

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19968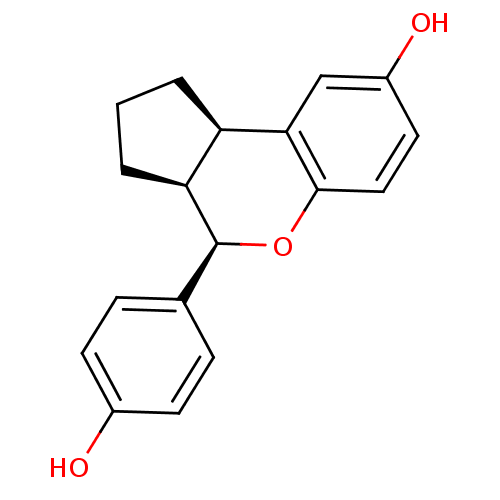 ((2R,6S,7R)-7-(4-hydroxyphenyl)-8-oxatricyclo[7.4.0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.190 | -54.9 | n/a | n/a | 0.660 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | J Med Chem 49: 6155-7 (2006) Article DOI: 10.1021/jm060491j BindingDB Entry DOI: 10.7270/Q2N014SV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.299 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110732 (CHEMBL3605927) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.543 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.998 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110730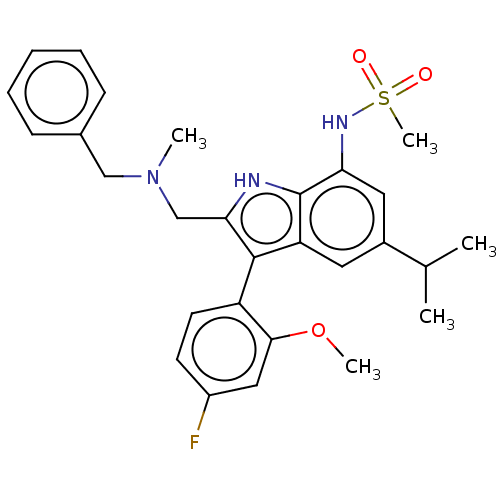 (CHEMBL3605919) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110733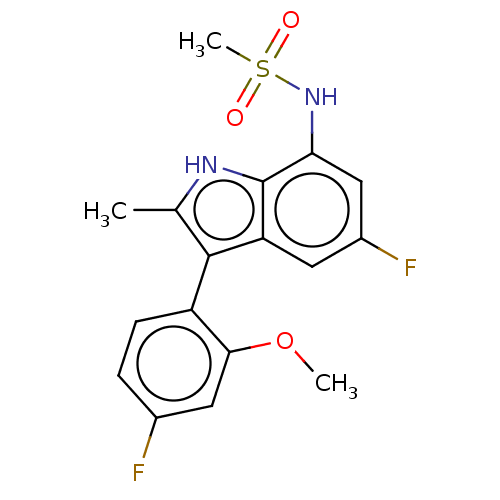 (CHEMBL3605929) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19969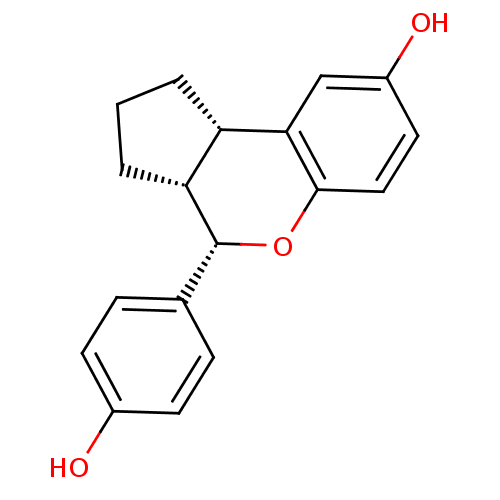 ((2S,6R,7S)-7-(4-hydroxyphenyl)-8-oxatricyclo[7.4.0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.54 | -49.8 | n/a | n/a | 3.61 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | J Med Chem 49: 6155-7 (2006) Article DOI: 10.1021/jm060491j BindingDB Entry DOI: 10.7270/Q2N014SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha/alpha (Homo sapiens (Human)) | BDBM50178953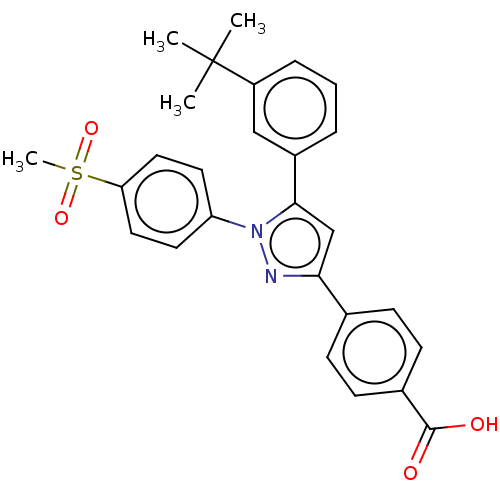 (CHEMBL3815166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay | Bioorg Med Chem Lett 26: 3274-3277 (2016) Article DOI: 10.1016/j.bmcl.2016.05.056 BindingDB Entry DOI: 10.7270/Q2FF3V9N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50110735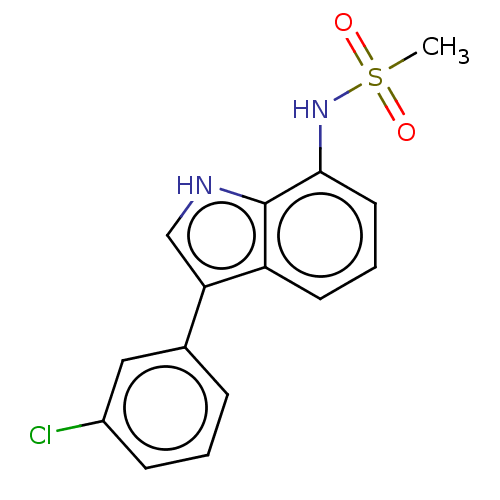 (CHEMBL3605932) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competit... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19968 ((2R,6S,7R)-7-(4-hydroxyphenyl)-8-oxatricyclo[7.4.0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.68 | -48.4 | n/a | n/a | 19.4 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | J Med Chem 49: 6155-7 (2006) Article DOI: 10.1021/jm060491j BindingDB Entry DOI: 10.7270/Q2N014SV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110731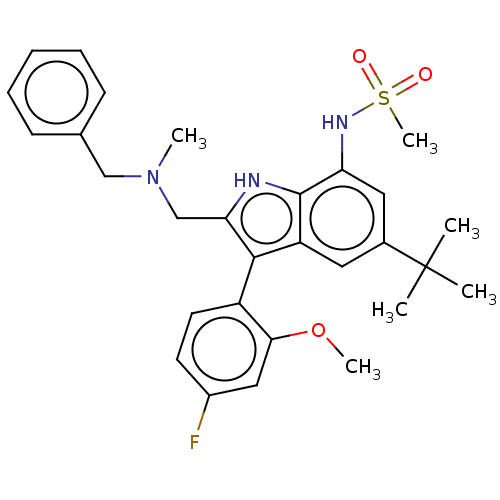 (CHEMBL3605926) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha/alpha (Homo sapiens (Human)) | BDBM50178952 (CHEMBL3814574) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay | Bioorg Med Chem Lett 26: 3274-3277 (2016) Article DOI: 10.1016/j.bmcl.2016.05.056 BindingDB Entry DOI: 10.7270/Q2FF3V9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110736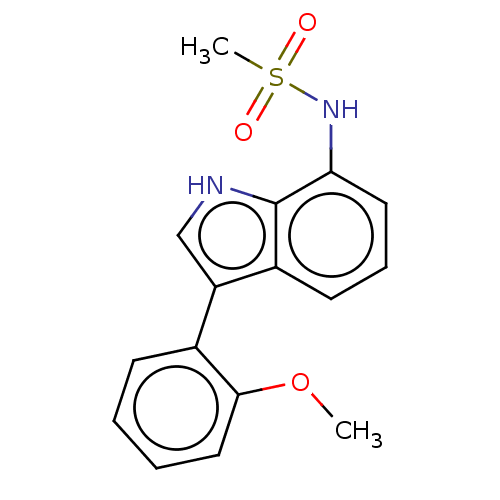 (CHEMBL3605933) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50586388 (CHEMBL5081557) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01918 BindingDB Entry DOI: 10.7270/Q2B85D1S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110735 (CHEMBL3605932) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110735 (CHEMBL3605932) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110734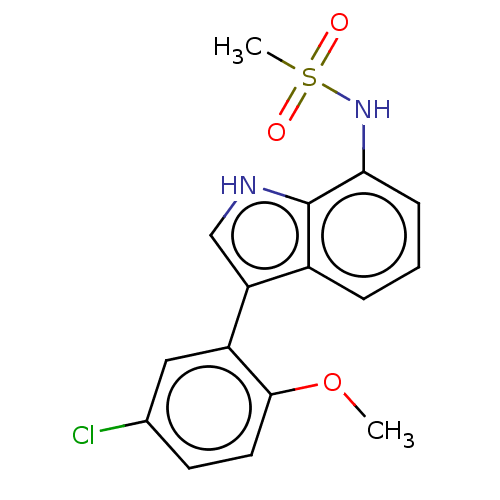 (CHEMBL3605930) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM498074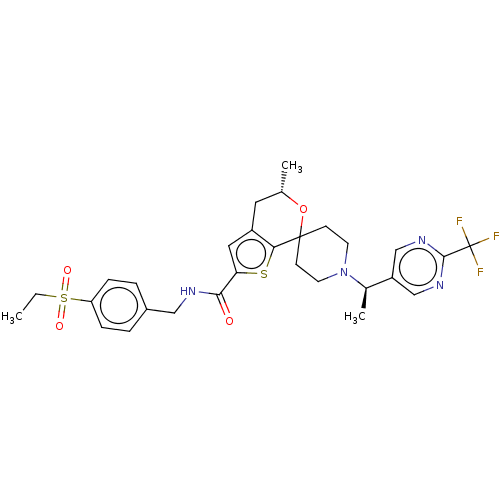 ((5′S)óN-[4-(Ethylsulfonyl)benzyl]-5′-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01918 BindingDB Entry DOI: 10.7270/Q2B85D1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha/alpha (Homo sapiens (Human)) | BDBM50178954 (CHEMBL3813779) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay | Bioorg Med Chem Lett 26: 3274-3277 (2016) Article DOI: 10.1016/j.bmcl.2016.05.056 BindingDB Entry DOI: 10.7270/Q2FF3V9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha/alpha (Homo sapiens (Human)) | BDBM50178961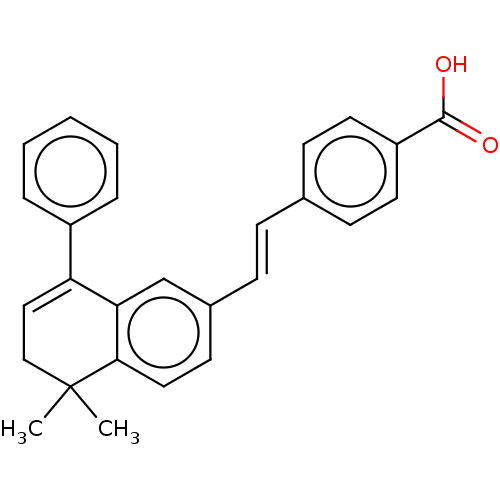 (CHEMBL2385268) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay | Bioorg Med Chem Lett 26: 3274-3277 (2016) Article DOI: 10.1016/j.bmcl.2016.05.056 BindingDB Entry DOI: 10.7270/Q2FF3V9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase (Plasmodium falciparum (isolate 3D7)) | BDBM50496902 (CHEMBL3237438) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum N-myristoyltransferase by CPM fluorescence assay | J Med Chem 57: 2773-88 (2014) Article DOI: 10.1021/jm500066b BindingDB Entry DOI: 10.7270/Q27084DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50211726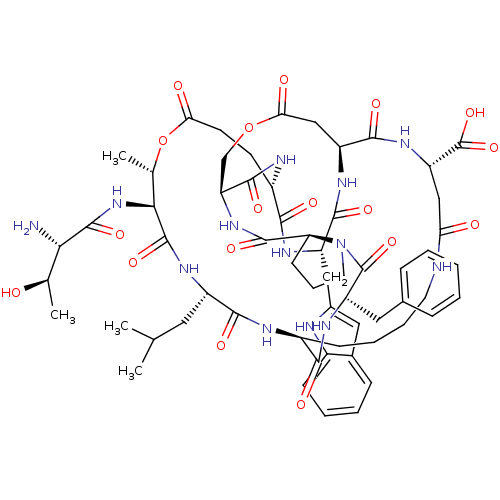 (CHEMBL403896 | FR-901451) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase | Bioorg Med Chem 15: 4618-28 (2007) Article DOI: 10.1016/j.bmc.2007.03.082 BindingDB Entry DOI: 10.7270/Q2D21ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50080264 (1N-[21-benzyl-29-[(Z)-ethylidene]-13,14,23-trihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase | Bioorg Med Chem 15: 4618-28 (2007) Article DOI: 10.1016/j.bmc.2007.03.082 BindingDB Entry DOI: 10.7270/Q2D21ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19969 ((2S,6R,7S)-7-(4-hydroxyphenyl)-8-oxatricyclo[7.4.0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 14.5 | -44.3 | n/a | n/a | 32.5 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | J Med Chem 49: 6155-7 (2006) Article DOI: 10.1021/jm060491j BindingDB Entry DOI: 10.7270/Q2N014SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase (Plasmodium falciparum (isolate 3D7)) | BDBM50496896 (CHEMBL3237425) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum N-myristoyltransferase by CPM fluorescence assay | J Med Chem 57: 2773-88 (2014) Article DOI: 10.1021/jm500066b BindingDB Entry DOI: 10.7270/Q27084DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase (Plasmodium falciparum (isolate 3D7)) | BDBM50496912 (CHEMBL3237422) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum N-myristoyltransferase by CPM fluorescence assay | J Med Chem 57: 2773-88 (2014) Article DOI: 10.1021/jm500066b BindingDB Entry DOI: 10.7270/Q27084DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50586394 (CHEMBL5078351) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01918 BindingDB Entry DOI: 10.7270/Q2B85D1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50586395 (CHEMBL5069696) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01918 BindingDB Entry DOI: 10.7270/Q2B85D1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50586396 (CHEMBL5079705) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01918 BindingDB Entry DOI: 10.7270/Q2B85D1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM302860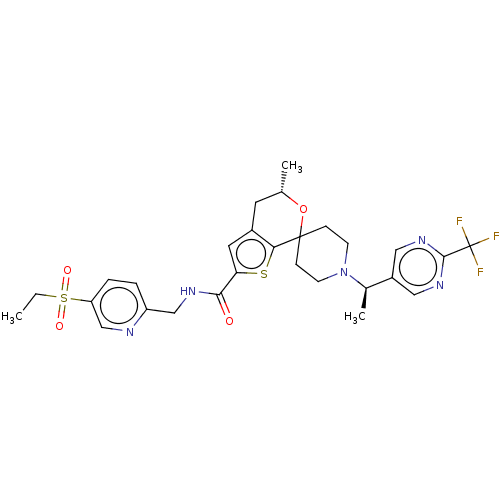 ((2S)-1-(3-Thienyl)propan-2-ol | US9598431, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01918 BindingDB Entry DOI: 10.7270/Q2B85D1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50006730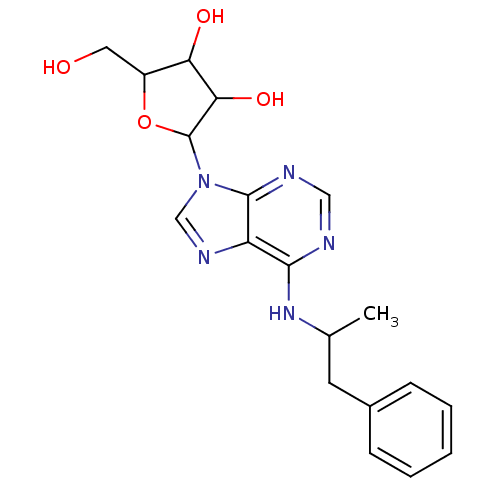 ((R)-2-Hydroxymethyl-5-[6-(1-methyl-2-phenyl-ethyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Whitby Research, Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 265: 227-36 (1993) BindingDB Entry DOI: 10.7270/Q23J3BGM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50148269 (CHEMBL3770219) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Binding affinity to human N-myristoyltransferase by fluorescence analysis | Medchemcomm 6: 1761-1766 (2016) BindingDB Entry DOI: 10.7270/Q2TB18R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50311138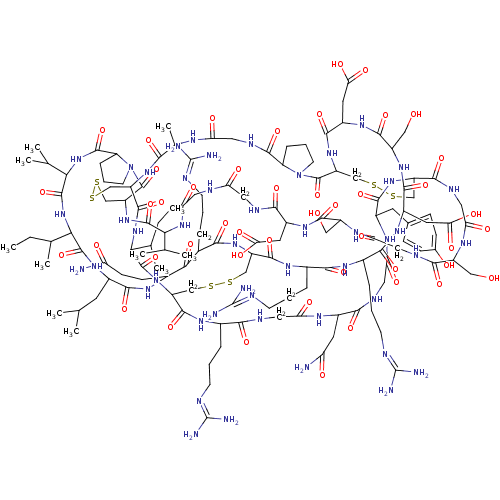 (CHEMBL1077421) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London SW7 2AZ Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase by substrate hydrolysis based fluorescence assay | J Med Chem 52: 6197-200 (2009) Article DOI: 10.1021/jm901233u BindingDB Entry DOI: 10.7270/Q2FT8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110733 (CHEMBL3605929) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110743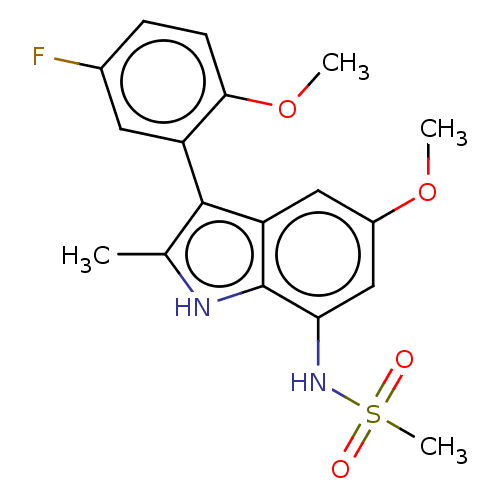 (CHEMBL3605924) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50135488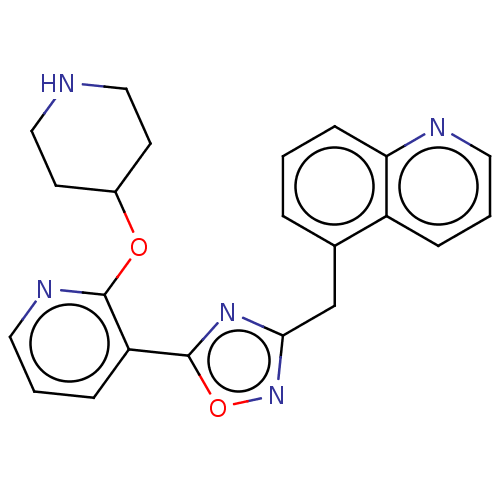 (CHEMBL3754571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human NMT1 by 7-diethylamine-3-(4'maleimidylphenyl)-4-methylcoumarin based fluorescence assay | Medchemcomm 6: 1767-1772 (2016) BindingDB Entry DOI: 10.7270/Q27M09RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase (Plasmodium falciparum (isolate 3D7)) | BDBM50496904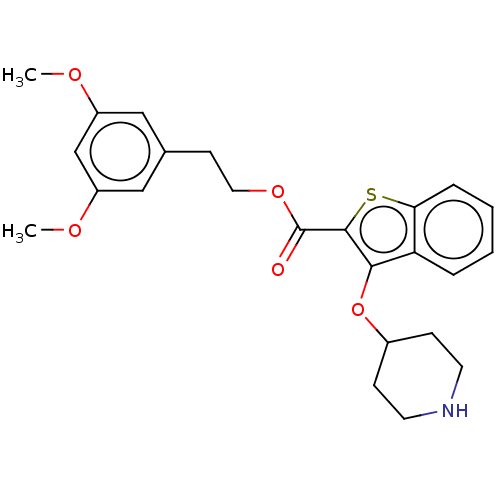 (CHEMBL3237412) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum N-myristoyltransferase by CPM fluorescence assay | J Med Chem 57: 2773-88 (2014) Article DOI: 10.1021/jm500066b BindingDB Entry DOI: 10.7270/Q27084DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50110736 (CHEMBL3605933) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competit... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50027680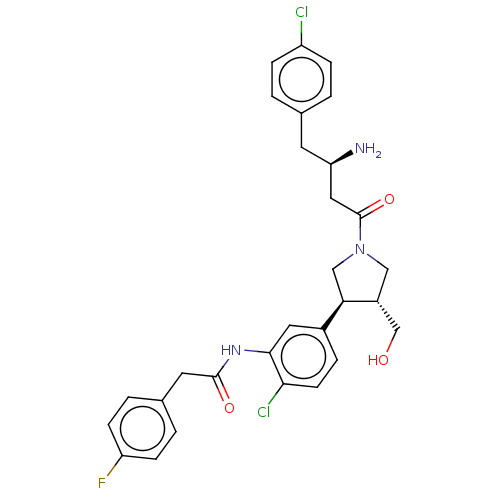 (CHEMBL3357975) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human NMT1 using GSNKSKPKDASQRRR-NH2 as substrate by CPM fluorescence assay | J Med Chem 57: 8664-70 (2014) Article DOI: 10.1021/jm5011397 BindingDB Entry DOI: 10.7270/Q2X63PJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50586389 (CHEMBL5093568) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01918 BindingDB Entry DOI: 10.7270/Q2B85D1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110789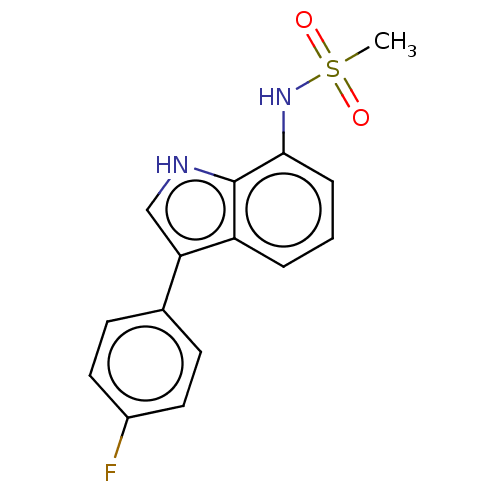 (CHEMBL3605934) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50311137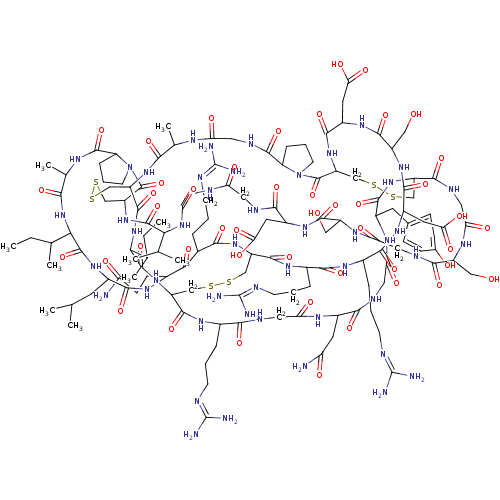 (CHEMBL1077420) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London SW7 2AZ Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase by substrate hydrolysis based fluorescence assay | J Med Chem 52: 6197-200 (2009) Article DOI: 10.1021/jm901233u BindingDB Entry DOI: 10.7270/Q2FT8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50039998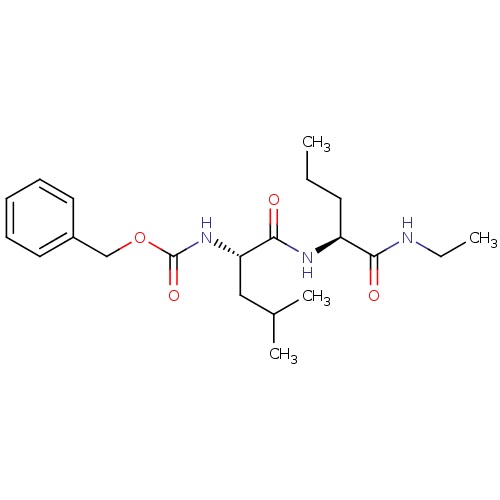 (CHEMBL99129 | [(S)-1-((S)-1-Ethylcarbamoyl-butylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alkermes, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of porcine erythrocyte calpain 1. | J Med Chem 37: 2918-29 (1994) BindingDB Entry DOI: 10.7270/Q2BR8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase (Plasmodium falciparum (isolate 3D7)) | BDBM50496914 (CHEMBL3237428) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum N-myristoyltransferase by CPM fluorescence assay | J Med Chem 57: 2773-88 (2014) Article DOI: 10.1021/jm500066b BindingDB Entry DOI: 10.7270/Q27084DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-2 catalytic subunit (Homo sapiens (Human)) | BDBM50039995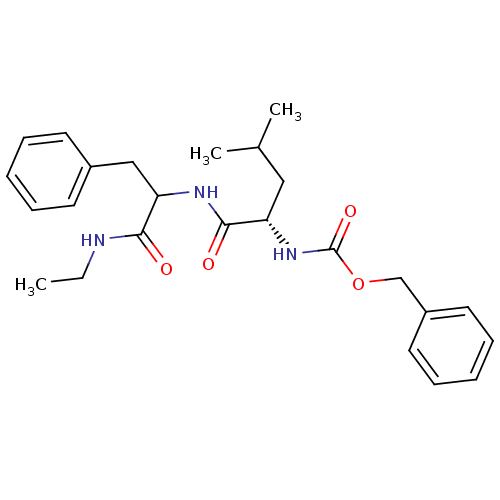 (CHEMBL98777 | [(S)-1-(1-Ethylcarbamoyl-2-phenyl-et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alkermes, Inc. Curated by ChEMBL | Assay Description Tested for inhibitory activity on human calpain 2 from placenta | J Med Chem 37: 2918-29 (1994) BindingDB Entry DOI: 10.7270/Q2BR8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50040007 (CHEMBL316932 | [(S)-1-((S)-1-Ethylcarbamoyl-2-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alkermes, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of porcine erythrocyte calpain 1. | J Med Chem 37: 2918-29 (1994) BindingDB Entry DOI: 10.7270/Q2BR8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50110743 (CHEMBL3605924) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competit... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-2 catalytic subunit (Bos taurus) | BDBM50039995 (CHEMBL98777 | [(S)-1-(1-Ethylcarbamoyl-2-phenyl-et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alkermes, Inc. Curated by ChEMBL | Assay Description Tested for inhibitory activity on bovine calpain 2 from heart | J Med Chem 37: 2918-29 (1994) BindingDB Entry DOI: 10.7270/Q2BR8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110739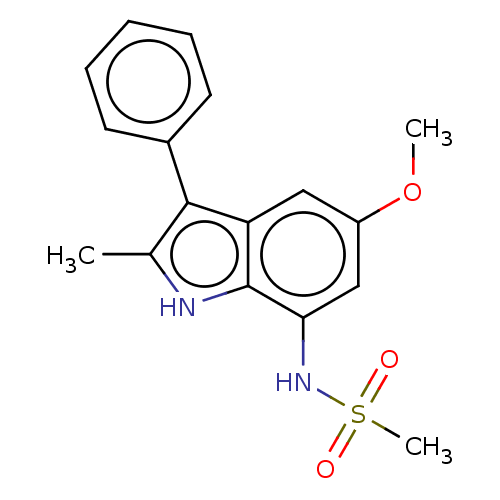 (CHEMBL3605923) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 790 total ) | Next | Last >> |