

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2A serotonin receptor. | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM82517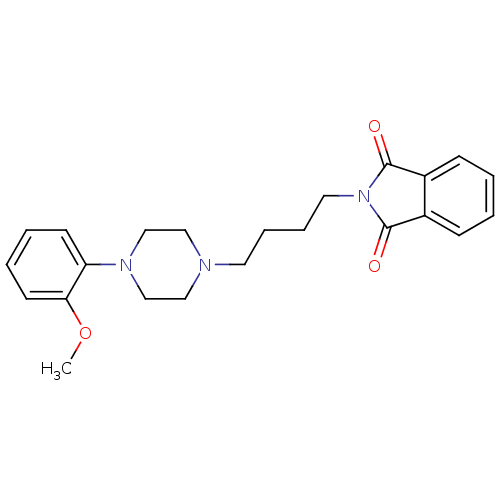 (2-{4-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-butyl}-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by PDSP Ki Database | Neuron 11: 449-58 (1993) Article DOI: 10.1016/0896-6273(93)90149-l BindingDB Entry DOI: 10.7270/Q269723D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM21392 (3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by PDSP Ki Database | Neuron 11: 449-58 (1993) Article DOI: 10.1016/0896-6273(93)90149-l BindingDB Entry DOI: 10.7270/Q269723D | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50028600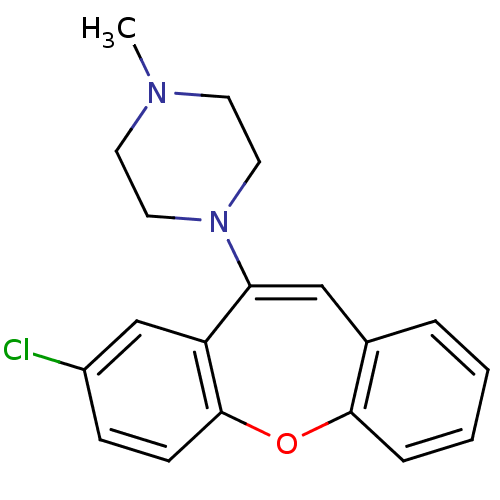 (1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiperone to the Dopamine receptor D4 in COS7 cells | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50028600 (1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-4 receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM78940 (METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by PDSP Ki Database | Neuron 11: 449-58 (1993) Article DOI: 10.1016/0896-6273(93)90149-l BindingDB Entry DOI: 10.7270/Q269723D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50028602 (1-(2-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-4 receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50028601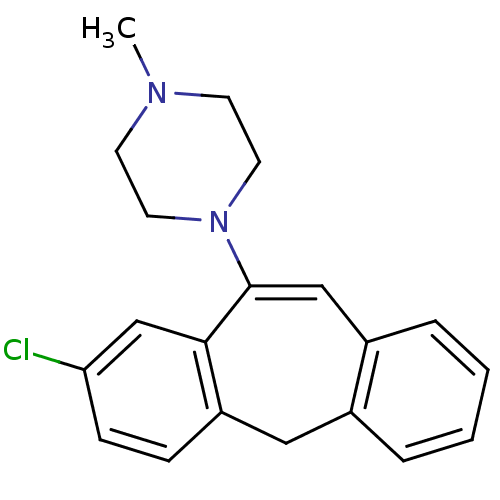 (1-(8-Chloro-5H-dibenzo[a,d]cyclohepten-10-yl)-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-2(long) receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50028601 (1-(8-Chloro-5H-dibenzo[a,d]cyclohepten-10-yl)-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-4 receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiperone to the Dopamine receptor D2L in COS7 cells | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50028598 (1-Dibenzo[b,f]oxepin-10-yl-4-methyl-piperazine | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiperone to the Dopamine receptor D4 in COS7 cells | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50028598 (1-Dibenzo[b,f]oxepin-10-yl-4-methyl-piperazine | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-4 receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50028601 (1-(8-Chloro-5H-dibenzo[a,d]cyclohepten-10-yl)-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [125I]-LSD binding to NIH 3T3 cells transfected with cloned rat 5-hydroxytryptamine 2A receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50028600 (1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-2(long) receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50028600 (1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiperone to the Dopamine receptor D2L in COS7 cells | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50028602 (1-(2-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [125I]-LSD binding to NIH 3T3 cells transfected with cloned rat 5-hydroxytryptamine 2A receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50028600 (1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity of the compound was evaluated by inhibition of [125I]-LSD binding to NIH 3T3 cells transfected with cloned rat 5-hydroxytryptamine 2C recept... | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50028600 (1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2C serotonin receptor in NIH3T3 cell line membr... | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50036437 (4-Dibenzo[b,f]oxepin-10-yl-1-methyl-piperidine; hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2A serotonin receptor in NIH3T3 cell line membr... | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50028976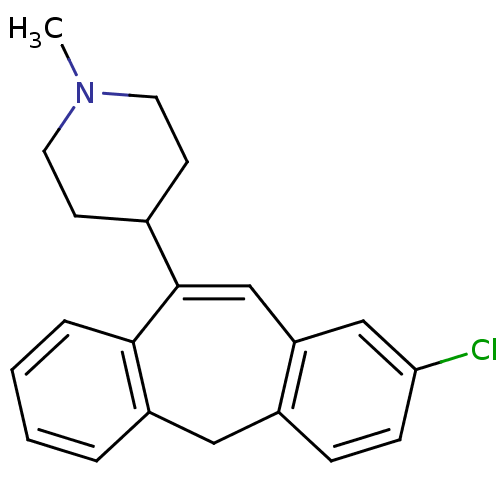 (4-(2-Chloro-5H-dibenzo[a,d]cyclohepten-10-yl)-1-me...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated as inhibition constant for serotonin 5-hydroxytryptamine 2C receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50027065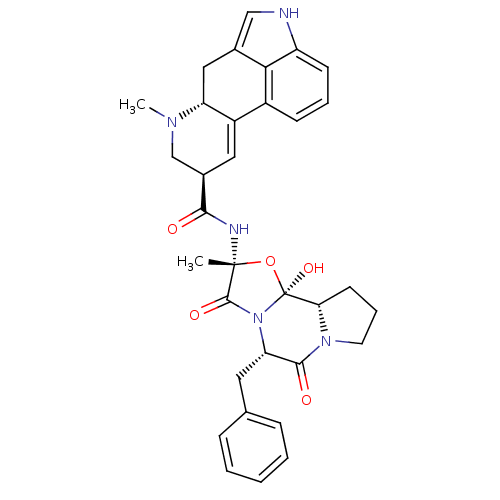 ((5'alpha)-12'-hydroxy-2'-methyl-5'-(phenylmethyl)e...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by PDSP Ki Database | Neuron 11: 449-58 (1993) Article DOI: 10.1016/0896-6273(93)90149-l BindingDB Entry DOI: 10.7270/Q269723D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50028600 (1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [125I]-LSD binding to NIH 3T3 cells transfected with cloned rat 5-hydroxytryptamine 2A receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50028599 (1-(2-Chloro-5H-dibenzo[a,d]cyclohepten-10-yl)-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [125I]-LSD binding to NIH 3T3 cells transfected with cloned rat 5-hydroxytryptamine 2A receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50028976 (4-(2-Chloro-5H-dibenzo[a,d]cyclohepten-10-yl)-1-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated as inhibition constant for serotonin 5-hydroxytryptamine 2A receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50028600 (1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2A serotonin receptor in NIH3T3 cell line membr... | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50028598 (1-Dibenzo[b,f]oxepin-10-yl-4-methyl-piperazine | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2A serotonin receptor in NIH3T3 cell line membr... | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50028598 (1-Dibenzo[b,f]oxepin-10-yl-4-methyl-piperazine | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [125I]-LSD binding to NIH 3T3 cells transfected with cloned rat 5-hydroxytryptamine 2A receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50036439 (4-(5H-Dibenzo[a,d]cyclohepten-10-yl)-1-ethyl-1,2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2A serotonin receptor. | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50010598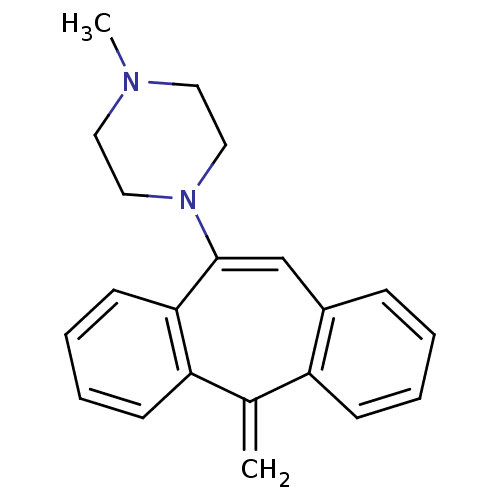 (1-Methyl-4-(5-methylene-5H-dibenzo[a,d]cyclohepten...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity of the compound was evaluated as inhibition constant for dopamine receptor D1 using [3H]-SCH- 23390 as radioligand | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM22871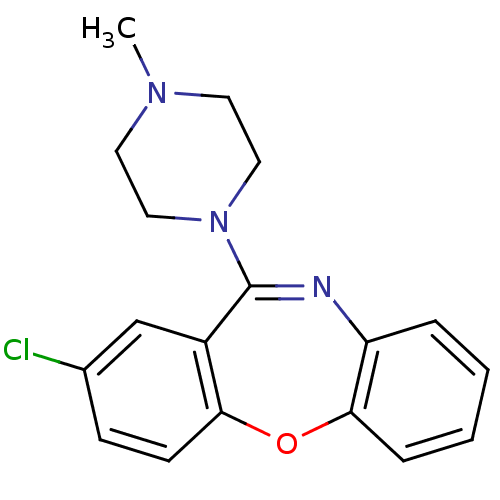 (13-chloro-10-(4-methylpiperazin-1-yl)-2-oxa-9-azat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-4 receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM22871 (13-chloro-10-(4-methylpiperazin-1-yl)-2-oxa-9-azat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiperone to the Dopamine receptor D4 in COS7 cells | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by PDSP Ki Database | Neuron 11: 449-58 (1993) Article DOI: 10.1016/0896-6273(93)90149-l BindingDB Entry DOI: 10.7270/Q269723D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50028602 (1-(2-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity of the compound was evaluated by inhibition of [125I]-LSD binding to NIH 3T3 cells transfected with cloned rat 5-hydroxytryptamine 2C recept... | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50036440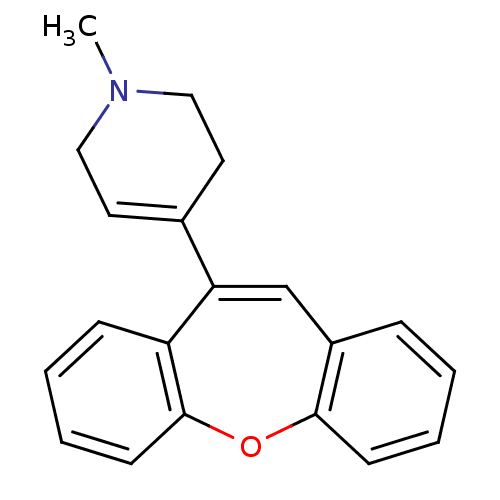 (4-Dibenzo[b,f]oxepin-10-yl-1-methyl-1,2,3,6-tetrah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2A serotonin receptor in NIH3T3 cell line membr... | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50028598 (1-Dibenzo[b,f]oxepin-10-yl-4-methyl-piperazine | C...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2C serotonin receptor in NIH3T3 cell line membr... | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50028598 (1-Dibenzo[b,f]oxepin-10-yl-4-methyl-piperazine | C...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity of the compound was evaluated by inhibition of [125I]-LSD binding to NIH 3T3 cells transfected with cloned rat 5-hydroxytryptamine 2C recept... | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50036441 (4-(5H-Dibenzo[a,d]cyclohepten-10-yl)-1-methyl-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2A serotonin receptor. | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50036442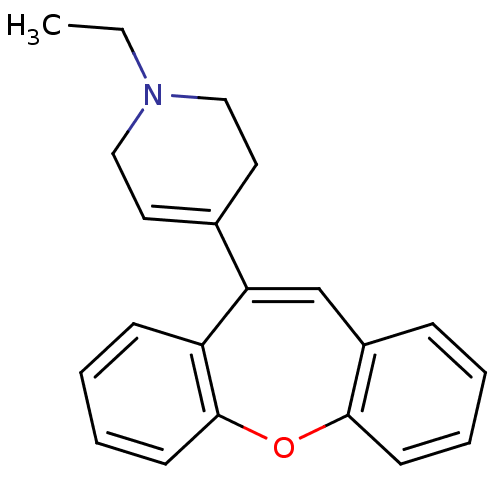 (4-Dibenzo[b,f]oxepin-10-yl-1-ethyl-1,2,3,6-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2A serotonin receptor in NIH3T3 cell line membr... | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50028601 (1-(8-Chloro-5H-dibenzo[a,d]cyclohepten-10-yl)-4-me...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity of the compound was evaluated by inhibition of [125I]-LSD binding to NIH 3T3 cells transfected with cloned rat 5-hydroxytryptamine 2C recept... | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5B (RAT) | BDBM50027065 ((5'alpha)-12'-hydroxy-2'-methyl-5'-(phenylmethyl)e...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 90: 3452-6 (1993) Article DOI: 10.1073/pnas.90.8.3452 BindingDB Entry DOI: 10.7270/Q21C1VCC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50010594 (2-Chloro-11-(4-methyl-piperazin-1-yl)-5H-dibenzo[b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated as inhibition constant for serotonin 5-hydroxytryptamine 2A receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50036439 (4-(5H-Dibenzo[a,d]cyclohepten-10-yl)-1-ethyl-1,2,3...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2C serotonin receptor. | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50028599 (1-(2-Chloro-5H-dibenzo[a,d]cyclohepten-10-yl)-4-me...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity of the compound was evaluated by inhibition of [125I]-LSD binding to NIH 3T3 cells transfected with cloned rat 5-hydroxytryptamine 2C recept... | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (RAT) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 90: 2184-8 (1993) Article DOI: 10.1073/pnas.90.6.2184 BindingDB Entry DOI: 10.7270/Q2765CVH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiperone to the Dopamine receptor D4 in COS7 cells | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated as inhibition constant for serotonin 5-hydroxytryptamine 2A receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2C serotonin receptor. | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2A serotonin receptor. | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50036437 (4-Dibenzo[b,f]oxepin-10-yl-1-methyl-piperidine; hy...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2C serotonin receptor in NIH3T3 cell line membr... | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated as inhibition constant for serotonin 5-hydroxytryptamine 2C receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 273 total ) | Next | Last >> |