17(24)9-12/h3-9,11,25-26H,2,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Campania Luigi Vanvitelli
Curated by ChEMBL
| Assay Description
Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... |
Eur J Med Chem 155: 946-960 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.041
BindingDB Entry DOI: 10.7270/Q2WM1H3T |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50462326

(CHEMBL4251024)Show SMILES CC(C)Cc1ccc(cc1)C(C)c1nc2cc(CCC(O)=O)ccc2n1Cc1ccccc1Cl Show InChI InChI=1S/C29H31ClN2O2/c1-19(2)16-21-8-12-23(13-9-21)20(3)29-31-26-17-22(11-15-28(33)34)10-14-27(26)32(29)18-24-6-4-5-7-25(24)30/h4-10,12-14,17,19-20H,11,15-16,18H2,1-3H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in A23187-stimulated human neutrophils assessed as reduction in 5-LO product formation preincubated for 15 mins followed by A23187... |
Eur J Med Chem 150: 876-899 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.045
BindingDB Entry DOI: 10.7270/Q25D8VHD |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50516943

(CHEMBL332610)Show InChI InChI=1S/C16H16O4/c1-19-13-7-12(8-14(10-13)20-2)4-3-11-5-6-15(17)16(18)9-11/h3-10,17-18H,1-2H3/b4-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania "L. Vanvitelli"
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as A23187-stimulated LTB4 production preincubated for 15 mins followed by A23187 addition and measur... |
Eur J Med Chem 180: 637-647 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.033
BindingDB Entry DOI: 10.7270/Q29S1VDF |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50462328

(CHEMBL4251469)Show SMILES CC(C)Cc1ccc(cc1)C(C)c1nc2cc(ccc2n1Cc1ccccc1Cl)-c1n[nH]c(=S)o1 Show InChI InChI=1S/C28H27ClN4OS/c1-17(2)14-19-8-10-20(11-9-19)18(3)26-30-24-15-21(27-31-32-28(35)34-27)12-13-25(24)33(26)16-22-6-4-5-7-23(22)29/h4-13,15,17-18H,14,16H2,1-3H3,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in A23187-stimulated human neutrophils assessed as reduction in 5-LO product formation preincubated for 15 mins followed by A23187... |
Eur J Med Chem 150: 876-899 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.045
BindingDB Entry DOI: 10.7270/Q25D8VHD |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50468648

(CHEMBL4291851)Show SMILES CCOC(=O)c1c(Cc2ccc(Cl)c(Cl)c2)[nH]c2c1cc(O)c1ccccc21 Show InChI InChI=1S/C22H17Cl2NO3/c1-2-28-22(27)20-15-11-19(26)13-5-3-4-6-14(13)21(15)25-18(20)10-12-7-8-16(23)17(24)9-12/h3-9,11,25-26H,2,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Campania Luigi Vanvitelli
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins followed by subs... |
Eur J Med Chem 155: 946-960 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.041
BindingDB Entry DOI: 10.7270/Q2WM1H3T |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50462326

(CHEMBL4251024)Show SMILES CC(C)Cc1ccc(cc1)C(C)c1nc2cc(CCC(O)=O)ccc2n1Cc1ccccc1Cl Show InChI InChI=1S/C29H31ClN2O2/c1-19(2)16-21-8-12-23(13-9-21)20(3)29-31-26-17-22(11-15-28(33)34)10-14-27(26)32(29)18-24-6-4-5-7-25(24)30/h4-10,12-14,17,19-20H,11,15-16,18H2,1-3H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LO in A23187-stimulated human neutrophils assessed as reduction in 5-LO product formation preincubated for 15 mins followed by A23187... |
Eur J Med Chem 150: 876-899 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.045
BindingDB Entry DOI: 10.7270/Q25D8VHD |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM22334

(BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...)Show InChI InChI=1S/C17H17NO3/c1-14(19)18(20)12-6-8-15-7-5-11-17(13-15)21-16-9-3-2-4-10-16/h2-11,13,20H,12H2,1H3/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of 5-Lipoxygenase in arachidonic acid-stimulated human neutrophils after 15 mins by HPLC analysis in presence of A23187 |
Eur J Med Chem 46: 5021-33 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.009
BindingDB Entry DOI: 10.7270/Q2445MVS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50325645
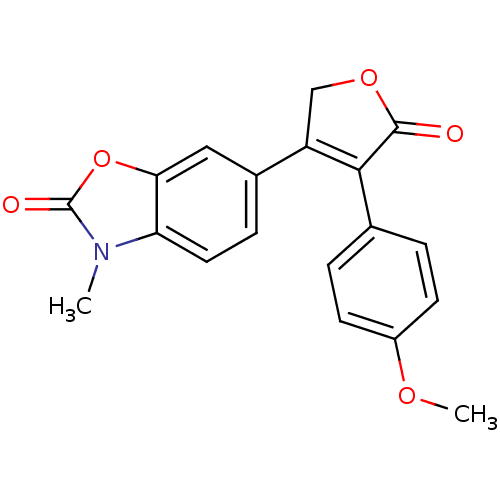
(3-(4-Methoxyphenyl)-4-(3-methyl-2-oxo-3H-benzoxazo...)Show SMILES COc1ccc(cc1)C1=C(COC1=O)c1ccc2n(C)c(=O)oc2c1 |t:9| Show InChI InChI=1S/C19H15NO5/c1-20-15-8-5-12(9-16(15)25-19(20)22)14-10-24-18(21)17(14)11-3-6-13(23-2)7-4-11/h3-9H,10H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX1 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM167032

(US9073876, 118 | US9732093, Compound 118)Show InChI InChI=1S/C18H16FN5O/c19-16-13(14-9-24-15(20)10-23-14)6-5-12(11-3-1-4-11)17(16)25-18-21-7-2-8-22-18/h2,5-11H,1,3-4H2,(H2,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX1 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of COX1-mediated 12-HHT production in human platelet after 5 mins by HPLC analysis |
Eur J Med Chem 46: 5021-33 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.009
BindingDB Entry DOI: 10.7270/Q2445MVS |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM31133
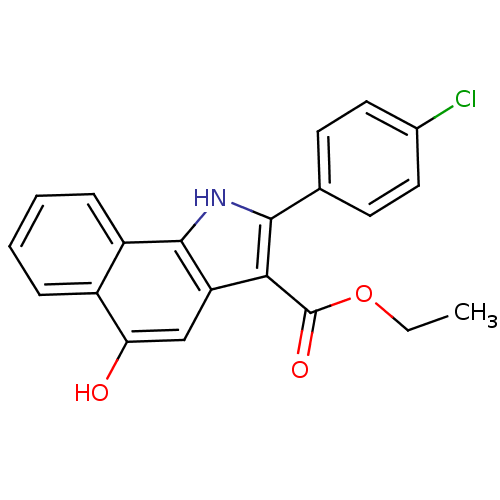
(5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11l)Show SMILES CCOC(=O)c1c([nH]c2c1cc(O)c1ccccc21)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H16ClNO3/c1-2-26-21(25)18-16-11-17(24)14-5-3-4-6-15(14)20(16)23-19(18)12-7-9-13(22)10-8-12/h3-11,23-24H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Campania Luigi Vanvitelli
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins followed by subs... |
Eur J Med Chem 155: 946-960 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.041
BindingDB Entry DOI: 10.7270/Q2WM1H3T |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM50611741
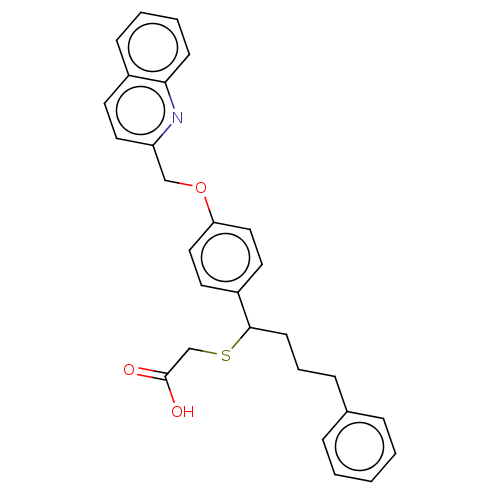
(AZ-12096971 | AZD-4769 | AZD4769 | Azd 4769 | Azd-...) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50516942

(CHEMBL119576 | NSC-381281)Show InChI InChI=1S/C17H18O4/c1-19-14-8-13(9-15(11-14)20-2)5-4-12-6-7-16(18)17(10-12)21-3/h4-11,18H,1-3H3/b5-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania "L. Vanvitelli"
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX in human PMNL cells assessed as A23187-stimulated LTB4 production preincubated for 15 mins followed by A23187 addition and measur... |
Eur J Med Chem 180: 637-647 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.033
BindingDB Entry DOI: 10.7270/Q29S1VDF |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50462326

(CHEMBL4251024)Show SMILES CC(C)Cc1ccc(cc1)C(C)c1nc2cc(CCC(O)=O)ccc2n1Cc1ccccc1Cl Show InChI InChI=1S/C29H31ClN2O2/c1-19(2)16-21-8-12-23(13-9-21)20(3)29-31-26-17-22(11-15-28(33)34)10-14-27(26)32(29)18-24-6-4-5-7-25(24)30/h4-10,12-14,17,19-20H,11,15-16,18H2,1-3H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in A23187-stimulated human monocytes assessed as reduction in 5-LO product formation preincubated for 15 mins followed by A23187 a... |
Eur J Med Chem 150: 876-899 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.045
BindingDB Entry DOI: 10.7270/Q25D8VHD |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50202473

(CHEMBL3954390)Show SMILES CC(C)Cc1ccc(cc1)C(C)c1nc2cc(ccc2n1Cc1ccccc1Cl)C#N Show InChI InChI=1S/C27H26ClN3/c1-18(2)14-20-8-11-22(12-9-20)19(3)27-30-25-15-21(16-29)10-13-26(25)31(27)17-23-6-4-5-7-24(23)28/h4-13,15,18-19H,14,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human neutrophils assessed as suppression of A23187-stimulated 5-LO product formation using arachidonic acid as substrate prein... |
Eur J Med Chem 122: 510-519 (2016)
Article DOI: 10.1016/j.ejmech.2016.07.004
BindingDB Entry DOI: 10.7270/Q2H41TCW |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50462356
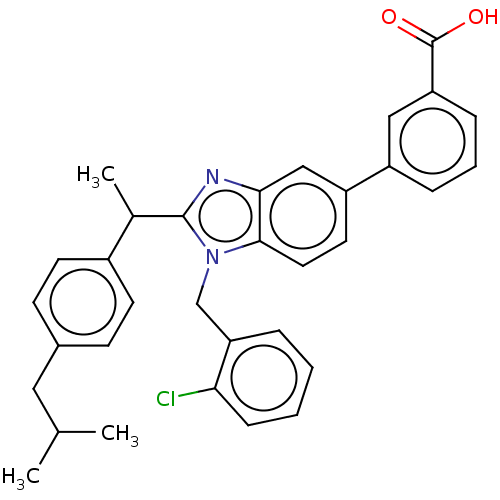
(CHEMBL4250561)Show SMILES CC(C)Cc1ccc(cc1)C(C)c1nc2cc(ccc2n1Cc1ccccc1Cl)-c1cccc(c1)C(O)=O Show InChI InChI=1S/C33H31ClN2O2/c1-21(2)17-23-11-13-24(14-12-23)22(3)32-35-30-19-26(25-8-6-9-27(18-25)33(37)38)15-16-31(30)36(32)20-28-7-4-5-10-29(28)34/h4-16,18-19,21-22H,17,20H2,1-3H3,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in A23187-stimulated human neutrophils assessed as reduction in 5-LO product formation preincubated for 15 mins followed by A23187... |
Eur J Med Chem 150: 876-899 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.045
BindingDB Entry DOI: 10.7270/Q25D8VHD |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50462340

(CHEMBL4247640)Show SMILES CC(C)Cc1ccc(cc1)C(C)c1nc2cc(ccc2n1Cc1ccccc1Cl)-c1nnco1 Show InChI InChI=1S/C28H27ClN4O/c1-18(2)14-20-8-10-21(11-9-20)19(3)27-31-25-15-22(28-32-30-17-34-28)12-13-26(25)33(27)16-23-6-4-5-7-24(23)29/h4-13,15,17-19H,14,16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in A23187-stimulated human neutrophils assessed as reduction in 5-LO product formation preincubated for 15 mins followed by A23187... |
Eur J Med Chem 150: 876-899 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.045
BindingDB Entry DOI: 10.7270/Q25D8VHD |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50603603
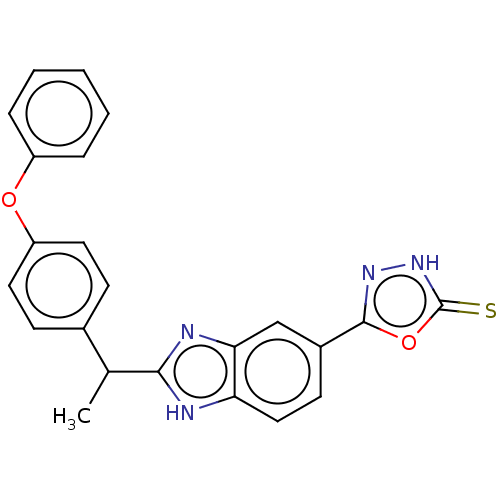
(CHEMBL5190547)Show SMILES CC(c1nc2cc(ccc2[nH]1)-c1n[nH]c(=S)o1)c1ccc(Oc2ccccc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114167
BindingDB Entry DOI: 10.7270/Q2TM7G6D |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM130674

(US8829200, 8)Show SMILES CNC(=O)c1ccc(cc1-c1nc2cc(ccc2n1C(C)(C)C)-c1cnc(N)nc1)C#N Show InChI InChI=1S/C24H23N7O/c1-24(2,3)31-20-8-6-15(16-12-28-23(26)29-13-16)10-19(20)30-21(31)18-9-14(11-25)5-7-17(18)22(32)27-4/h5-10,12-13H,1-4H3,(H,27,32)(H2,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50468659

(CHEMBL4286295)Show SMILES CCOC(=O)c1c(Cc2c(F)cccc2F)[nH]c2c1cc(O)c1ccccc21 Show InChI InChI=1S/C22H17F2NO3/c1-2-28-22(27)20-15-11-19(26)12-6-3-4-7-13(12)21(15)25-18(20)10-14-16(23)8-5-9-17(14)24/h3-9,11,25-26H,2,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Campania Luigi Vanvitelli
Curated by ChEMBL
| Assay Description
Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... |
Eur J Med Chem 155: 946-960 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.041
BindingDB Entry DOI: 10.7270/Q2WM1H3T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data